Abstract
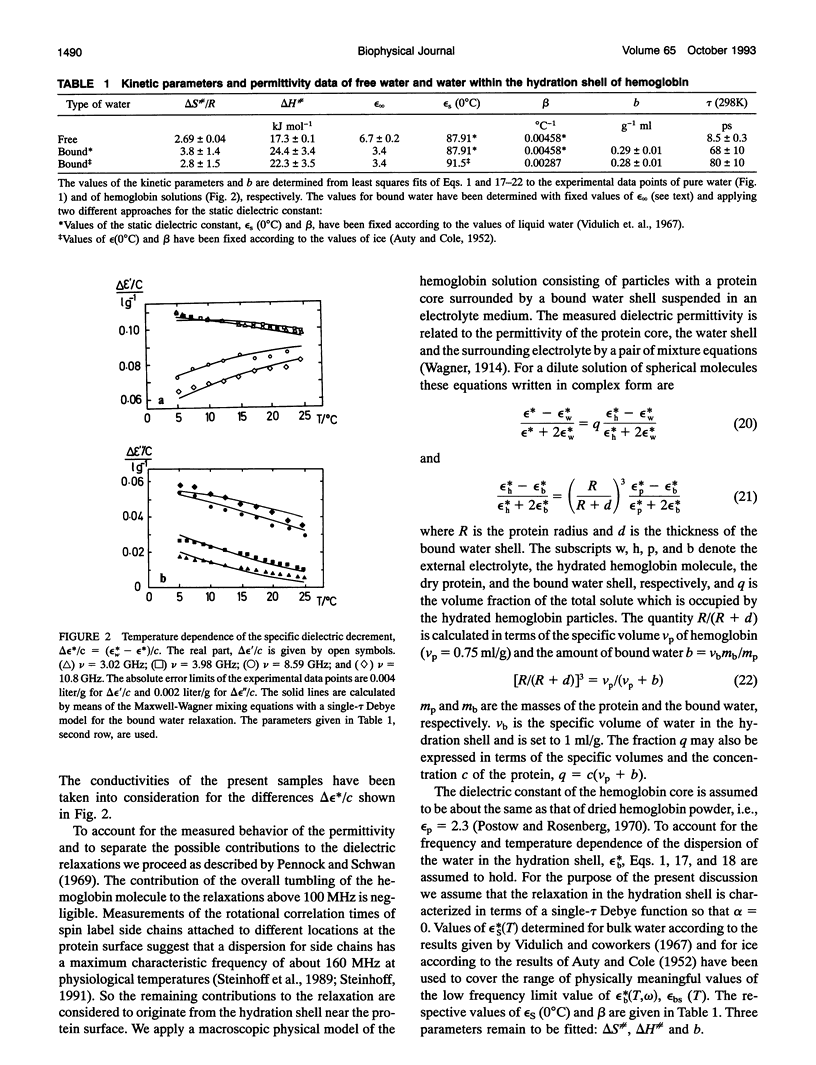
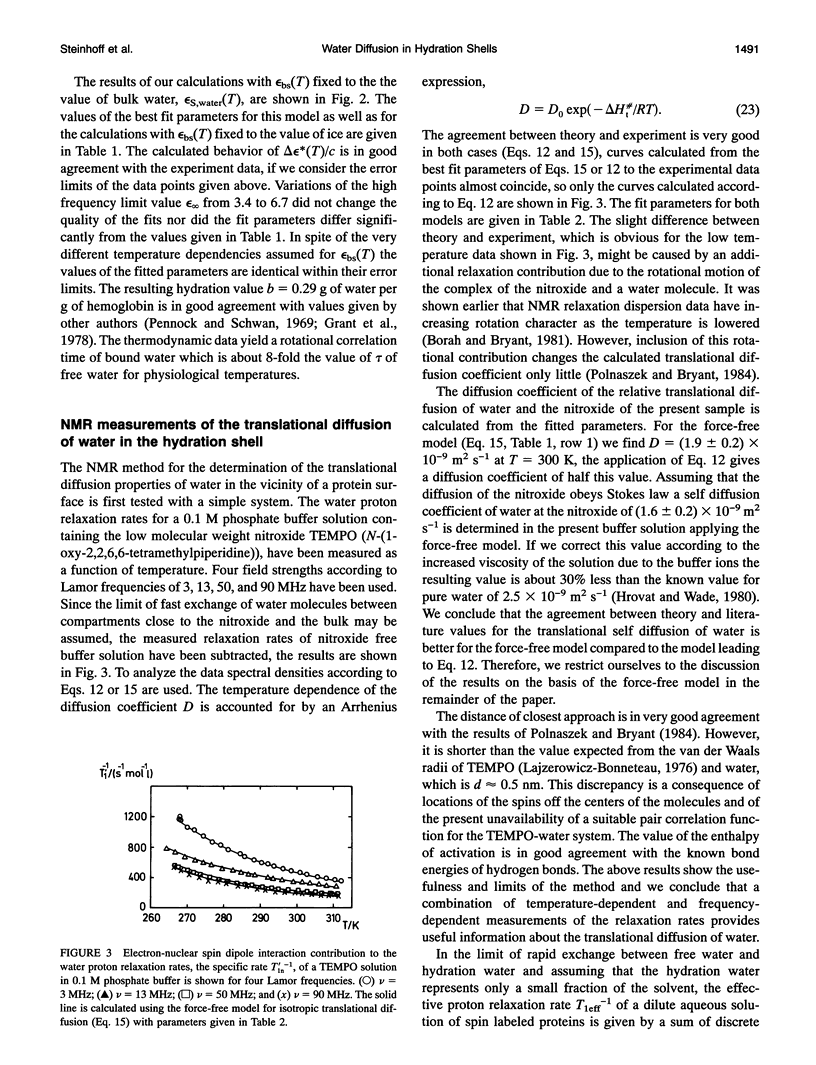
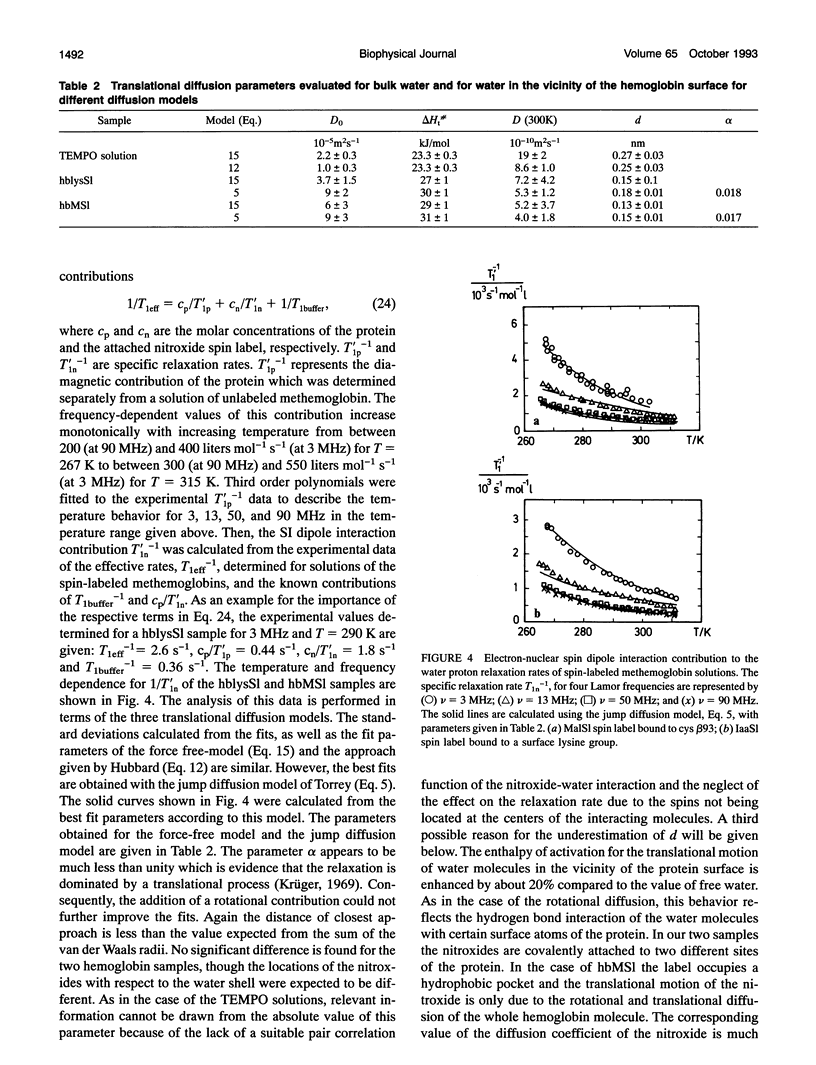
The dynamic properties of water in the hydration shell of hemoglobin have been studied by means of dielectric permittivity measurements and nuclear magnetic resonance spectroscopy. The temperature behavior of the complex permittivity of hemoglobin solutions has been measured at 3.02, 3.98, 8.59, and 10.80 GHz. At a temperature of 298 K the average rotational correlation time tau of water within a hydration shell of 0.5-nm thickness is determined from the activation parameters to be 68 +/- 10 ps, which is 8-fold the corresponding value of bulk water. Solvent proton magnetic relaxation induced by electron-nuclear dipole interaction between hemoglobin bound nitroxide spin labels and water protons is used to determine the translational diffusion coefficient D(T) of the hydration water. The temperature dependent relaxation behavior for Lamor frequencies between 3 and 90 MHz yields an average value D(298K) = (5 +/- 2) x 10(-10)m2 s-1, which is about one-fifth of the corresponding value of bulk water. The decrease of the water mobility in the hydration shell compared to the bulk is mainly due to an enhanced activation enthalpy.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks C. L., 3rd, Karplus M. Solvent effects on protein motion and protein effects on solvent motion. Dynamics of the active site region of lysozyme. J Mol Biol. 1989 Jul 5;208(1):159–181. doi: 10.1016/0022-2836(89)90093-4. [DOI] [PubMed] [Google Scholar]
- Careri G., Gratton E., Yang P. H., Rupley J. A. Correlation of IR spectroscopic, heat capacity, diamagnetic susceptibility and enzymatic measurements on lysozyme powder. Nature. 1980 Apr 10;284(5756):572–573. doi: 10.1038/284572a0. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Gratton E. Protein dynamics and hydration. Methods Enzymol. 1986;127:207–216. doi: 10.1016/0076-6879(86)27017-2. [DOI] [PubMed] [Google Scholar]
- Goldanskii V. I., Krupyanskii Y. F. Protein and protein-bound water dynamics studied by Rayleigh scattering of Mössbauer radiation (RSMR). Q Rev Biophys. 1989 Feb;22(1):39–92. doi: 10.1017/s003358350000336x. [DOI] [PubMed] [Google Scholar]
- Grant E. H., McClean V. E., Nightingale N. R., Sheppard R. J., Chapman M. J. Dielectric behavior of water in biological solutions: studies on myoglobin, human low-density lipoprotein, and polyvinylpyrrolidone. Bioelectromagnetics. 1986;7(2):151–162. doi: 10.1002/bem.2250070206. [DOI] [PubMed] [Google Scholar]
- Moffat J. K. Spin-labelled haemoglobins: a structural interpretation of electron paramagnetic resonance spectra based on X-ray analysis. J Mol Biol. 1971 Jan 28;55(2):135–146. doi: 10.1016/0022-2836(71)90187-2. [DOI] [PubMed] [Google Scholar]
- Pennock B. E., Schwan H. P. Further observations on the electrical properties of hemoglobin-bound water. J Phys Chem. 1969 Aug;73(8):2600–2610. doi: 10.1021/j100842a024. [DOI] [PubMed] [Google Scholar]
- Redhardt A., Daseler W. A new method for absolute determination of radical concentrations of EPR. J Biochem Biophys Methods. 1987 Nov;15(2):71–83. doi: 10.1016/0165-022x(87)90035-2. [DOI] [PubMed] [Google Scholar]
- SCHWAN H. P. Electrical properties of tissue and cell suspensions. Adv Biol Med Phys. 1957;5:147–209. doi: 10.1016/b978-1-4832-3111-2.50008-0. [DOI] [PubMed] [Google Scholar]
- Steinhoff HJn, Dombrowsky O., Karin C., Schneiderhahn C. Two dimensional diffusion of small molecules on protein surfaces: an EPR study of the restricted translational diffusion of protein-bound spin labels. Eur Biophys J. 1991;20(5):293–303. doi: 10.1007/BF00450565. [DOI] [PubMed] [Google Scholar]
- Steinhoff H. J. Residual motion of hemoglobin-bound spin labels and protein dynamics: viscosity dependence of the rotational correlation times. Eur Biophys J. 1990;18(1):57–62. doi: 10.1007/BF00185420. [DOI] [PubMed] [Google Scholar]
- Steinhoff H., Lieutenant K., Schlitter J. Residual motion of hemoglobin-bound spin labels as a probe for protein dynamics. Z Naturforsch C. 1989 Mar-Apr;44(3-4):280–288. doi: 10.1515/znc-1989-3-417. [DOI] [PubMed] [Google Scholar]
- Teeter M. M. Water-protein interactions: theory and experiment. Annu Rev Biophys Biophys Chem. 1991;20:577–600. doi: 10.1146/annurev.bb.20.060191.003045. [DOI] [PubMed] [Google Scholar]


