Abstract
We construct a minimal model of cytosolic free Ca2+ oscillations based on Ca2+ release via the inositol 1,4,5-trisphosphate (IP3) receptor/Ca2+ channel (IP3R) of a single intracellular Ca2+ pool. The model relies on experimental evidence that the cytosolic free calcium concentration ([Ca2+]c) modulates the IP3R in a biphasic manner, with Ca2+ release inhibited by low and high [Ca2+]c and facilitated by intermediate [Ca2+]c, and that channel inactivation occurs on a slower time scale than activation. The model produces [Ca2+]c oscillations at constant [IP3] and reproduces a number of crucial experiments. The two-dimensional spatial model with IP3 dynamics, cytosolic diffusion of IP3 (Dp = 300 microns 2 s-1), and cytosolic diffusion of Ca2+ (Dc = 20 microns 2 s-1) produces circular, planar, and spiral waves of Ca2+ with speeds of 7-15 microns.s-1, which annihilate upon collision. Increasing extracellular [Ca2+] influx increases wave speed and baseline [Ca2+]c. A [Ca2+]c-dependent Ca2+ diffusion coefficient does not alter the qualitative behavior of the model. An important model prediction is that channel inactivation must occur on a slower time scale than activation in order for waves to propagate. The model serves to capture the essential macroscopic mechanisms that are involved in the production of intracellular Ca2+ oscillations and traveling waves in the Xenopus laevis oocyte.
Full text
PDF

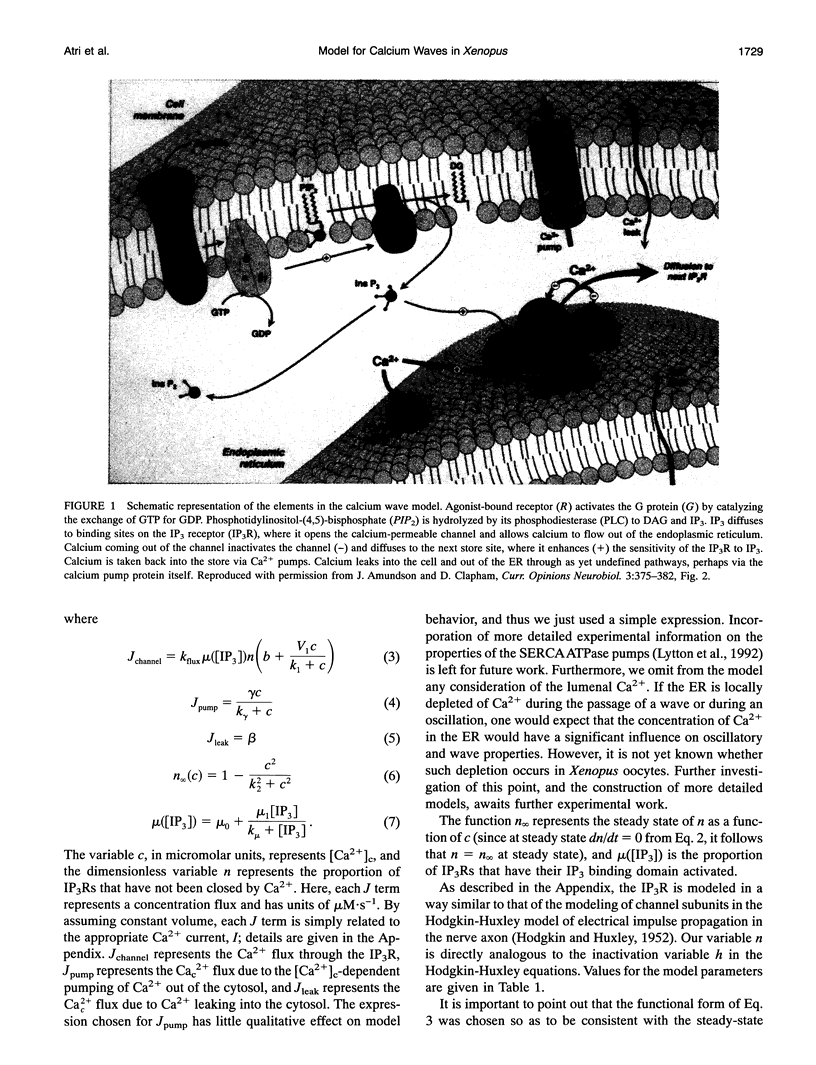
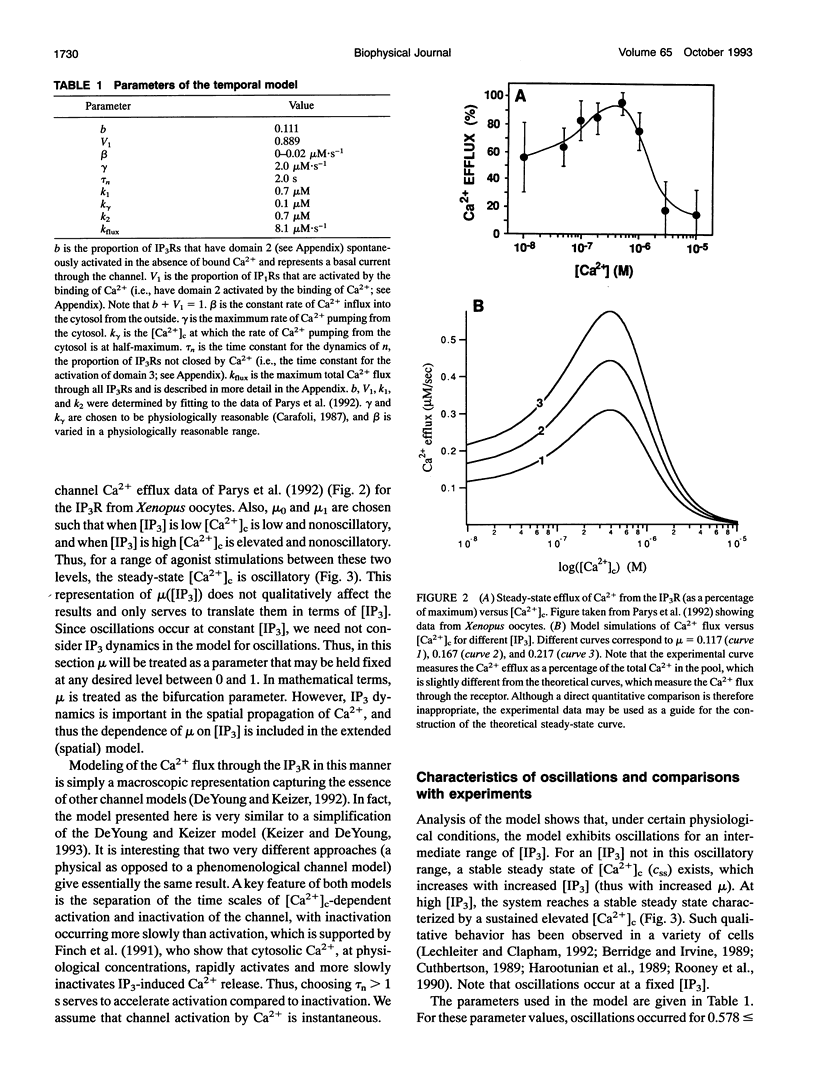









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992 Dec 11;258(5089):1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Galione A. Cytosolic calcium oscillators. FASEB J. 1988 Dec;2(15):3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. The molecular basis of communication within the cell. Sci Am. 1985 Oct;253(4):142–152. doi: 10.1038/scientificamerican1085-142. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Boitano S., Dirksen E. R., Sanderson M. J. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992 Oct 9;258(5080):292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- Camacho P., Lechleiter J. D. Increased frequency of calcium waves in Xenopus laevis oocytes that express a calcium-ATPase. Science. 1993 Apr 9;260(5105):226–229. doi: 10.1126/science.8385800. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Charles A. C., Merrill J. E., Dirksen E. R., Sanderson M. J. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991 Jun;6(6):983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- Cobbold P. H., Cuthbertson K. S. Calcium oscillations: phenomena, mechanisms and significance. Semin Cell Biol. 1990 Aug;1(4):311–321. [PubMed] [Google Scholar]
- Cuthbertson K. S., Chay T. R. Modelling receptor-controlled intracellular calcium oscillators. Cell Calcium. 1991 Feb-Mar;12(2-3):97–109. doi: 10.1016/0143-4160(91)90012-4. [DOI] [PubMed] [Google Scholar]
- Davis T. N. What's new with calcium? Cell. 1992 Nov 13;71(4):557–564. doi: 10.1016/0092-8674(92)90590-9. [DOI] [PubMed] [Google Scholar]
- De Young G. W., Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9895–9899. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle S., Welsh M. J. Inositol trisphosphate is required for the propagation of calcium waves in Xenopus oocytes. J Biol Chem. 1992 Apr 25;267(12):7963–7966. [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Girard S., Clapham D. Acceleration of intracellular calcium waves in Xenopus oocytes by calcium influx. Science. 1993 Apr 9;260(5105):229–232. doi: 10.1126/science.8385801. [DOI] [PubMed] [Google Scholar]
- Girard S., Lückhoff A., Lechleiter J., Sneyd J., Clapham D. Two-dimensional model of calcium waves reproduces the patterns observed in Xenopus oocytes. Biophys J. 1992 Feb;61(2):509–517. doi: 10.1016/S0006-3495(92)81855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeter A., Dupont G., Berridge M. J. Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1461–1465. doi: 10.1073/pnas.87.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Tsien R. Y. Agonist-induced calcium oscillations in depolarized fibroblasts and their manipulation by photoreleased Ins(1,4,5)P3, Ca++, and Ca++ buffer. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):935–943. doi: 10.1101/sqb.1988.053.01.108. [DOI] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990 Jun;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-triphosphate-induced Ca2+ release. Nature. 1992 Nov 5;360(6399):76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- Lechleiter J. D., Clapham D. E. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992 Apr 17;69(2):283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Burk S. E., Shull G. E., MacLennan D. H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem. 1992 Jul 15;267(20):14483–14489. [PubMed] [Google Scholar]
- Meyer T., Stryer L. Calcium spiking. Annu Rev Biophys Biophys Chem. 1991;20:153–174. doi: 10.1146/annurev.bb.20.060191.001101. [DOI] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Characteristics of membrane currents evoked by photoreleased inositol trisphosphate in Xenopus oocytes. Am J Physiol. 1992 Jul;263(1 Pt 1):C154–C165. doi: 10.1152/ajpcell.1992.263.1.C154. [DOI] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci U S A. 1990 Jan;87(1):260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Yao Y. Regenerative release of calcium from functionally discrete subcellular stores by inositol trisphosphate. Proc Biol Sci. 1991 Dec 23;246(1317):269–274. doi: 10.1098/rspb.1991.0154. [DOI] [PubMed] [Google Scholar]
- Parys J. B., Sernett S. W., DeLisle S., Snyder P. M., Welsh M. J., Campbell K. P. Isolation, characterization, and localization of the inositol 1,4,5-trisphosphate receptor protein in Xenopus laevis oocytes. J Biol Chem. 1992 Sep 15;267(26):18776–18782. [PubMed] [Google Scholar]
- Petersen O. H., Wakui M. Oscillating intracellular Ca2+ signals evoked by activation of receptors linked to inositol lipid hydrolysis: mechanism of generation. J Membr Biol. 1990 Nov;118(2):93–105. doi: 10.1007/BF01868467. [DOI] [PubMed] [Google Scholar]
- Quinn S. J., Williams G. H., Tillotson D. L. Calcium oscillations in single adrenal glomerulosa cells stimulated by angiotensin II. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5754–5758. doi: 10.1073/pnas.85.15.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney T. A., Sass E. J., Thomas A. P. Characterization of cytosolic calcium oscillations induced by phenylephrine and vasopressin in single fura-2-loaded hepatocytes. J Biol Chem. 1989 Oct 15;264(29):17131–17141. [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. The kinetics of changes in intracellular calcium concentration in fura-2-loaded human platelets. J Biol Chem. 1987 Dec 5;262(34):16364–16369. [PubMed] [Google Scholar]
- Sanderson M. J., Charles A. C., Dirksen E. R. Mechanical stimulation and intercellular communication increases intracellular Ca2+ in epithelial cells. Cell Regul. 1990 Jul;1(8):585–596. doi: 10.1091/mbc.1.8.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneyd J., Girard S., Clapham D. Calcium wave propagation by calcium-induced calcium release: an unusual excitable system. Bull Math Biol. 1993 Mar;55(2):315–344. doi: 10.1007/BF02460886. [DOI] [PubMed] [Google Scholar]
- Somogyi R., Stucki J. W. Hormone-induced calcium oscillations in liver cells can be explained by a simple one pool model. J Biol Chem. 1991 Jun 15;266(17):11068–11077. [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Tsunoda Y. Oscillatory Ca2+ signaling and its cellular function. New Biol. 1991 Jan;3(1):3–17. [PubMed] [Google Scholar]






