Abstract
Using a heat conduction calorimeter with very high resolution (+/- 0.00005 J/degrees C.cm3), we have measured the specific heat CpL between 25 and 35 degrees C of dimyristoylphosphatidylcholine (DMPC) in aqueous dispersions. Previous studies of the temperature dependence of the chemical potential of DMPC in the L alpha phase (lamellar, liquid crystalline) indicated that a dispersion consisting only of unilamellar vesicles forms spontaneously at a critical temperature T* of 29.0 degrees C. Our present measurements show an anomaly in CpL between 28.70 and 29.50 degrees C: the curve for CpL versus T first decreases and then exhibits an inflection point at 28.96 degrees C before it flattens. This anomaly is attributed to the transformation from multilamellar dispersion to unilamellar vesicles at T* = 28.96 degrees C. Two independent properties of the CpL data also indicate T* is a critical point for the formation of unilamellar vesicles: (a) the time to reach equilibrium upon changing temperature increased dramatically between 28.7 and 28.96 degrees C, increasing as (T* - T)-1; at T > T* the dramatic "slowing-down" phenomenon was not observed. This slowing-down near T* is a general characteristic of critical phenomena. (b) The free energy change for the multilamellar-unilamellar transformation was obtained from the CpL-T data over this temperature interval and found to be 3.2 J/mol or 0.016 ergs/cm2 of bilayer, in agreement with other estimates of the interaction energy between neutral bilayers. We conclude with a discussion of the implications for membrane bilayer stability of these newly identified dynamic properties of the transformation.
Full text
PDF

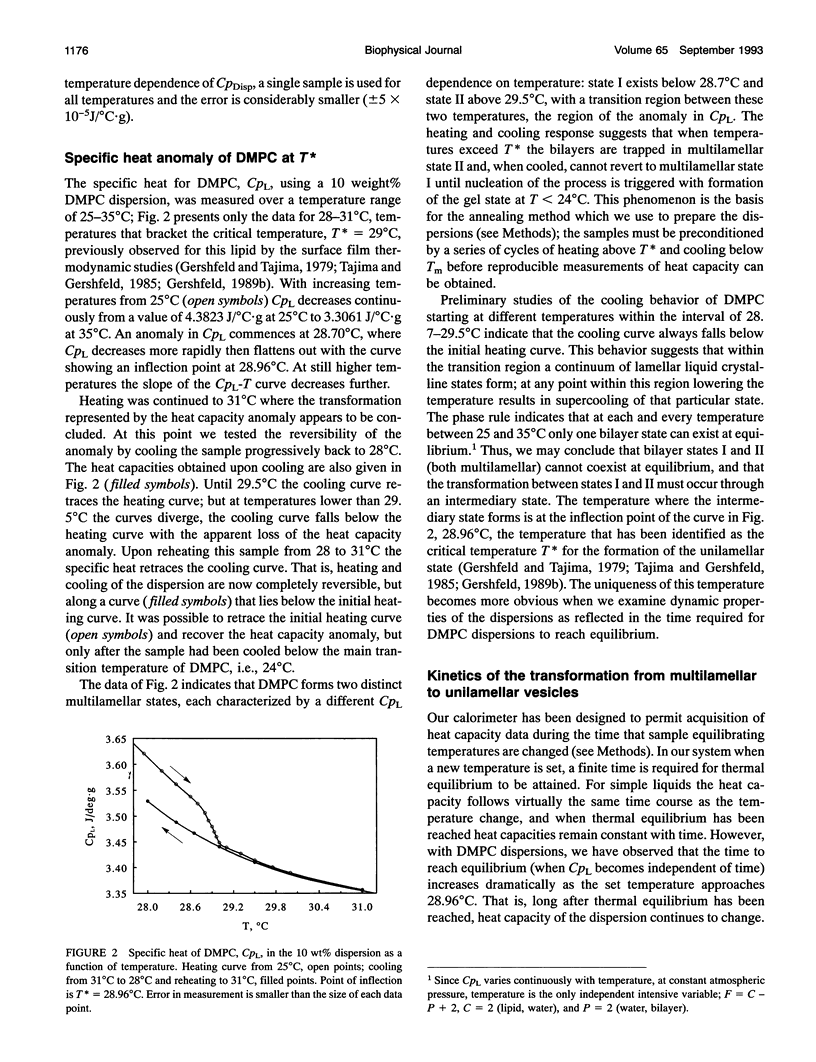

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cevc G., Fenzl W., Sigl L. Surface-induced x-ray reflection visualization of membrane orientation and fusion into multibilayers. Science. 1990 Sep 7;249(4973):1161–1163. doi: 10.1126/science.249.4973.1161. [DOI] [PubMed] [Google Scholar]
- Evans E., Metcalfe M. Free energy potential for aggregation of giant, neutral lipid bilayer vesicles by Van der Waals attraction. Biophys J. 1984 Sep;46(3):423–426. doi: 10.1016/S0006-3495(84)84039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey P. F., Webb W. W. Lateral diffusion in phospholipid bilayer membranes and multilamellar liquid crystals. Biochemistry. 1978 Jul 25;17(15):3046–3053. doi: 10.1021/bi00608a016. [DOI] [PubMed] [Google Scholar]
- Gershfeld N. L. Equilibrium studies of lecithin-cholesterol interactions I. Stoichiometry of lecithin-cholesterol complexes in bulk systems. Biophys J. 1978 Jun;22(3):469–488. doi: 10.1016/S0006-3495(78)85500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershfeld N. L., Murayama M. Thermal instability of red blood cell membrane bilayers: temperature dependence of hemolysis. J Membr Biol. 1988;101(1):67–72. doi: 10.1007/BF01872821. [DOI] [PubMed] [Google Scholar]
- Gershfeld N. L. Phospholipid surface bilayers at the air-water interface. III. Relation between surface bilayer formation and lipid bilayer assembly in cell membranes. Biophys J. 1986 Sep;50(3):457–461. doi: 10.1016/S0006-3495(86)83482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershfeld N. L., Stevens W. F., Jr, Nossal R. J. Equilibrium studies of phospholipid bilayer assembly. Coexistence of surface bilayers and unilamellar vesicles. Faraday Discuss Chem Soc. 1986;(81):19–28. doi: 10.1039/dc9868100019. [DOI] [PubMed] [Google Scholar]
- Gershfeld N. L., Tajima K. Spontaneous formation of lecithin bilayers at the air-water surface. Nature. 1979 Jun 21;279(5715):708–709. doi: 10.1038/279708a0. [DOI] [PubMed] [Google Scholar]
- Gershfeld N. L. The critical unilamellar lipid state: a perspective for membrane bilayer assembly. Biochim Biophys Acta. 1989 Dec 6;988(3):335–350. doi: 10.1016/0304-4157(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Ginsberg L., Atack J. R., Rapoport S. I., Gershfeld N. L. Evidence for a membrane lipid defect in Alzheimer disease. Mol Chem Neuropathol. 1993 May-Jun;19(1-2):37–46. doi: 10.1007/BF03160167. [DOI] [PubMed] [Google Scholar]
- Ginsberg L., Gershfeld N. L. Membrane bilayer instability and the pathogenesis of disorders of myelin. Neurosci Lett. 1991 Sep 2;130(1):133–136. doi: 10.1016/0304-3940(91)90245-o. [DOI] [PubMed] [Google Scholar]
- Ginsberg L., Gershfeld N. L. Phospholipid surface bilayers at the air-water interface. II. Water permeability of dimyristoylphosphatidylcholine surface bilayers. Biophys J. 1985 Feb;47(2 Pt 1):211–215. doi: 10.1016/s0006-3495(85)83893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg L., Gilbert D. L., Gershfeld N. L. Membrane bilayer assembly in neural tissue of rat and squid as a critical phenomenon: influence of temperature and membrane proteins. J Membr Biol. 1991 Jan;119(1):65–73. doi: 10.1007/BF01868541. [DOI] [PubMed] [Google Scholar]
- Karle H. Effect on red cells of a small rise in temperature: in vitro studies. Br J Haematol. 1969 Apr;16(4):409–419. doi: 10.1111/j.1365-2141.1969.tb00418.x. [DOI] [PubMed] [Google Scholar]
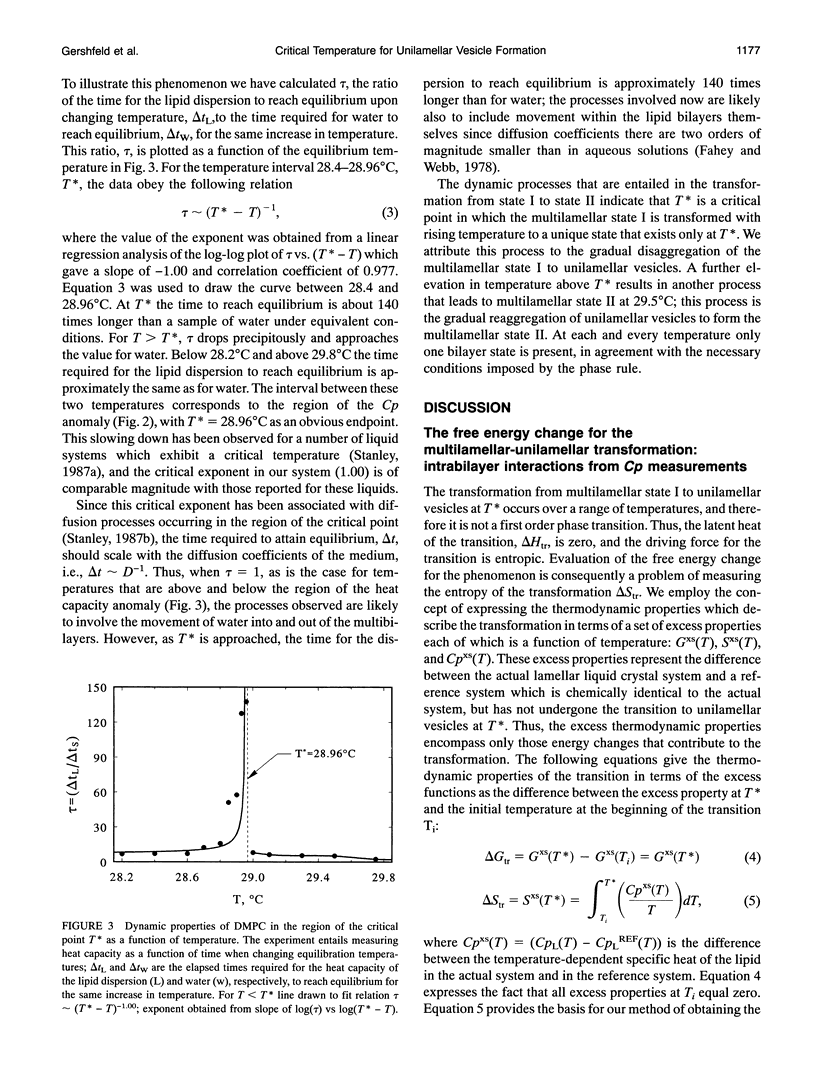
- Mudd C. P., Gershfeld N. L., Berger R. L., Tajima K. A differential heat-conduction microcalorimeter for heat-capacity measurements of fluids. J Biochem Biophys Methods. 1993 May;26(2-3):149–171. doi: 10.1016/0165-022x(93)90045-p. [DOI] [PubMed] [Google Scholar]
- Mudd C., Berger R. L., Hopkins H. P., Friauf W. S., Gibson C. An optimized differential heat conduction solution microcalorimeter for thermal kinetic measurements. J Biochem Biophys Methods. 1982 Aug;6(3):179–203. doi: 10.1016/0165-022x(82)90042-2. [DOI] [PubMed] [Google Scholar]
- Tajima K., Gershfeld N. L. Phospholipid surface bilayers at the air-water interface. I. Thermodynamic properties. Biophys J. 1985 Feb;47(2 Pt 1):203–209. doi: 10.1016/s0006-3495(85)83892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]