Abstract
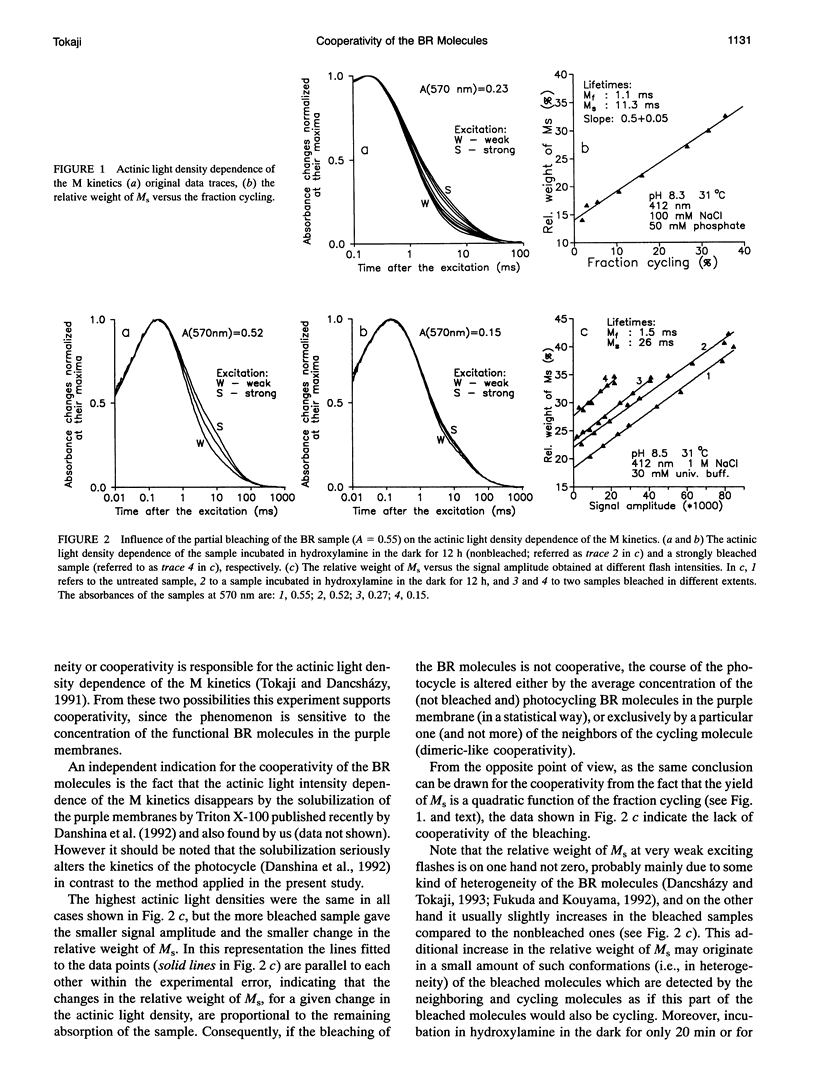
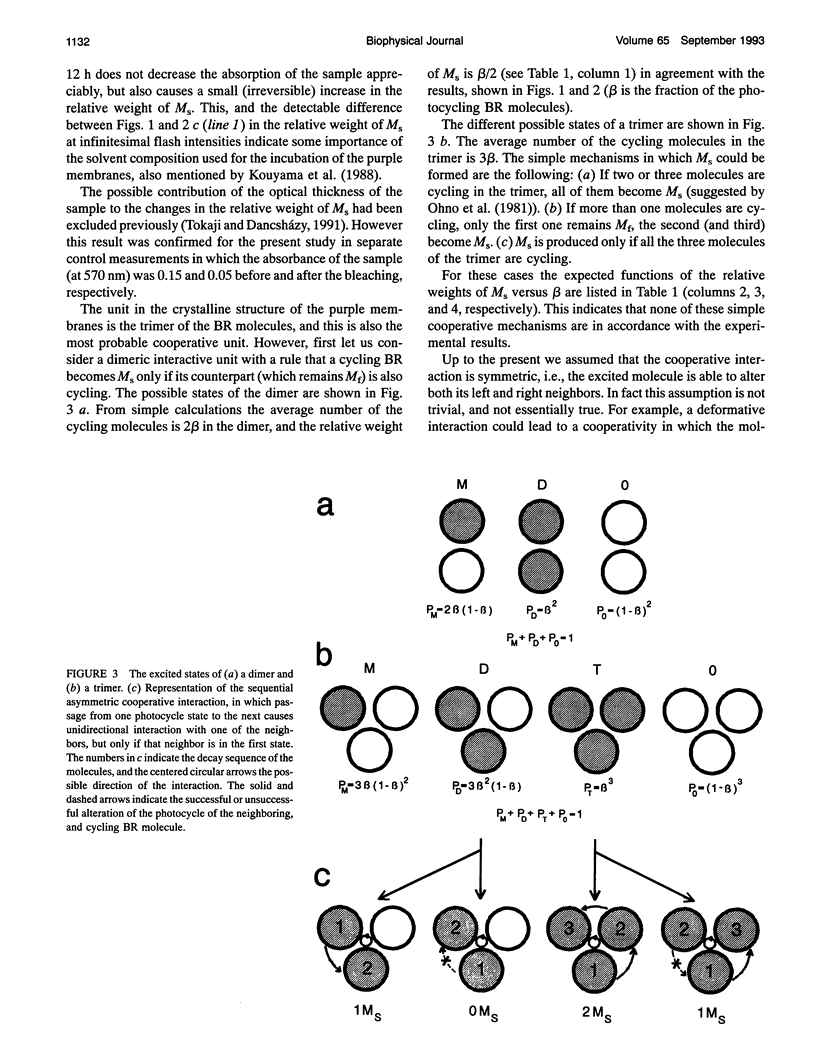
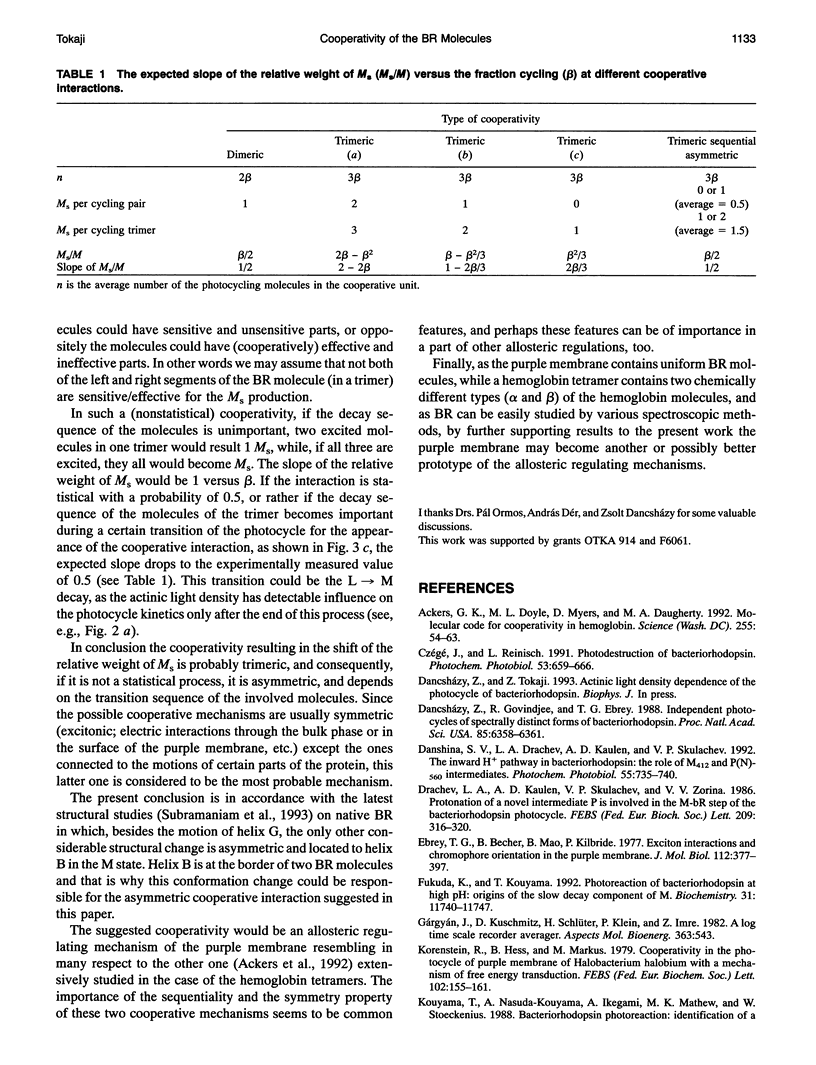
The kinetics of the absorption changes accompanying the photocycle of bacteriorhodopsin (BR) strongly depend on the intensity of the exciting short laser pulse. The decrease in the flash intensity dependence of the M kinetics after different extents of bleaching of the purple membranes by hydroxylamine proves the existence of a cooperative interaction between the photocycling BR molecules. The yield of the slow component of the M decay (M(s)) is a quadratic function of the extent of the fraction cycling. The slope of the relative weight of M(s) versus the fraction cycling is 0.5. This slope indicates a dimeric-like cooperative interaction, although the structural units of the purple membranes are the trimers of the BR molecules. For the most probable cooperative mechanism an asymmetric trimeric interaction is suggested, which accounts for the apparently dimeric features. A photocycling molecule may influence only one of its two neighbors in the trimer. From this asymmetric feature a deformative interaction is expected to be the cooperative mechanism, which would be an allosteric regulating mechanism in the purple membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K., Doyle M. L., Myers D., Daugherty M. A. Molecular code for cooperativity in hemoglobin. Science. 1992 Jan 3;255(5040):54–63. doi: 10.1126/science.1553532. [DOI] [PubMed] [Google Scholar]
- Dancsházy Z., Govindjee R., Ebrey T. G. Independent photocycles of the spectrally distinct forms of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6358–6361. doi: 10.1073/pnas.85.17.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrey T. G., Becher B., Mao B., Kilbride P., Honig B. Exciton interactions and chromophore orientation in the purple membrane. J Mol Biol. 1977 May 25;112(3):377–397. doi: 10.1016/s0022-2836(77)80188-5. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Kouyama T. Photoreaction of bacteriorhodopsin at high pH: origins of the slow decay component of M. Biochemistry. 1992 Dec 1;31(47):11740–11747. doi: 10.1021/bi00162a010. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Nasuda-Kouyama A., Ikegami A., Mathew M. K., Stoeckenius W. Bacteriorhodopsin photoreaction: identification of a long-lived intermediate N (P,R350) at high pH and its M-like photoproduct. Biochemistry. 1988 Aug 9;27(16):5855–5863. doi: 10.1021/bi00416a006. [DOI] [PubMed] [Google Scholar]
- Marinetti T. Nonproton ion release by purple membranes exhibits cooperativity as shown by determination of the optical cross-section. Biophys J. 1988 Aug;54(2):197–204. doi: 10.1016/S0006-3495(88)82948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S., Gerstein M., Oesterhelt D., Henderson R. Electron diffraction analysis of structural changes in the photocycle of bacteriorhodopsin. EMBO J. 1993 Jan;12(1):1–8. doi: 10.1002/j.1460-2075.1993.tb05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokaji Z., Dancsházy Z. Light-induced, long-lived perturbation of the photocycle of bacteriorhodopsin. FEBS Lett. 1991 Apr 9;281(1-2):170–172. doi: 10.1016/0014-5793(91)80385-g. [DOI] [PubMed] [Google Scholar]
- Xie A. H., Nagle J. F., Lozier R. H. Flash spectroscopy of purple membrane. Biophys J. 1987 Apr;51(4):627–635. doi: 10.1016/S0006-3495(87)83387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


