Abstract
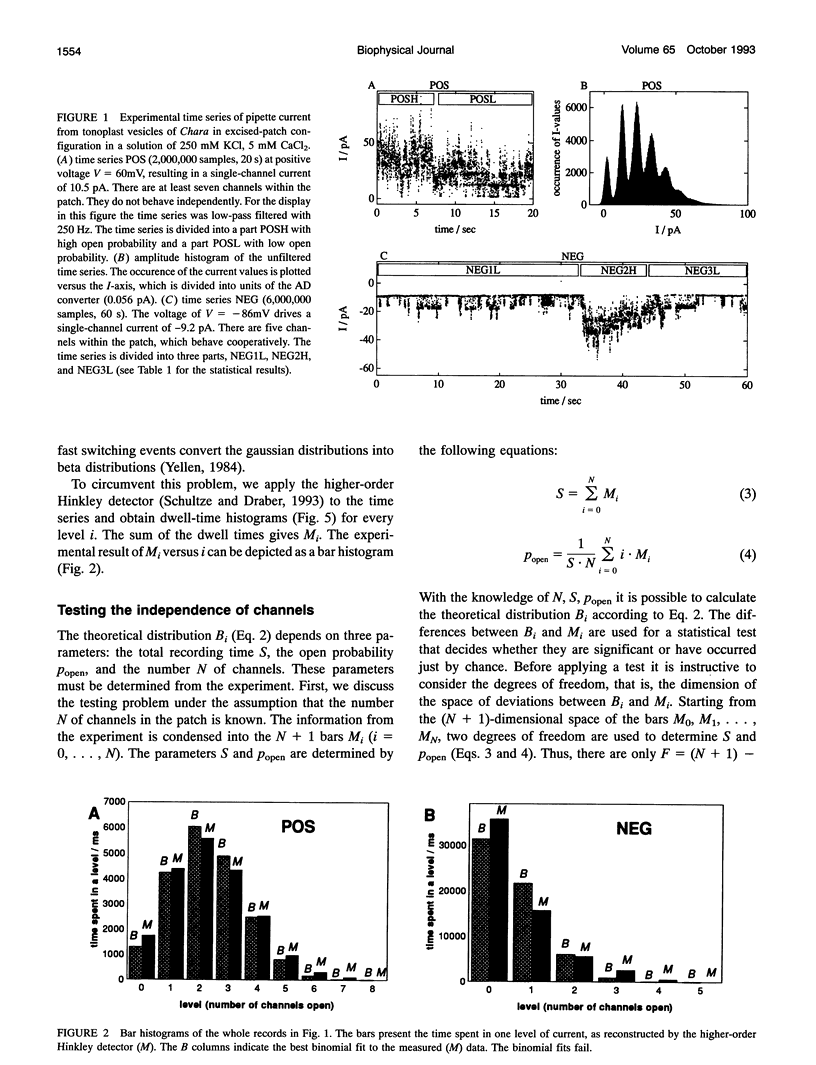
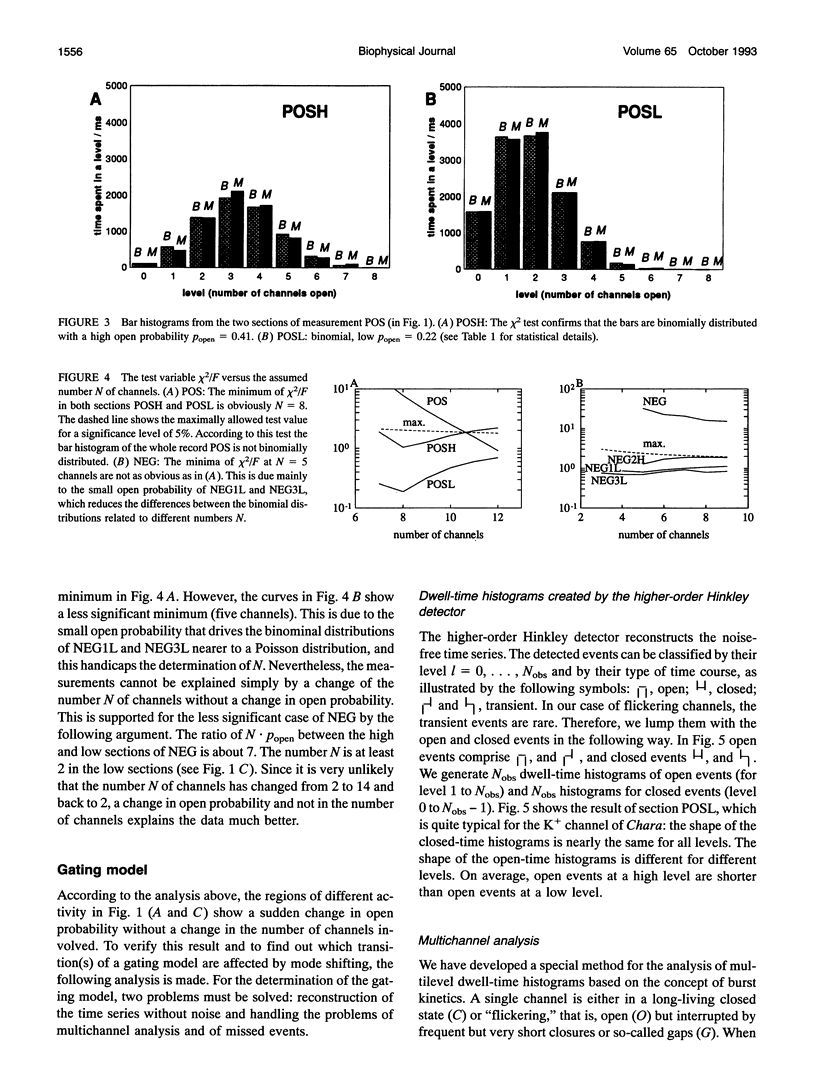
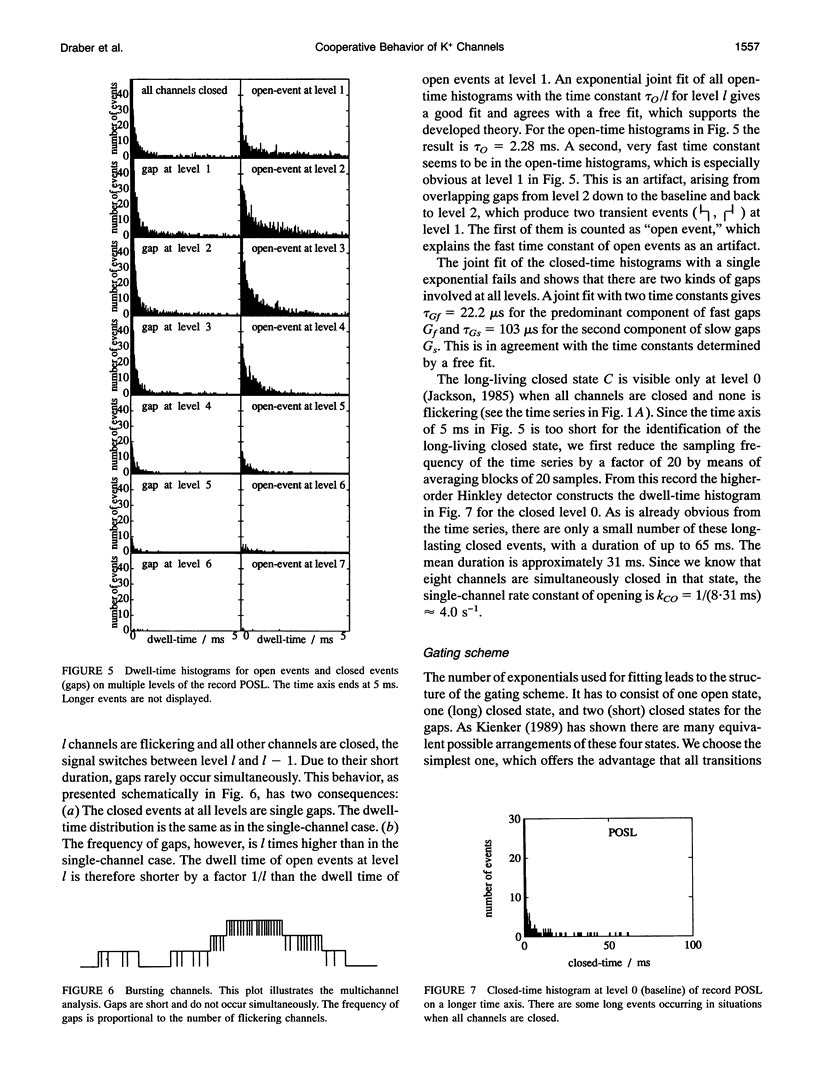
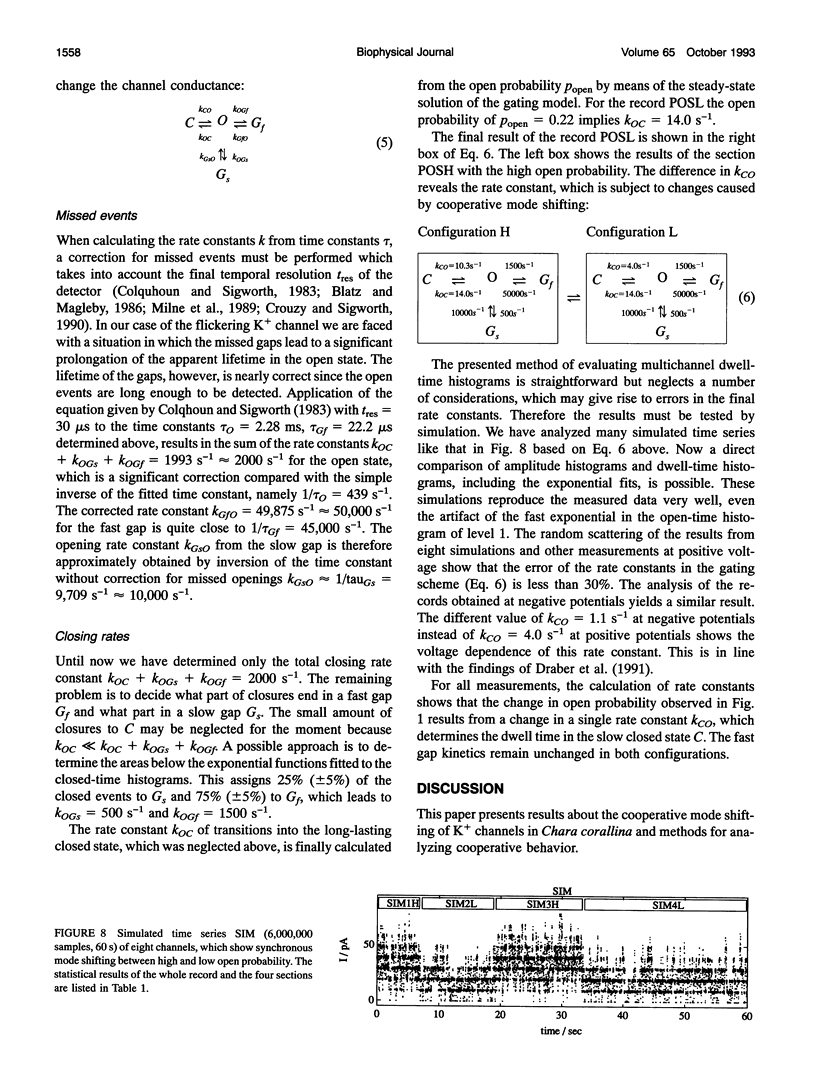
Spontaneous cooperatively of K+ channels is studied in excised patches of Chara corallina tonoplasts. Bar histograms (dwell time versus number of open channels) are constructed from the time series of current by means of the higher-order Hinkley detector (R. Schultze and S. Draber. 1993. J. Membr. Biol. 132:41-52). A statistical test, based on these bar histograms, shows that the channels are not independent. Further analysis reveals that the channels are cooperatively changing their open probability, which leads to the idea of cooperative mode shifting.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzy S. C., Sigworth F. J. Yet another approach to the dwell-time omission problem of single-channel analysis. Biophys J. 1990 Sep;58(3):731–743. doi: 10.1016/S0006-3495(90)82416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draber S., Schultze R., Hansen U. P. Patch-clamp studies on the anomalous mole fraction effect of the K+ channel in cytoplasmic droplets of Nitella: an attempt to distinguish between a multi-ion single-file pore and an enzyme kinetic model with lazy state. J Membr Biol. 1991 Aug;123(2):183–190. doi: 10.1007/BF01998088. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Horn R. Estimating the number of channels in patch recordings. Biophys J. 1991 Aug;60(2):433–439. doi: 10.1016/S0006-3495(91)82069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa K., Ehrenstein G., Moran N., Jia M. Evidence for interactions between batrachotoxin-modified channels in hybrid neuroblastoma cells. Biophys J. 1986 Sep;50(3):531–537. doi: 10.1016/S0006-3495(86)83491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Stochastic behavior of a many-channel membrane system. Biophys J. 1985 Feb;47(2 Pt 1):129–137. doi: 10.1016/s0006-3495(85)83886-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler R. W., Brink P. R., Dewey M. M. The septum of the lateral axon of the earthworm: a thin section and freeze-fracture study. J Neurocytol. 1979 Oct;8(5):565–590. doi: 10.1007/BF01208510. [DOI] [PubMed] [Google Scholar]
- Kienker P. Equivalence of aggregated Markov models of ion-channel gating. Proc R Soc Lond B Biol Sci. 1989 Apr 22;236(1284):269–309. doi: 10.1098/rspb.1989.0024. [DOI] [PubMed] [Google Scholar]
- Kiss T., Nagy K. Interaction between sodium channels in mouse neuroblastoma cells. Eur Biophys J. 1985;12(1):13–18. doi: 10.1007/BF00254090. [DOI] [PubMed] [Google Scholar]
- Liu Y., Dilger J. P. Application of the one- and two-dimensional Ising models to studies of cooperativity between ion channels. Biophys J. 1993 Jan;64(1):26–35. doi: 10.1016/S0006-3495(93)81337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manivannan K., Ramanan S. V., Mathias R. T., Brink P. R. Multichannel recordings from membranes which contain gap junctions. Biophys J. 1992 Jan;61(1):216–227. doi: 10.1016/S0006-3495(92)81828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne R. K., Yeo G. F., Madsen B. W., Edeson R. O. Estimation of single channel kinetic parameters from data subject to limited time resolution. Biophys J. 1989 Apr;55(4):673–676. doi: 10.1016/S0006-3495(89)82865-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M. R., Hess P. Reversible uncoupling of inactivation in N-type calcium channels. Nature. 1991 Jun 20;351(6328):657–659. doi: 10.1038/351657a0. [DOI] [PubMed] [Google Scholar]
- Schultze R., Draber S. A nonlinear filter algorithm for the detection of jumps in patch-clamp data. J Membr Biol. 1993 Feb;132(1):41–52. doi: 10.1007/BF00233050. [DOI] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeramian E., Trautmann A., Claverie P. Acetylcholine receptors are not functionally independent. Biophys J. 1986 Aug;50(2):253–263. doi: 10.1016/S0006-3495(86)83459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. Y., Potts J. F., Trimmer J. S., Agnew W. S., Sigworth F. J. Multiple gating modes and the effect of modulating factors on the microI sodium channel. Neuron. 1991 Nov;7(5):775–785. doi: 10.1016/0896-6273(91)90280-d. [DOI] [PubMed] [Google Scholar]


