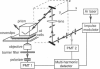
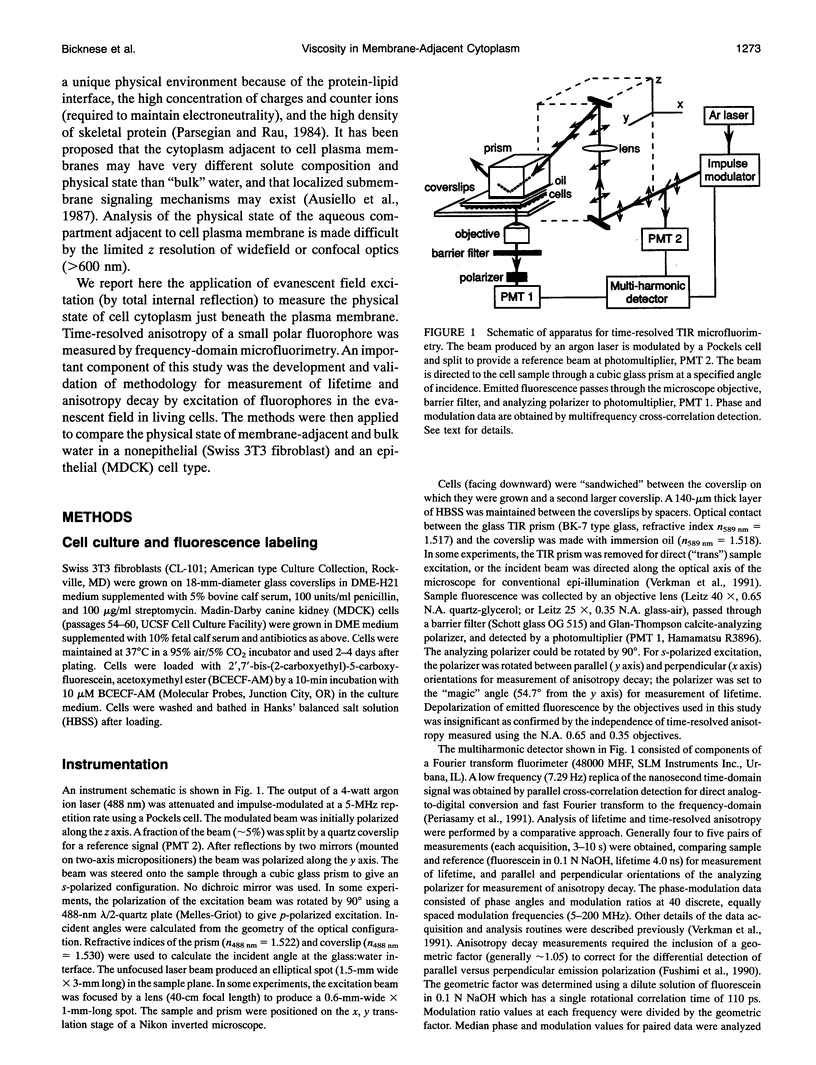
Abstract
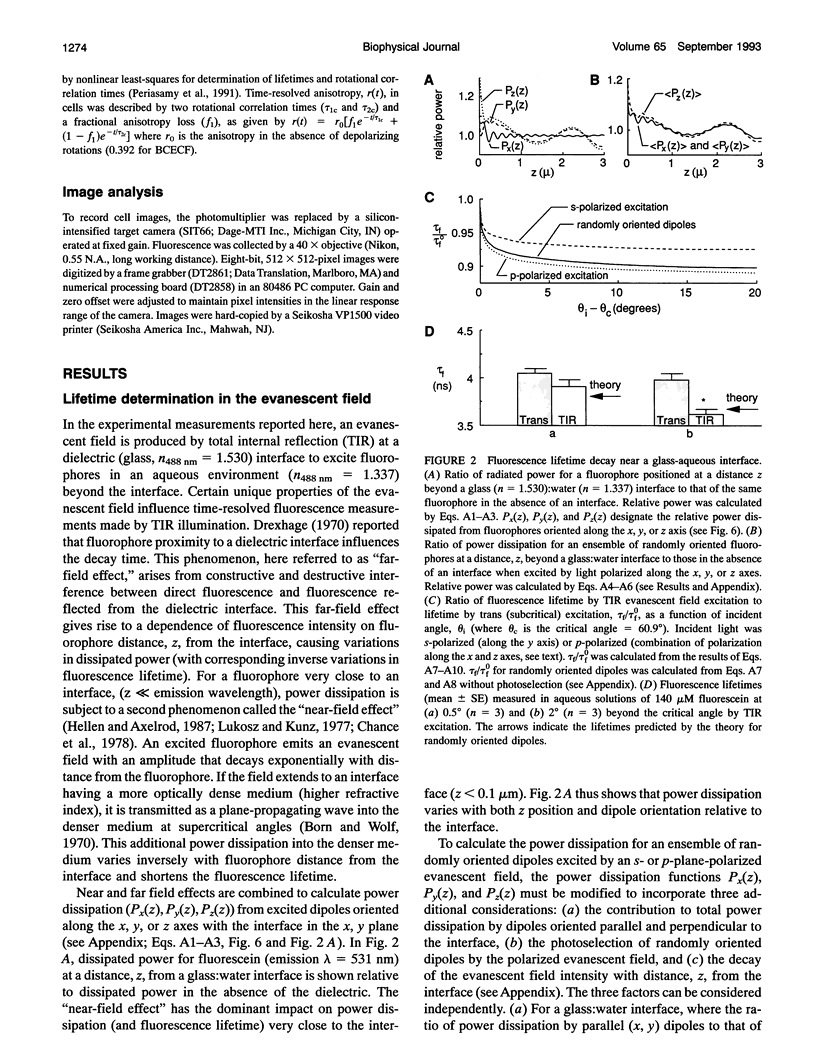
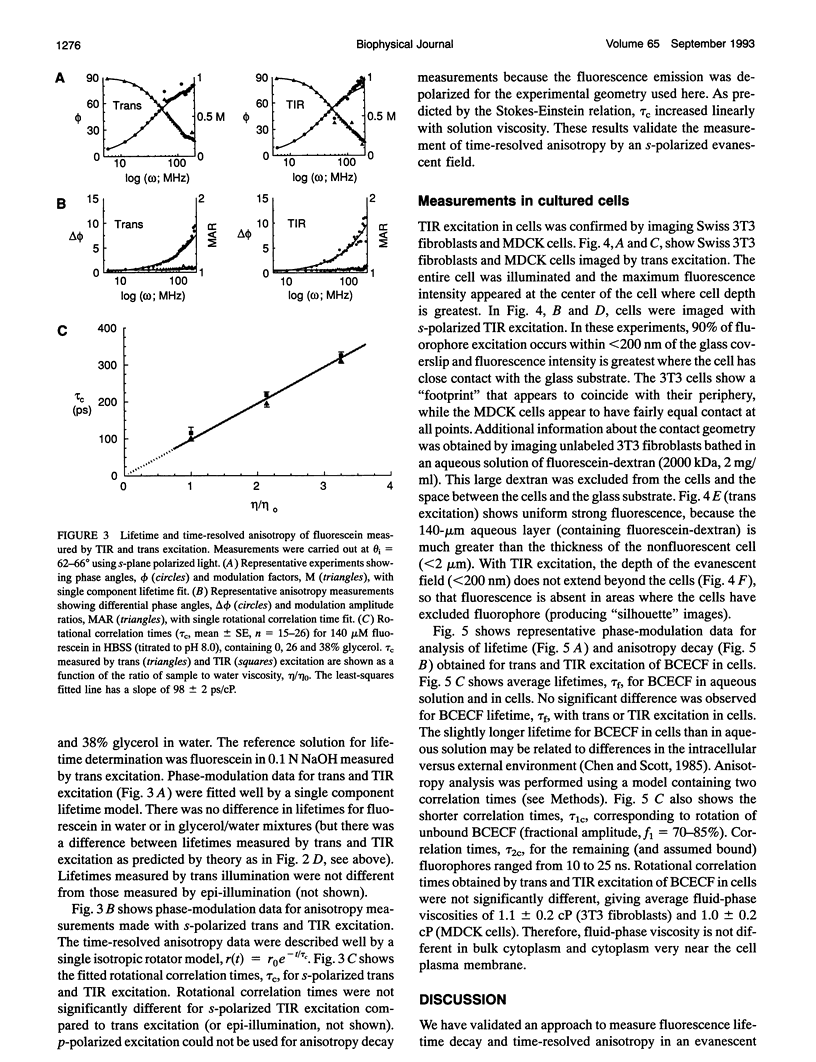
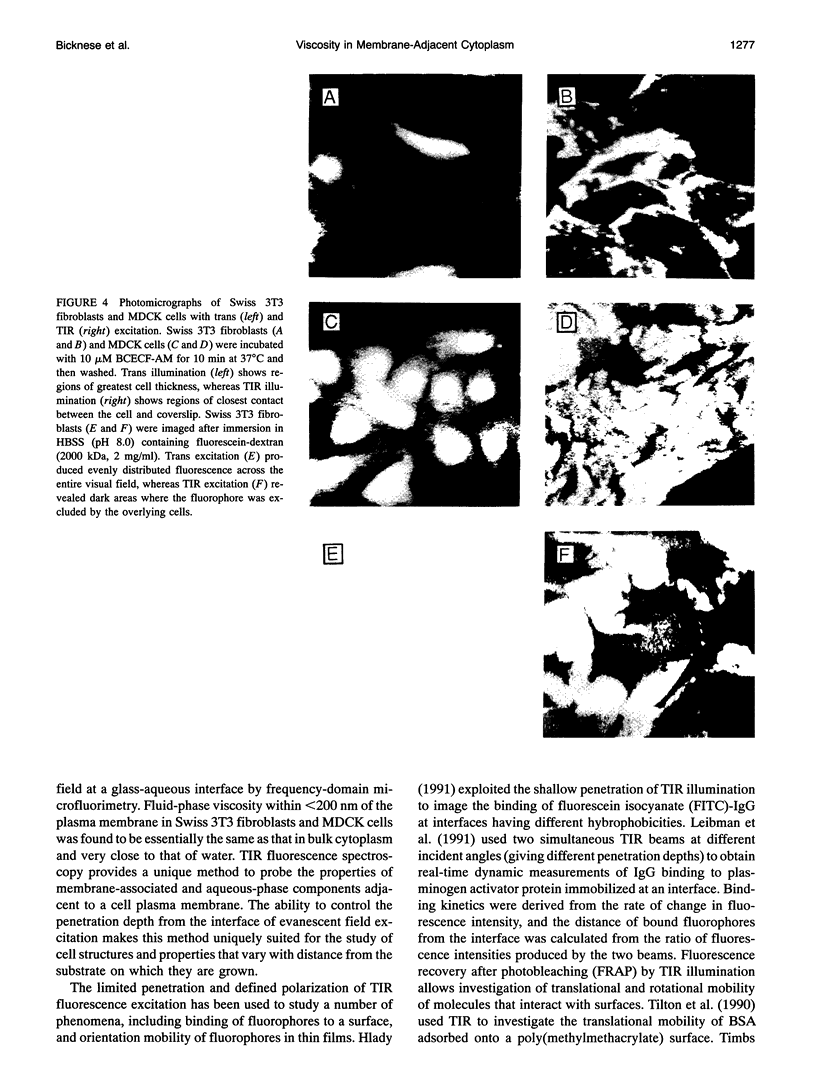
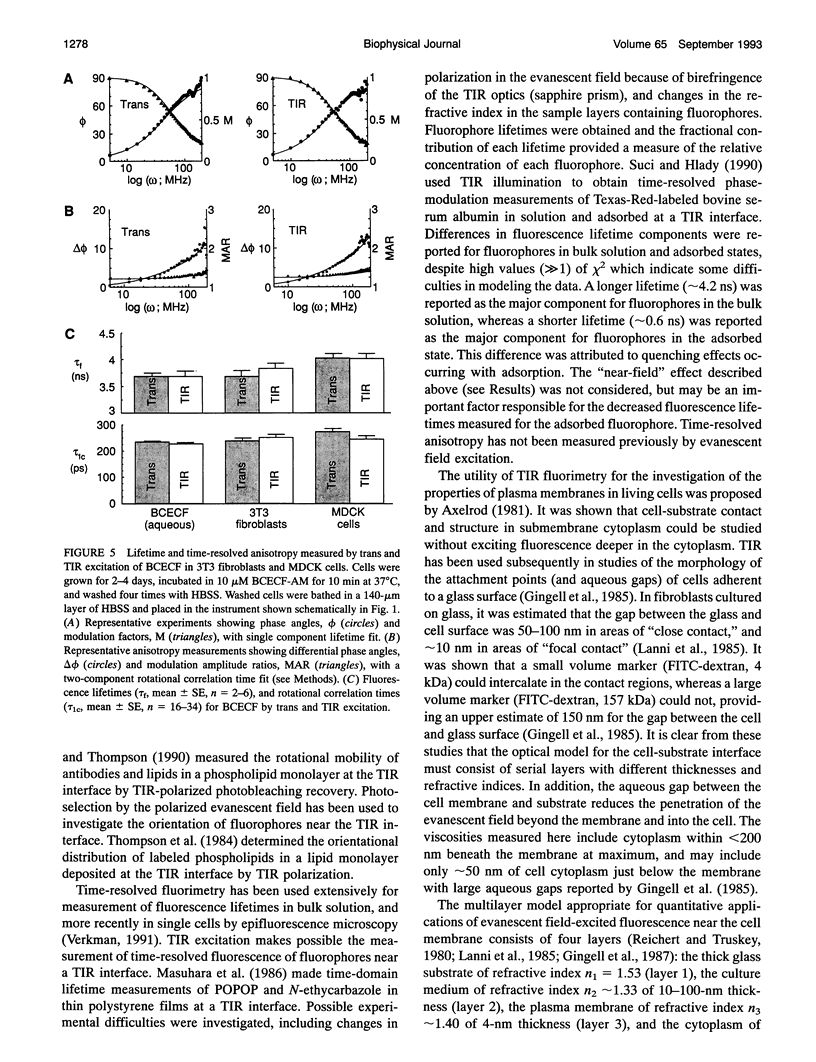
The purpose of this study was to determine whether the unique physical milieu just beneath the cell plasma membrane influences the rheology of fluid-phase cytoplasm. Cytoplasmic viscosity was evaluated from the picosecond rotation of the small fluorophore 2',7'-bis-(2-carboxyethyl)-5-carboxyfluorescein (BCECF) by parallel-acquisition Fourier transform microfluorimetry (Fushimi and Verkman, 1991). Information about viscosity within < 200 nm of cell plasma membranes was obtained by selective excitation of fluorophores in an evanescent field created by total internal reflection (TIR) of impulse-modulated s-plane-polarized laser illumination (488 nm) at a glass-aqueous interface. Measurements of fluorescence lifetime and time-resolved anisotropy were carried out in solutions containing fluorescein or BCECF at known viscosities, and monolayers of BCECF-labeled Swiss 3T3 fibroblasts and Madin-Darby canine kidney (MDCK) cells. Specific concerns associated with time-resolved fluorescence measurements in the evanescent field were examined theoretically and/or experimentally, including variations in lifetime due to fluorophore proximity to the interface, and the use of the s and p polarized excitation. In fluorescein solutions excited with s-plane polarized light, there was a 5-10% decrease in fluorescein lifetime with TIR compared to trans (subcritical) illumination, but no change in rotational correlation time (approximately 98 ps/cP). Intracellular BCECF had a single lifetime of 3.7 +/- 0.1 ns near the cell plasma membrane. Apparent fluid-phase viscosity near the cell plasma membrane was 1.1 +/- 0.2 cP (fibroblast) and 1.0 +/- 0.2 cP (MDCK), not significantly different from the viscosity measured in bulk cytoplasm far from the plasma membrane. The results establish the methodology for time-resolved microfluorimetric measurement of polarization in the evanescent field and demonstrate that the cell plasma membrane has little effect on the fluid-phase viscosity of adjacent cytoplasm.
Full text
PDF


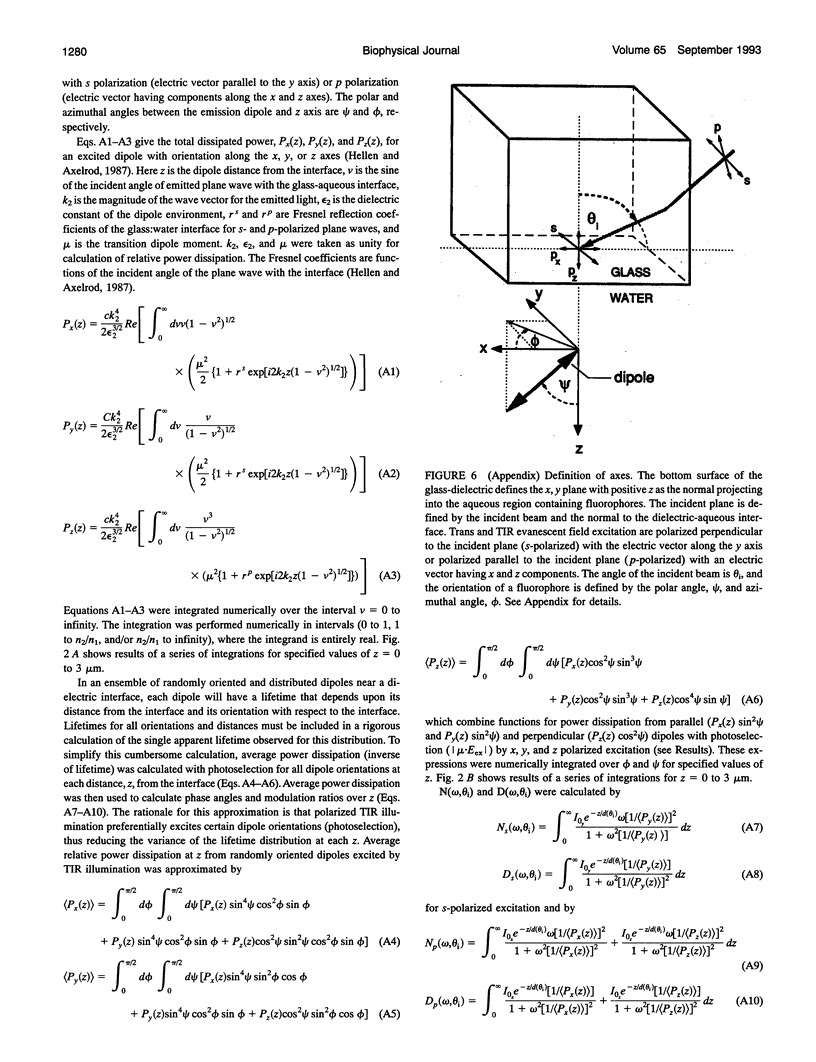


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausiello D. A., Hartwig J., Brown D. Membrane and microfilament organization and vasopressin action in transporting epithelia. Soc Gen Physiol Ser. 1987;42:259–275. [PubMed] [Google Scholar]
- Axelrod D., Burghardt T. P., Thompson N. L. Total internal reflection fluorescence. Annu Rev Biophys Bioeng. 1984;13:247–268. doi: 10.1146/annurev.bb.13.060184.001335. [DOI] [PubMed] [Google Scholar]
- Axelrod D. Cell-substrate contacts illuminated by total internal reflection fluorescence. J Cell Biol. 1981 Apr;89(1):141–145. doi: 10.1083/jcb.89.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D. Total internal reflection fluorescence microscopy. Methods Cell Biol. 1989;30:245–270. doi: 10.1016/s0091-679x(08)60982-6. [DOI] [PubMed] [Google Scholar]
- Clegg J. S. Properties and metabolism of the aqueous cytoplasm and its boundaries. Am J Physiol. 1984 Feb;246(2 Pt 2):R133–R151. doi: 10.1152/ajpregu.1984.246.2.R133. [DOI] [PubMed] [Google Scholar]
- Dix J. A., Verkman A. S. Mapping of fluorescence anisotropy in living cells by ratio imaging. Application to cytoplasmic viscosity. Biophys J. 1990 Feb;57(2):231–240. doi: 10.1016/S0006-3495(90)82526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton A. B. How crowded is the cytoplasm? Cell. 1982 Sep;30(2):345–347. doi: 10.1016/0092-8674(82)90231-8. [DOI] [PubMed] [Google Scholar]
- Fushimi K., Dix J. A., Verkman A. S. Cell membrane fluidity in the intact kidney proximal tubule measured by orientation-independent fluorescence anisotropy imaging. Biophys J. 1990 Feb;57(2):241–254. doi: 10.1016/S0006-3495(90)82527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushimi K., Verkman A. S. Low viscosity in the aqueous domain of cell cytoplasm measured by picosecond polarization microfluorimetry. J Cell Biol. 1991 Feb;112(4):719–725. doi: 10.1083/jcb.112.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingell D., Heavens O. S., Mellor J. S. General electromagnetic theory of total internal reflection fluorescence: the quantitative basis for mapping cell-substratum topography. J Cell Sci. 1987 Jun;87(Pt 5):677–693. doi: 10.1242/jcs.87.5.677. [DOI] [PubMed] [Google Scholar]
- Gingell D., Todd I., Bailey J. Topography of cell-glass apposition revealed by total internal reflection fluorescence of volume markers. J Cell Biol. 1985 Apr;100(4):1334–1338. doi: 10.1083/jcb.100.4.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao H. P., Abney J. R., Verkman A. S. Determinants of the translational mobility of a small solute in cell cytoplasm. J Cell Biol. 1993 Jan;120(1):175–184. doi: 10.1083/jcb.120.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni F., Waggoner A. S., Taylor D. L. Structural organization of interphase 3T3 fibroblasts studied by total internal reflection fluorescence microscopy. J Cell Biol. 1985 Apr;100(4):1091–1102. doi: 10.1083/jcb.100.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepock J. R., Cheng K. H., Campbell S. D., Kruuv J. Rotational diffusion of TEMPONE in the cytoplasm of Chinese hamster lung cells. Biophys J. 1983 Dec;44(3):405–412. doi: 10.1016/S0006-3495(83)84314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K., Mujumdar S., Mujumdar R. B., Ernst L. A., Galbraith W., Waggoner A. S. A novel fluorescence ratiometric method confirms the low solvent viscosity of the cytoplasm. Biophys J. 1993 Jul;65(1):236–242. doi: 10.1016/S0006-3495(93)81075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K., Taylor D. L., Lanni F. Probing the structure of cytoplasm. J Cell Biol. 1986 Jun;102(6):2015–2022. doi: 10.1083/jcb.102.6.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastro A. M., Keith A. D. Diffusion in the aqueous compartment. J Cell Biol. 1984 Jul;99(1 Pt 2):180s–187s. doi: 10.1083/jcb.99.1.180s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian V. A., Rau D. C. Water near intracellular surfaces. J Cell Biol. 1984 Jul;99(1 Pt 2):196s–200s. doi: 10.1083/jcb.99.1.196s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy N., Armijo M., Verkman A. S. Picosecond rotation of small polar fluorophores in the cytosol of sea urchin eggs. Biochemistry. 1991 Dec 24;30(51):11836–11841. doi: 10.1021/bi00115a600. [DOI] [PubMed] [Google Scholar]
- Periasamy N., Kao H. P., Fushimi K., Verkman A. S. Organic osmolytes increase cytoplasmic viscosity in kidney cells. Am J Physiol. 1992 Oct;263(4 Pt 1):C901–C907. doi: 10.1152/ajpcell.1992.263.4.C901. [DOI] [PubMed] [Google Scholar]
- Porter K. R. The cytomatrix: a short history of its study. J Cell Biol. 1984 Jul;99(1 Pt 2):3s–12s. doi: 10.1083/jcb.99.1.3s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert W. M., Truskey G. A. Total internal reflection fluorescence (TIRF) microscopy. I. Modelling cell contact region fluorescence. J Cell Sci. 1990 Jun;96(Pt 2):219–230. doi: 10.1242/jcs.96.2.219. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Burghardt T. P. Total internal reflection fluorescence. Measurement of spatial and orientational distributions of fluorophores near planar dielectric interfaces. Biophys Chem. 1986 Nov;25(1):91–97. doi: 10.1016/0301-4622(86)85069-4. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., McConnell H. M., Burhardt T. P. Order in supported phospholipid monolayers detected by the dichroism of fluorescence excited with polarized evanescent illumination. Biophys J. 1984 Dec;46(6):739–747. doi: 10.1016/S0006-3495(84)84072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton R. D., Gast A. P., Robertson C. R. Surface diffusion of interacting proteins. Effect of concentration on the lateral mobility of adsorbed bovine serum albumin. Biophys J. 1990 Nov;58(5):1321–1326. doi: 10.1016/S0006-3495(90)82473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbs M. M., Thompson N. L. Slow rotational mobilities of antibodies and lipids associated with substrate-supported phospholipid monolayers as measured by polarized fluorescence photobleaching recovery. Biophys J. 1990 Aug;58(2):413–428. doi: 10.1016/S0006-3495(90)82387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman A. S., Armijo M., Fushimi K. Construction and evaluation of a frequency-domain epifluorescence microscope for lifetime and anisotropy decay measurements in subcellular domains. Biophys Chem. 1991 Apr;40(1):117–125. doi: 10.1016/0301-4622(91)85036-p. [DOI] [PubMed] [Google Scholar]