Abstract
The results of variable dielectric coefficient Poisson-Boltzmann calculations of the counter-ion concentration in the vicinity of an all-atom model of the B-form of DNA are presented with an emphasis on the importance of spatial variations in the dielectric properties of the solvent, particularly at the macro-ion-solvent interface. Calculations of the distribution of hard-sphere electrolyte ions of various dimensions are reported. The presence of a dielectric boundary significantly increases the magnitude of the electrostatic potential with a concomitant increase in the accumulation of small counter-ions in the groove regions of DNA. Because ions with radii greater than 2 A have restricted access to the minor groove, the effect there is less significant than it is within the major groove. Changes in the dielectric coefficient for the electrolyte solution, allowing variation from 10 to 25, 40, 60, and 78.5 within the first 7.4 A of the surface of DNA, substantially increases the calculated surface concentration of counter-ions of all sizes. A lower dielectric coefficient near the macro-ion surface also tends to increase the counter-ion density in regions where the electrostatic potential is more negative than -kT. Regardless of the choice of dielectric coefficient, the number of ions in regions where the electrostatic potential is less than -kT remains the same for 0.153 M added 1-1 monovalent electrolyte as for the case without added salt. The strong dependence of the calculated distribution of counter-ion density on the choice of dielectric coefficients representing the solvent continuum suggests that care must be taken to properly characterize the physical system when studying electrostatic properties using these methods.
Full text
PDF

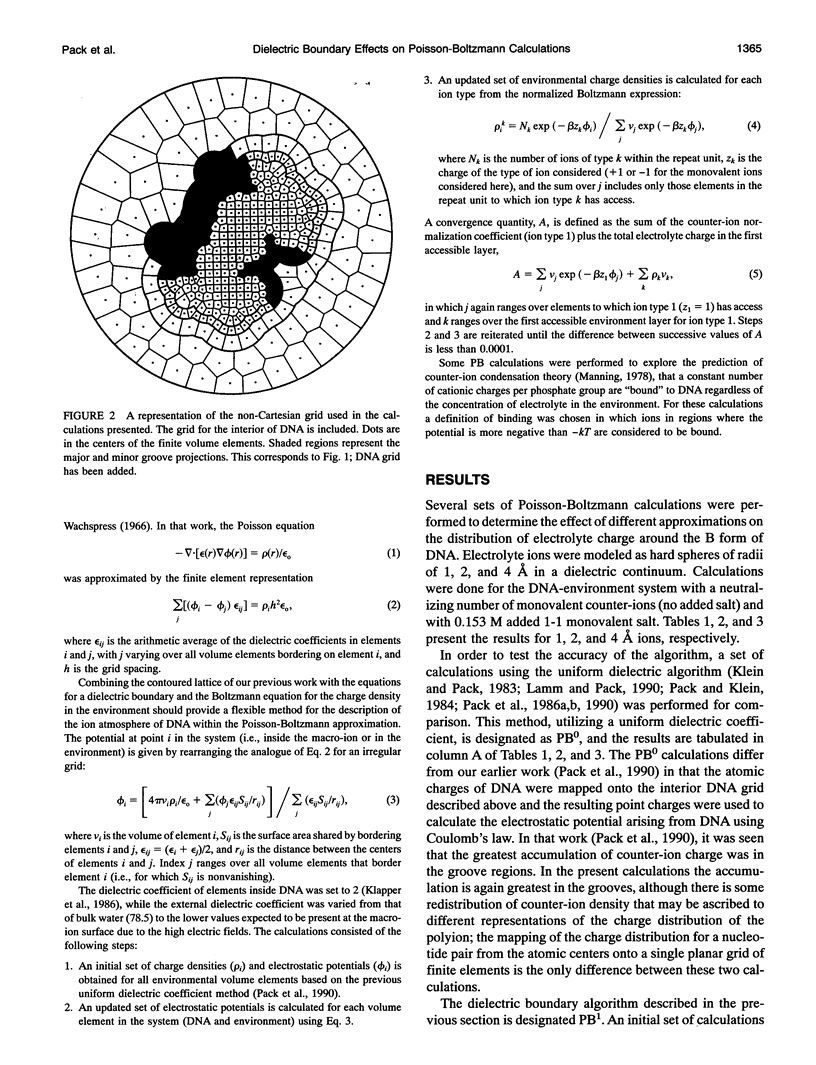
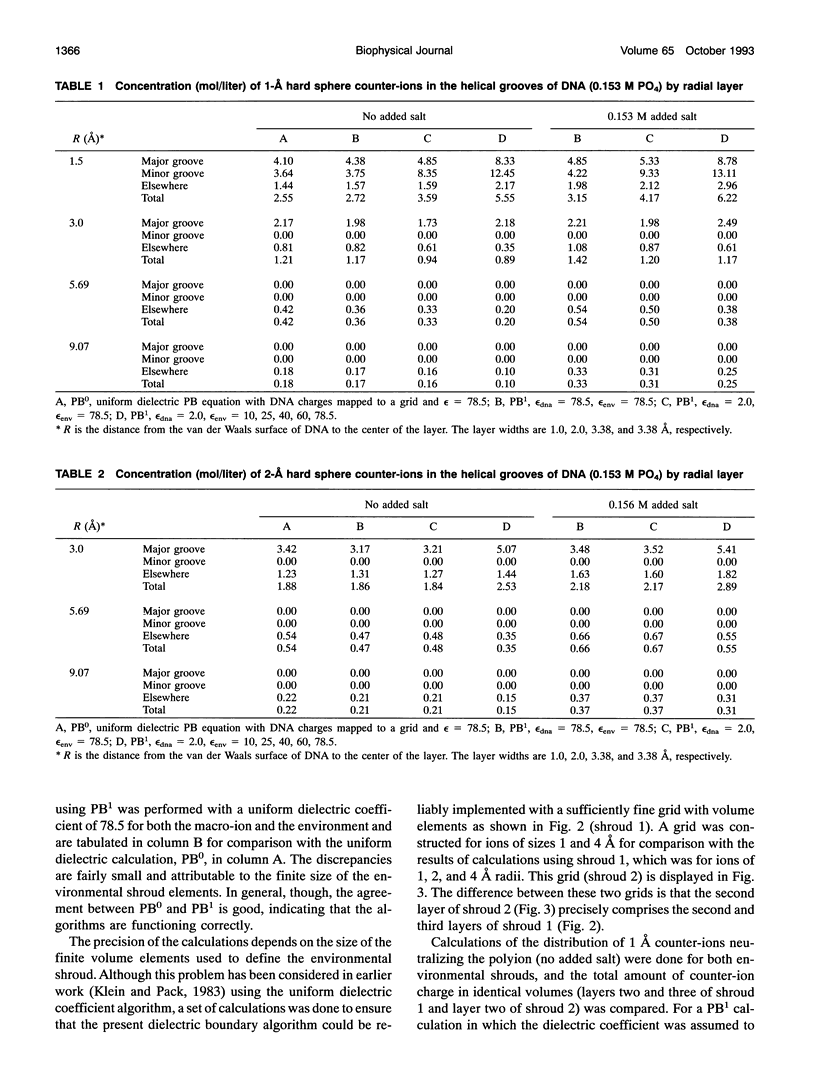
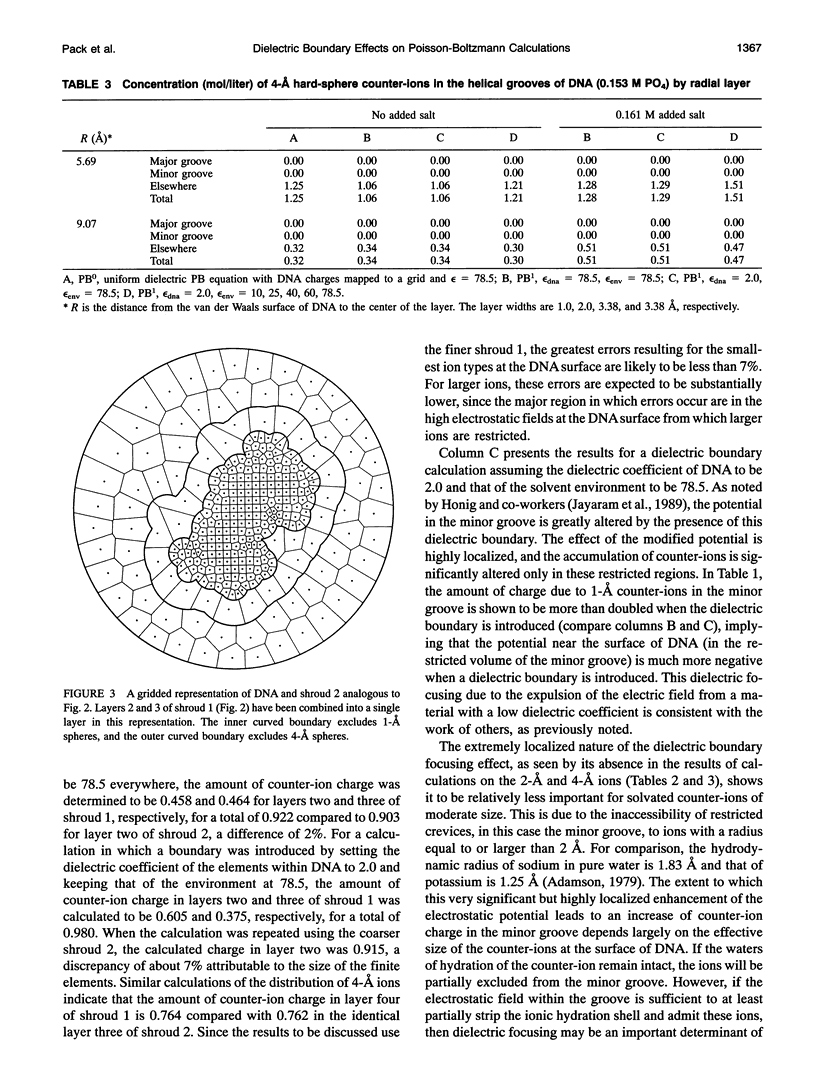



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gilson M. K., Rashin A., Fine R., Honig B. On the calculation of electrostatic interactions in proteins. J Mol Biol. 1985 Aug 5;184(3):503–516. doi: 10.1016/0022-2836(85)90297-9. [DOI] [PubMed] [Google Scholar]
- Jayaram B., Sharp K. A., Honig B. The electrostatic potential of B-DNA. Biopolymers. 1989 May;28(5):975–993. doi: 10.1002/bip.360280506. [DOI] [PubMed] [Google Scholar]
- Klapper I., Hagstrom R., Fine R., Sharp K., Honig B. Focusing of electric fields in the active site of Cu-Zn superoxide dismutase: effects of ionic strength and amino-acid modification. Proteins. 1986 Sep;1(1):47–59. doi: 10.1002/prot.340010109. [DOI] [PubMed] [Google Scholar]
- Klein B. J., Pack G. R. Calculations of the spatial distribution of charge density in the environment of DNA. Biopolymers. 1983 Nov;22(11):2331–2352. doi: 10.1002/bip.360221103. [DOI] [PubMed] [Google Scholar]
- Lamm G., Pack G. R. Acidic domains around nucleic acids. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9033–9036. doi: 10.1073/pnas.87.22.9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bret M., Zimm B. H. Monte Carlo determination of the distribution of ions about a cylindrical polyelectrolyte. Biopolymers. 1984 Feb;23(2):271–285. doi: 10.1002/bip.360230208. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Pack G. R., Klein B. J. Generalized Poisson-Boltzmann calculation of the distribution of electrolyte ions around the B- and Z-conformers of DNA. Biopolymers. 1984 Dec;23(12):2801–2823. doi: 10.1002/bip.360231208. [DOI] [PubMed] [Google Scholar]
- Pack G. R., Prasad C. V., Salafsky J. S., Wong L. Calculations on the effect of methylation on the electrostatic stability of the B- and Z-conformers of DNA. Biopolymers. 1986 Sep;25(9):1697–1715. doi: 10.1002/bip.360250912. [DOI] [PubMed] [Google Scholar]
- Pack G. R., Wong L., Prasad C. V. Counterion distribution around DNA: variation with conformation and sequence. Nucleic Acids Res. 1986 Feb 11;14(3):1479–1493. doi: 10.1093/nar/14.3.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers N. K., Moore G. R., Sternberg M. J. Electrostatic interactions in globular proteins: calculation of the pH dependence of the redox potential of cytochrome c551. J Mol Biol. 1985 Apr 20;182(4):613–616. doi: 10.1016/0022-2836(85)90248-7. [DOI] [PubMed] [Google Scholar]
- Warwicker J. Continuum dielectric modelling of the protein-solvent system, and calculation of the long-range electrostatic field of the enzyme phosphoglycerate mutase. J Theor Biol. 1986 Jul 21;121(2):199–210. doi: 10.1016/s0022-5193(86)80093-5. [DOI] [PubMed] [Google Scholar]
- Warwicker J., Ollis D., Richards F. M., Steitz T. A. Electrostatic field of the large fragment of Escherichia coli DNA polymerase I. J Mol Biol. 1985 Dec 5;186(3):645–649. doi: 10.1016/0022-2836(85)90136-6. [DOI] [PubMed] [Google Scholar]
- Warwicker J., Watson H. C. Calculation of the electric potential in the active site cleft due to alpha-helix dipoles. J Mol Biol. 1982 Jun 5;157(4):671–679. doi: 10.1016/0022-2836(82)90505-8. [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Rau D. C., Bloomfield V. A. Comparison of polyelectrolyte theories of the binding of cations to DNA. Biophys J. 1980 May;30(2):317–325. doi: 10.1016/S0006-3495(80)85097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauhar R. J., Morgan R. S. A new method for computing the macromolecular electric potential. J Mol Biol. 1985 Dec 20;186(4):815–820. doi: 10.1016/0022-2836(85)90399-7. [DOI] [PubMed] [Google Scholar]