Abstract
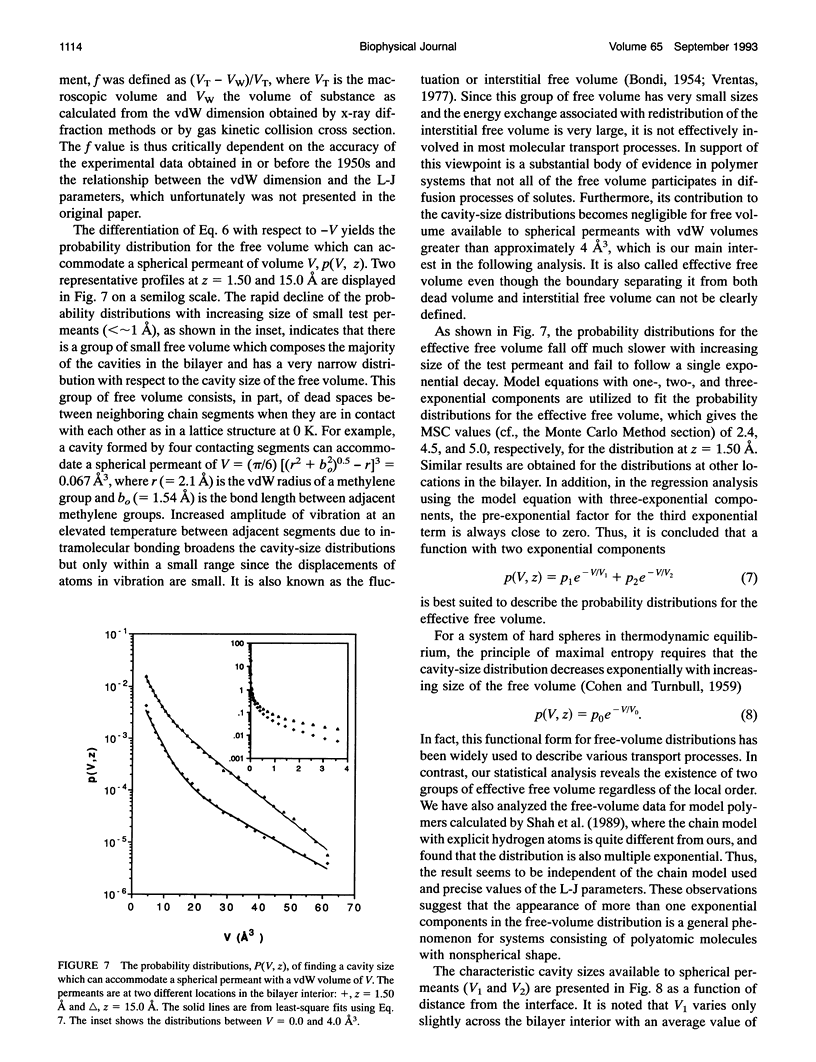
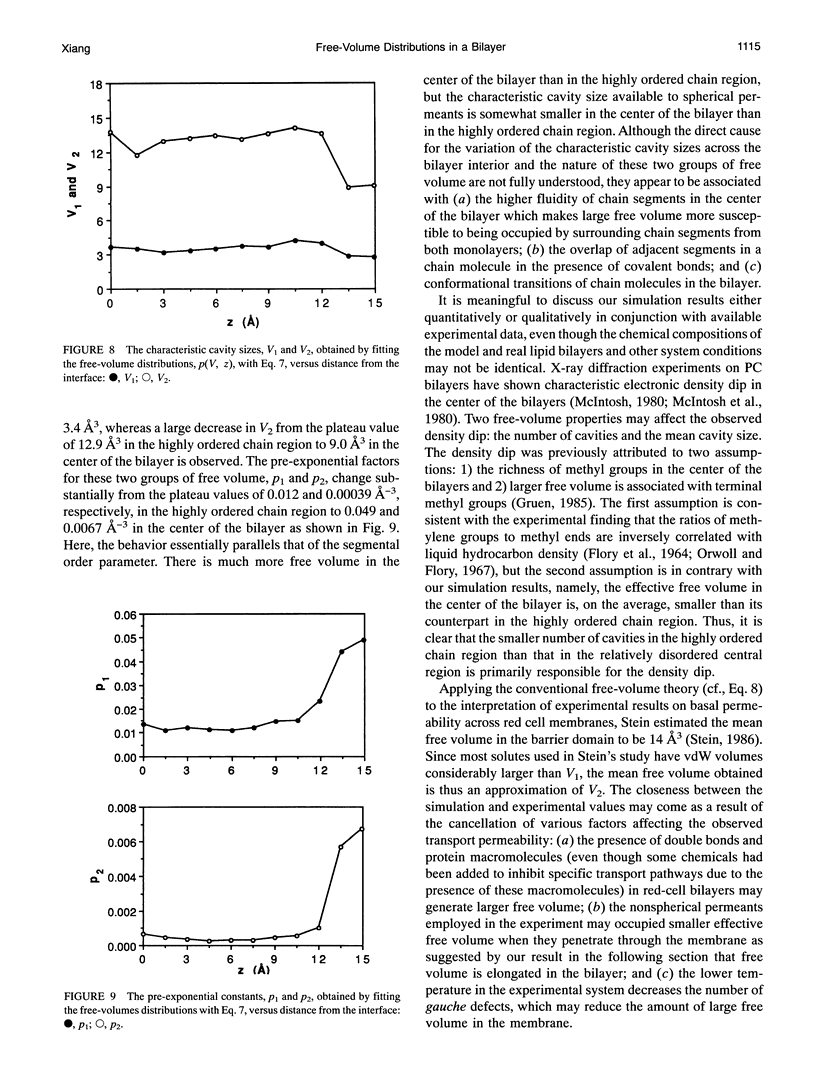
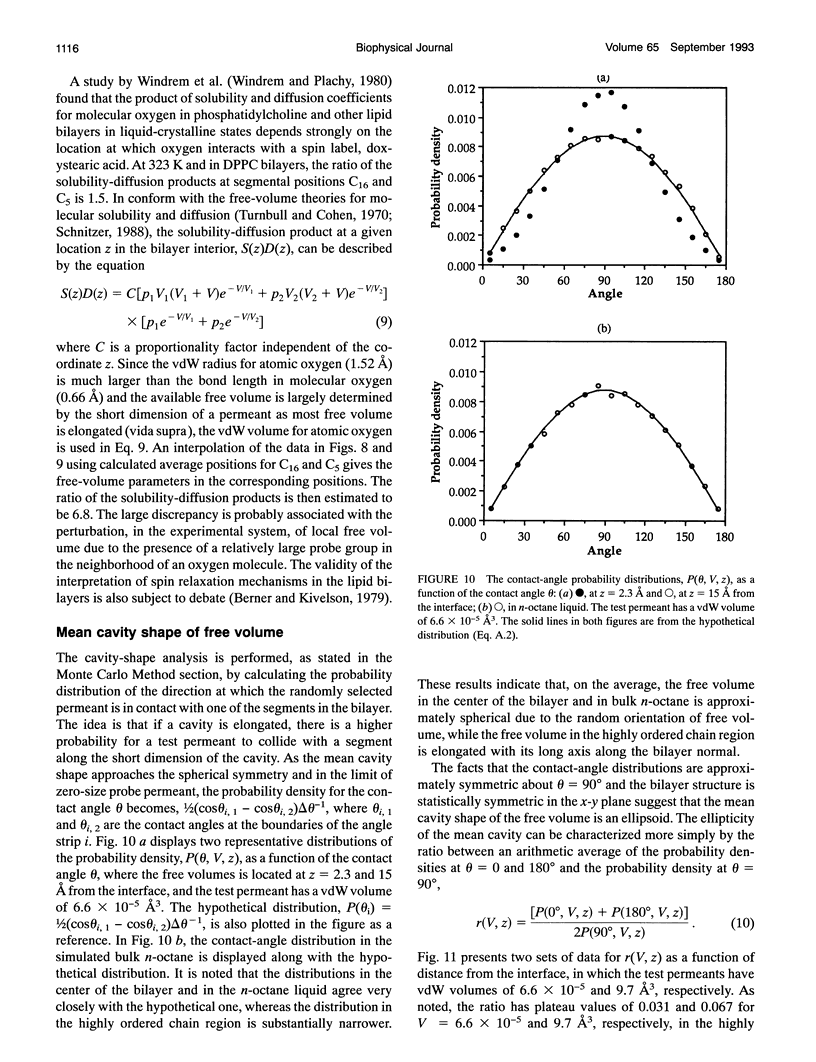
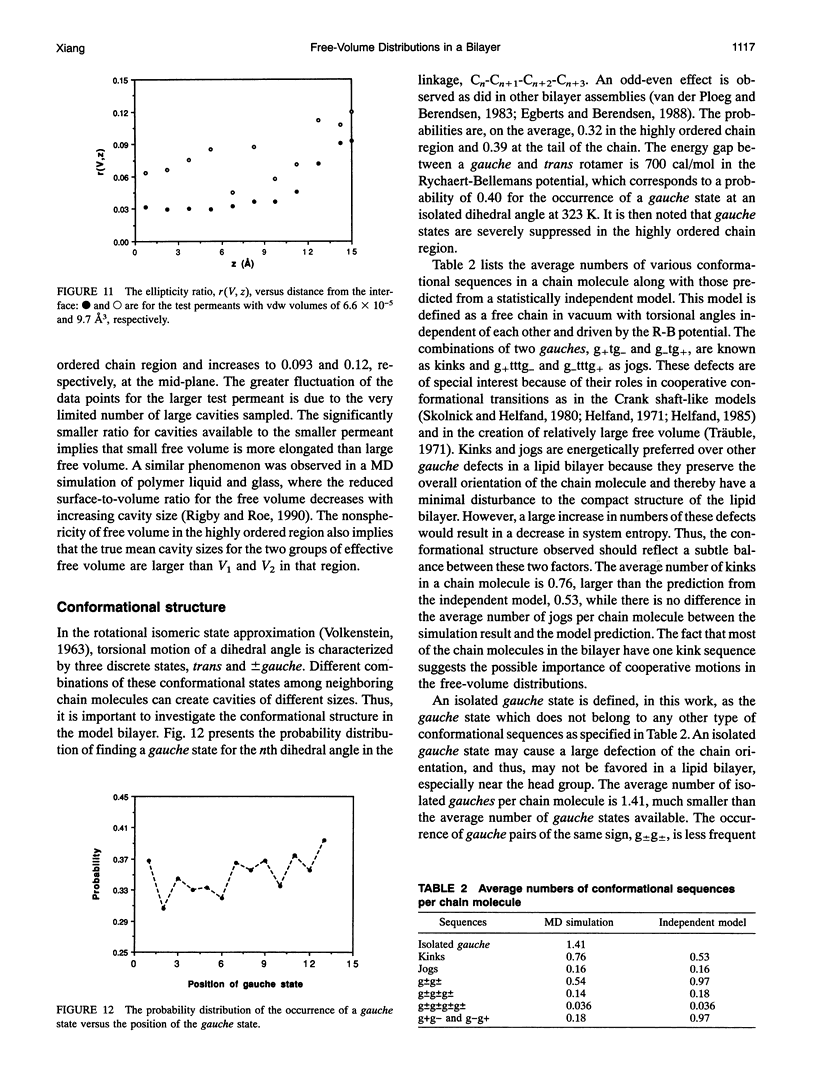
A novel combined approach of molecular dynamics (MD) and Monte Carlo simulations is developed to calculate various free-volume distributions as a function of position in a lipid bilayer membrane at 323 K. The model bilayer consists of 2 x 100 chain molecules with each chain molecule having 15 carbon segments and one head group and subject to forces restricting bond stretching, bending, and torsional motions. At a surface density of 30 A2/chain molecule, the probability density of finding effective free volume available to spherical permeants displays a distribution with two exponential components. Both pre-exponential factors, p1 and p2, remain roughly constant in the highly ordered chain region with average values of 0.012 and 0.00039 A-3, respectively, and increase to 0.049 and 0.0067 A-3 at the mid-plane. The first characteristic cavity size V1 is only weakly dependent on position in the bilayer interior with an average value of 3.4 A3, while the second characteristic cavity size V2 varies more dramatically from a plateau value of 12.9 A3 in the highly ordered chain region to 9.0 A3 in the center of the bilayer. The mean cavity shape is described in terms of a probability distribution for the angle at which the test permeant is in contact with one of and does not overlap with anyone of the chain segments in the bilayer. The results show that (a) free volume is elongated in the highly ordered chain region with its long axis normal to the bilayer interface approaching spherical symmetry in the center of the bilayer and (b) small free volume is more elongated than large free volume. The order and conformational structures relevant to the free-volume distributions are also examined. It is found that both overall and internal motions have comparable contributions to local disorder and couple strongly with each other, and the occurrence of kink defects has higher probability than predicted from an independent-transition model.
Full text
PDF


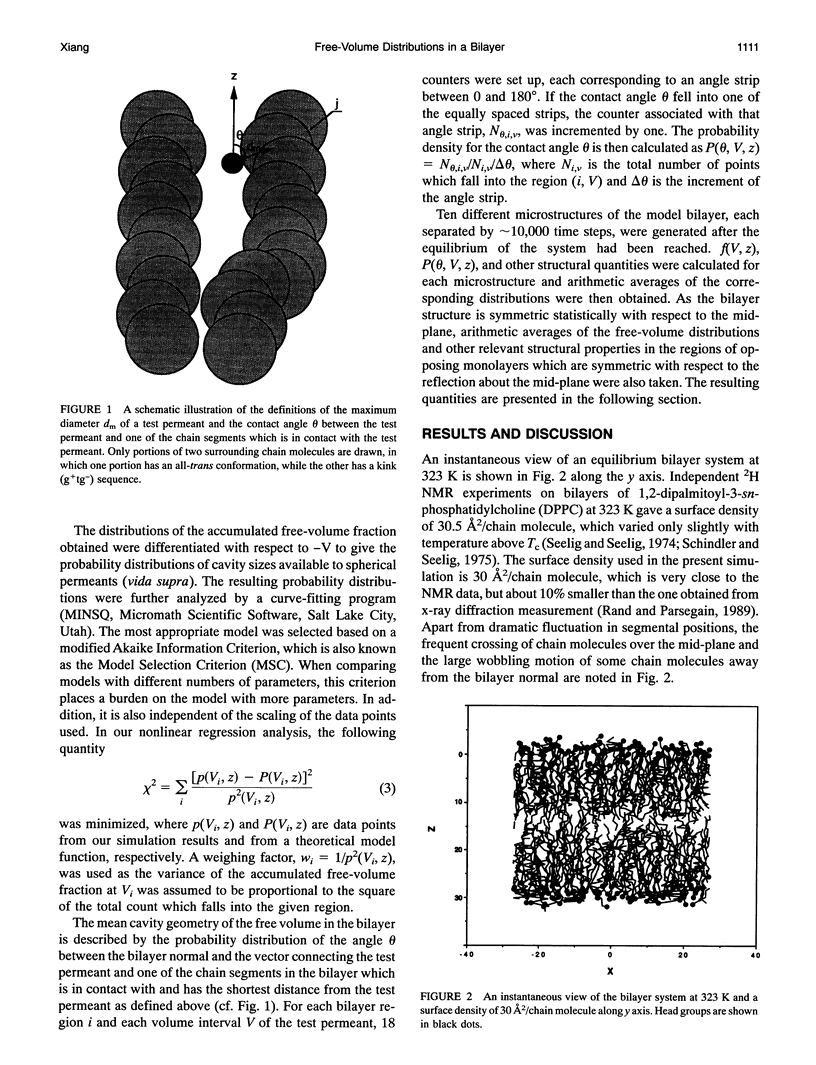
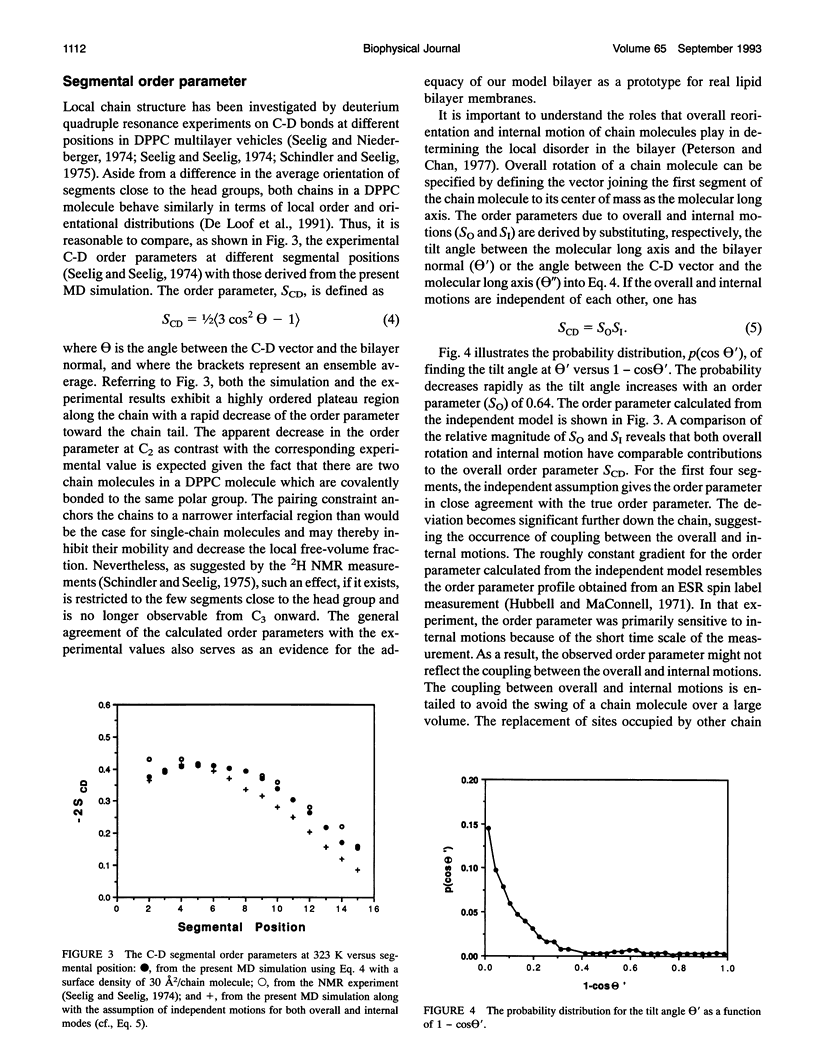
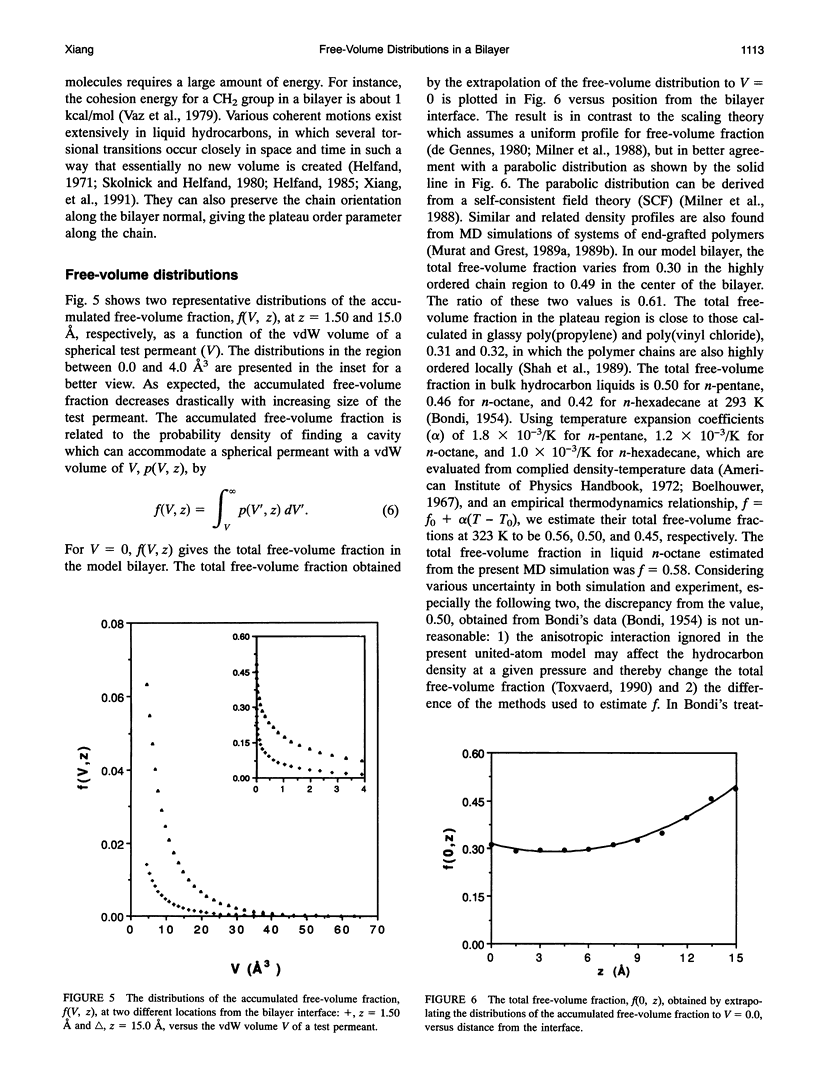



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Loof H., Harvey S. C., Segrest J. P., Pastor R. W. Mean field stochastic boundary molecular dynamics simulation of a phospholipid in a membrane. Biochemistry. 1991 Feb 26;30(8):2099–2113. doi: 10.1021/bi00222a015. [DOI] [PubMed] [Google Scholar]
- Fixman M. Classical statistical mechanics of constraints: a theorem and application to polymers. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3050–3053. doi: 10.1073/pnas.71.8.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. H., Dehlinger P. J., Van S. P. Shape of the hydrophobic barrier of phospholipid bilayers (evidence for water penetration in biological membranes). J Membr Biol. 1974;15(2):159–192. doi: 10.1007/BF01870086. [DOI] [PubMed] [Google Scholar]
- Helfand E. Dynamics of conformational transitions in polymers. Science. 1984 Nov 9;226(4675):647–650. doi: 10.1126/science.226.4675.647. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Jacobs R. E., Hudson B. S., Andersen H. C. A theory of phase transitions and phase diagrams for one- and two-component phospholipid bilayers. Biochemistry. 1977 Oct 4;16(20):4349–4359. doi: 10.1021/bi00639a004. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J. Differences in hydrocarbon chain tilt between hydrated phosphatidylethanolamine and phosphatidylcholine bilayers. A molecular packing model. Biophys J. 1980 Feb;29(2):237–245. doi: 10.1016/S0006-3495(80)85128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A., MacDonald R. C. The organization of n-alkanes in lipid bilayers. Biochim Biophys Acta. 1980 Apr 24;597(3):445–463. doi: 10.1016/0005-2736(80)90219-9. [DOI] [PubMed] [Google Scholar]
- Murat M, Grest GS. Interaction between grafted polymeric brushes: A molecular-dynamics study. Phys Rev Lett. 1989 Sep 4;63(10):1074–1077. doi: 10.1103/PhysRevLett.63.1074. [DOI] [PubMed] [Google Scholar]
- Pastor R. W., Venable R. M., Karplus M. Model for the structure of the lipid bilayer. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):892–896. doi: 10.1073/pnas.88.3.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. O., Chan S. I. More on the motional state of lipid bilayer membranes: interpretation of order parameters obtained from nuclear magnetic resonance experiments. Biochemistry. 1977 Jun 14;16(12):2657–2667. doi: 10.1021/bi00631a012. [DOI] [PubMed] [Google Scholar]
- Schindler H., Seelig J. Deuterium order parameters in relation to thermodynamic properties of a phospholiped bilayer. A statistical mechanical interpretation. Biochemistry. 1975 Jun 3;14(11):2283–2287. doi: 10.1021/bi00682a001. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. E. Analysis of steric partition behavior of molecules in membranes using statistical physics. Application to gel chromatography and electrophoresis. Biophys J. 1988 Dec;54(6):1065–1076. doi: 10.1016/S0006-3495(88)83043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H. L., Jr A theoretical model for lipid monolayer phase transitions. Biochim Biophys Acta. 1975 Oct 17;406(3):329–346. doi: 10.1016/0005-2736(75)90014-0. [DOI] [PubMed] [Google Scholar]
- Seelig A., Seelig J. The dynamic structure of fatty acyl chains in a phospholipid bilayer measured by deuterium magnetic resonance. Biochemistry. 1974 Nov 5;13(23):4839–4845. doi: 10.1021/bi00720a024. [DOI] [PubMed] [Google Scholar]
- Seelig J., Niederberger W. Two pictures of a lipid bilayer. A comparison between deuterium label and spin-label experiments. Biochemistry. 1974 Apr 9;13(8):1585–1588. doi: 10.1021/bi00705a005. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Clegg R. M., Hallmann D. Translational diffusion of lipids in liquid crystalline phase phosphatidylcholine multibilayers. A comparison of experiment with theory. Biochemistry. 1985 Jan 29;24(3):781–786. doi: 10.1021/bi00324a037. [DOI] [PubMed] [Google Scholar]
- Walter A., Gutknecht J. Permeability of small nonelectrolytes through lipid bilayer membranes. J Membr Biol. 1986;90(3):207–217. doi: 10.1007/BF01870127. [DOI] [PubMed] [Google Scholar]
- Wang D. C., Taraschi T. F., Rubin E., Janes N. Configurational entropy is the driving force of ethanol action on membrane architecture. Biochim Biophys Acta. 1993 Jan 18;1145(1):141–148. doi: 10.1016/0005-2736(93)90391-c. [DOI] [PubMed] [Google Scholar]
- Windrem D. A., Plachy W. Z. The diffusion-solubility of oxygen in lipid bilayers. Biochim Biophys Acta. 1980 Aug 14;600(3):655–665. doi: 10.1016/0005-2736(80)90469-1. [DOI] [PubMed] [Google Scholar]
- Xiang T. X., Chen X., Anderson B. D. Transport methods for probing the barrier domain of lipid bilayer membranes. Biophys J. 1992 Jul;63(1):78–88. doi: 10.1016/S0006-3495(92)81581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]