Abstract
The neuroendocrine ATP-sensitive K+ (KATP) channel comprises four pore-forming subunits (Kir6.2), and four modulatory sulfonylurea receptor subunits (SUR1). ATP/ADP binding to Kir6.2 inhibits KATP, whereas MgATP/MgADP binding to two sites on SUR1 promotes activation. As SUR1 is part of the ABC transporter family, it can hydrolyze MgATP to MgADP. Whether or not enzymatic activity is required for KATP activation remains controversial. Non-hydrolyzable ATP analogs do not activate KATP, which may reflect an inability of these compounds to bind to SUR1, their inability to promote a conformational change in SUR1 that leads to channel activation, or a requirement for ATP hydrolysis during channel gating. To explore this, we synthesized a fluorescent trinitrophenyl (TNP) derivative of the non-hydrolyzable ATP analog β,γ-methyleneadenosine 5’-triphosphate (AMP-PCP). Synthesis was verified by UV-visible absorbance, fluorescence, 1H NMR, and mass spectrometry. Purity was assessed using TLC and reversed-phase HPLC. We can measure real-time binding of fluorescent nucleotide derivatives to intact KATP channels in cell membranes using FRET between channels labeled with a fluorescent, non-canonical amino acid and TNP-nucleotide derivatives. This technique provides us with sufficient spatial resolution to discriminate between binding to each site on KATP. Using this approach we measured TNP-ATP binding to nucleotide binding site 1 on SUR1 in fluorescently labeled Kir6.2/SUR1 channels in unroofed membranes of HEK293T cells. TNP-AMP-PCP binds to both nucleotide binding sites on SUR1 in the absence of Mg2+. AMP-PCP was able to compete with TNP-ATP for binding to NBS2, suggesting that it, too, binds NBS2. We conclude that the failure of non-hydrolyzable ATP analogs to activate KATP does not stem from an inability of these nucleotides to bind to the channel, leaving open the possibilities that they are unable to induce an activating conformational change in SUR1 or that nucleotide hydrolysis by SUR1 is a prerequisite for channel activation.
Keywords: ion channel, enzyme, fluorescence, ATP
SUMMARY
Rubio et al. synthesize, purify, and characterize a novel, fluorescent non-hydrolyzable ATP derivative and use it to probe the nucleotide-binding sites of KATP.
INTRODUCTION
ATP sensitive K+ (KATP) channels are endowed with a unique physiological gift: the ability to directly translate a change in a cell’s metabolic state into a change in its electrical excitability. Whereas KATP plays crucial roles in cardiovascular and neuronal tissues, its best understood task is in pancreatic β-cells, where changes in KATP open probability during digestion trigger insulin secretion (Puljung, 2023). Underlying this crucial function, mutations in the genes that encode the pancreatic KATP channel cause inherited diseases of insulin secretion including neonatal diabetes and congenital hyperinsulinism. Mutations that severely disrupt KATP function can also cause neuronal complications including developmental delay and epilepsy (Ashcroft et al., 2017).
The pancreatic KATP subtype is a hetero-octameric complex comprising four inward-rectifier K+ channel subunits (Kir6.2), each associated with a modulatory sulfonylurea receptor (SUR1) (Figure 1A,B) (Inagaki et al., 1997; Shyng and Nichols, 1997). Metabolic sensitivity is conferred on KATP through the binding of intracellular adenine nucleotides (ATP/ADP) to three classes of intracellular nucleotide-binding site (NBS), twelve sites in all, when accounting for the complex’s four-fold symmetry (Lee et al., 2017). The cytoplasmic domain of each Kir6.2 subunit contains a binding site for ATP/ADP. Nucleotide binding to Kir6.2 closes the channel in a reaction that does not require Mg2+ (Tucker et al., 1997). Nucleotides bind to two NBSs on SUR1, formed at the interface between its cytoplasmic nucleotide binding domains (NBDs, Figure 1C) (Vedovato et al., 2015). NBD dimerization drives a conformational change in the transmembrane domains of SUR1 that is coupled to an increase in KATP’s open probability (Gribble et al., 1998). Whereas nucleotides bind to both NBSs of SUR1 in the presence or absence of Mg2+, Mg2+ is required to stabilize the NBD dimers and promote channel activation (Puljung et al., 2019).
Figure 1. Structure of the pancreatic KATP channel.

A. Cartoon showing the transmembrane topology of the pancreatic KATP channel. Two (of four) Kir6.2 subunits are shown along with one (of four) SUR1 subunits. Red and green circles mark the inhibitory and excitatory nucleotide binding sites (respectively). B. Cryo-EM structure of the pancreatic KATP channel determined in the presence of MgATP/ADP and viewed from the cytoplasmic side (Lee et al., 2017). The color scheme matches that of the schematic in panel A. PDB accession number 6C3O. C. The nucleotide binding domains of one SUR1 subunit viewed from the cytoplasmic side. ATP (bound to NBS1) and ADP (bound to NBS2) are shown as sticks. W688 and T1397 mark the sites that will be replaced with ANAP in our experiments.
SUR1 is part of the ABC family of transporters (Aguilar-Bryan et al., 1995; Tusnády et al., 1997). Unlike most ABC proteins, SUR1 does not have any intrinsic transport activity, evolving only to modulate trafficking and opening of the Kir6.2 pore (Sakura et al., 1995; Inagaki et al., 1995). Like other members of the ABC transporter family, SUR1 is an ATPase. NBS2, formed by the conserved, catalytic WalkerA and WalkerB motifs on NBD2 and an ABC signature sequence on NBD1 is competent to hydrolyze ATP. This is evident from in vitro ATPase activity in isolated NBD2 constructs and SUR1 subunits as well as a cryo-EM structure determined in the presence of MgATP, which surprisingly showed density for MgADP at NBS2 (Matsuo et al., 1999; de Wet et al., 2007; Lee et al., 2017). NBS1, on the other hand, is thought to be a degenerate site with no ATPase activity due to amino acid substitutions in its WalkerB motif and ABC signature sequence (Vedovato et al., 2015). Degenerate sites are found in other ABC-family proteins including the closely related cystic fibrosis transmembrane conductance regulator (CFTR) channel and the bacterial transporter TM287/288 (Hohl et al., 2012; Aleksandrov et al., 2002).
Whereas SUR1 retains the ability to hydrolyze ATP to ADP, the relevance of this enzymatic activity for channel gating is unclear. KATP is directly activated by MgADP, implying that ATP hydrolysis is not required to provide a “power-stroke” coupled to channel activation (Proks et al., 2010). However, it remains an intriguing possibility that MgATP must first be hydrolyzed to MgADP to promote channel gating. Consistent with this idea, opening of cardiac KATP channels (formed by Kir6.2 and SUR2A) is promoted by vanadate, which stabilizes ABC proteins in a post-hydrolytic conformation (Zingman et al.). However, the time course of the current increase in vanadate was slow (taking 8.8 ± 0.9 min to develop) compared to channel gating. For comparison, direct activation of Kir6.2-G334D/SUR1 by MgATP in inside-out patches was much faster, following a biexponential time course with time constants of 730 ms and 4.060 s (Proks et al., 2010). Further discounting the hypothesis that hydrolysis is required for gating, analysis of single-channel KATP currents do not show any out-of-equilibrium steps in the gating process as one would expect for a process that involves an irreversible hydrolysis step (Choi et al., 2008; Csanady et al., 2010). Finally, ATP was able to promote a conformational change in isolated SUR1 subunits that displaced binding of tritiated glibenclamide (a KATP antagonist that binds SUR1) in the absence of Mg2+, i.e. under conditions that do not support hydrolysis (Ortiz et al., 2012; Martin et al., 2017). However, this effect required very high concentrations of ATP. Furthermore, these experiments were performed in isolated SUR1 subunits in Pichia membranes under equilibrium binding conditions, not in intact-functioning channels under physiological conditions.
To probe the requirement for nucleotide hydrolysis by SUR1, we have decided to evaluate the effects of non-hydrolyzable ATP analogs in real-time using intact, functional channels in a membrane environment. Neither β,γ-methyleneadenosine 5’-triphosphate (AMP-PCP), which has a methylene group replacing the oxygen between the β and γ phosphates of ATP nor adenylyl-imidodiphosphate (AMP-PNP), which has an NH group at the same location, appear to support KATP activation (Dunne, 1989; Kozlowski and Ashford, 1992; Proks et al., 2010; Findlay, 1987; Ashcroft and Kakei, 1989; Treherne and Ashford, 1992; Jiang and Haddad, 1997; Schwanstecher and Panten, 1994; Schwanstecher et al., 1994; Hehl and Neumcke, 1994; Schwanstecher et al., 1992). Adenosine 5’-O-(3-thiotriphosphate) (ATP-γ-S) activates KATP channels for which the inhibitory nucleotide binding site on Kir6.2 has been removed (Kir6.2-G334D/SUR1) (Proks et al., 2010). However, this analog is considered to be weakly hydrolyzable.
There are at least three possible explanations for the inability of AMP-PCP and AMP-PNP to activate KATP. 1) The nucleotides fail to bind to the NBSs of SUR1. 2) The nucleotides bind but fail to induce the conformational change (NBD dimerization) in SUR1 that promotes channel activation. 3). The nucleotides bind and dimerize the NBDs, but there is a bona fide requirement for ATP hydrolysis during the channel gating cycle. In this paper, we will address the first of these possibilities: binding. We have previously developed a technique that allows us to measure site-specific nucleotide binding to intact, functional KATP channels in the plasma membrane (Puljung et al., 2019; Usher et al., 2020b; a). This technique employs Förster resonance energy transfer (FRET) between the non-canonical amino acid l-3-(6-acetylnaphthalen-2-ylamino)-2-aminopropionic acid (ANAP), which can be incorporated directly into SUR1 using amber-stop-codon suppression, and fluorescent, trinitrophenyl (TNP) derivatives of ATP and ADP (Chatterjee et al., 2013; Puljung, 2021; Puljung et al., 2019; Usher et al., 2020a). The steep distance dependence of FRET allows us to discriminate binding to each NBS. To evaluate non-hydrolyzable nucleotide effects on functional KATP channels in the plasma membrane, we synthesized, purified, and characterized a trinitrophenyl derivative of AMP-PCP (TNP-AMP-PCP). This derivative binds both NBSs of SUR1. Furthermore, we used a competition assay to demonstrate that non-fluorescent AMP-PCP can bind directly to NBS2. We conclude that the inability of non-hydrolyzable nucleotides to activate KATP does not arise from an inability to bind to SUR1 and either reflects an inability of these derivatives to change the conformation of SUR1 or a requirement for ATP hydrolysis to ADP to promote channel activation.
MATERIALS AND METHODS
TNP-AMP-PCP synthesis.
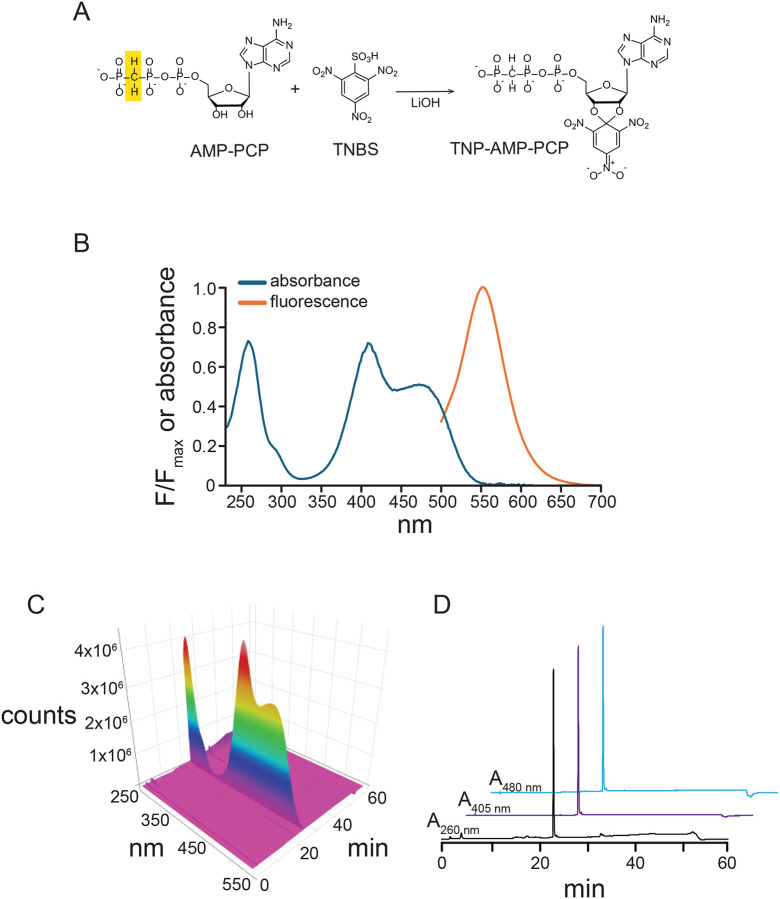
We synthesized TNP-AMP-PCP using a modified version of the protocol for TNP-ATP synthesis published by Hiratsuka and Uchida (Figure 2A) (Hiratsuka and Uchida, 1973). 25 mg of the Na+ salt of β,γ-methyleneadenosine 5’-triphosphate (AMP-PCP; Enzo Biochem; Farmingdale, NY) were dissolved in 1 mL of deionized water and the concentration was verified by UV-vis spectroscopy (λmax = 259 nm, ε = 15,400 M−1cm−1) using a Hitachi U-3010 UV-visible (Hitachi, Tokyo, Japan) and UV Solutions software. The measured concentration of AMP-PCP was 0.032 M (a total of 32 μmoles of AMP-PCP). 130 μL of a 1 M stock solution of 2,4,6-trinitrobenzenesulfonic acid (TNBS; Sigma-Aldrich, St. Louis, MO) in water were added to the AMP-PCP solution while stirring. The pH was adjusted to ~9.5 with 240 μL of 1 M LiOH (Sigma-Aldrich). The reaction was stirred in the dark for four days at room temperature. Basic pH was maintained with daily additions of 1 M LiOH (an additional volume of 260 μL). Reaction progress was evident by the appearance of a deep orange color, resulting from the formation of a Meisenheimer complex subsequent to trinitrophenylation of the ribose (Azegami and Iwai, 1975).
Figure 2. Synthesis and characterization of TNP-AMP-PCP.
A. General reaction scheme for synthesis of TNP-AMP-PCP. The methylene group between the β and γ phosphates of AMP-PCP is highlighted in yellow. B. UV/Vis absorbance and normalized fluorescence emission spectra of purified TNP-AMP-PCP in 1M Tris, pH 8.0. C. Reversed-phase HPLC on purified TNP-AMP-PCP. The sample was applied to a C18 in 0.1 M TEAB buffer and eluted on a gradient of 0% to 60% acetonitrile in TEAB buffer. D. Cross sections of the chromatogram in D showing the elution of a single peak detected at 260 nm, 405 nm, and 480 nm. The traces are offset vertically and horizontally (by 5 min and 10 min for the 405 nm and 480 nm trace, respectively) for display purposes.
The product was purified by precipitation with 10 mL of 95% ethanol and 100 μL of 3 M sodium acetate, pH 5.2 (EMD Millipore Co.; Billerica, MA) and stored at −20 °C. The precipitate was collected by centrifugation at 13,500 xg in a Labnet Prism microcentrifuge (Labnet International; Edison, NJ). The product was washed twice with 95% ethanol, dried, and resuspended in deionized water for subsequent applications. Based on A259 (see below) we estimate our yield to be 79.3%.
We also attempted to synthesize trinitrophenyl derivatives of adenylyl-imidodiphosphate (AMP-PNP) and adenosine 5’-O-(3-thiotriphosphate) (ATP-γ-S). Whereas the reaction clearly proceeded and led to the formation of a Meisenheimer complex, subsequent mass spectrometric and thin-layer chromatography analyses suggested that one or more phosphate groups were lost during the reaction or purification process. Thus, we did not pursue these compounds any further.
Thin layer chromatography (TLC).
Purified samples of TNP-AMP-PCP (and the products from our reactions involving AMP-PNP and ATP-γ-S) were spotted on MilliporeSigma (Burlington, MA) TLC Silica gel 60 F254 plates (mobile phase 40:10:25 n-butanol: glacial acetic acid: water). Commercially available TNP-ATP (Tocris Bioscience; Minneapolis, MN) and TNP-ADP (Axxora LLC; Farmingdale, NY) were run for comparison. Retention fraction values were as follows: TNP-ATP, 0; TNP-ADP, 0.006; TNP-AMP-PCP, 0; AMP-PCP product 0.35; ATP-γ-S product (0.5).
UV-visible spectrophotometry.
Aqueous solutions of TNP-AMP-PCP were quantified by absorbance at 259 nm, using the published ε259 nm value for TNP-ATP of 26,400 M−1cm−1 (Hiratsuka and Uchida, 1973). Stock solutions were diluted 1:100 in water and absorbance was measured using a Red Tide UBS650U spectrometer (Ocean Optics; Orlando, FL) and LoggerPro software (Vernier; Beaverton, OR). Trinitrophenylation of the ribose of TNP-AMP-PCP induces the formation of a Meisenheimer complex and two additional absorbance peaks in the visible spectrum (ε408 nm = 26,400 M−1cm−1, ε470 nm = 18,500 M−1cm−1; published values for TNP-ATP). This complex is reversibly protonated with a pKa around 4.5 (Azegami and Iwai, 1975). To study pH effects on the absorbance of the Meisenheimer complex an 8.3 mM stock of TNP-AMP-PCP was diluted 1:200 in 1.0 M tris(hydroxymethyl)aminomethane (Tris), pH 8.0, 1.0 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.3, or 0.5 M 2-((morpholino)ethyl)sulfonic acid (MES), pH 5.3. Absorbance spectra were blank subtracted using buffer-only samples.
Fluorescence emission.
The fluorescence emission of a 41.5 μM solution of TNP-AMP-PCP in 1 M Tris, pH 8.0 was measured using a Hitachi F-7000 fluorescence spectrophotometer and FL Solutions software. The samples were excited at 400 nm and emission was measured between 450 nm and 700 nm. Slit widths were 5 nm on the excitation and emission side and the PMT voltage was set to 950 V. Spectra were blank subtracted using a 1.0 M Tris solution.
High performance liquid chromatography (HPLC).
Sample purity was assessed by reversed-phase HPLC using an Econosphere C18 5μ column (Grace; Columbia, MD). Samples were run on a linear gradient from 0–60% acetonitrile in 0.1 M triethylammonium bicarbonate (TEAB, pH ~7.5) at a flow rate of 1 mL/min using a Hitachi Elite LaChrome HPLC equipped with an L-2450 diode-array detector. Data were acquired using EZChrom Elite software (Agilent Technologies; Santa Clara, CA).
Mass spectrometry.
TNP-nucleotide samples were diluted to a concentration of 20 μM in 1:1 water:acetonitrile plus 0.1% triethylamine. Samples were analyzed via direct-infusion electrospray ionization using an AB SCIEX 4000 QTRAP mass spectrometer (Framingham, MA). The sample was delivered to the mass spectrometer via a syringe pump operating at a flow rate of 10 μL/min. Electrospray ionization was conducted in negative mode with an inlet spray voltage of −4500 V, a declustering potential of −140 V and a source temperature of 70 °C. Analysis was conducted in enhanced mass spectrometry mode using a dynamic fill time for the ion trap, scanning over a range of 200 to 800 m/z at a rate of 1000 m/z per second. Data were acquired and analyzed using Analyst software (Sciex). Peaks and intensities are reported in Table 1.
Table 1.
Mass spec peaks.
| m/z | height | m/z | height |
|---|---|---|---|
| 205.2 | 117790 | 396.96 | 182690 |
| 219.04 | 115380 | 413.04 | 103370 |
| 228 | 983170 | 416.96 | 137020 |
| 228.64 | 146630 | 439.04 | 117790 |
| 228.96 | 204330 | 455.12 | 112980 |
| 229.44 | 127400 | 473.28 | 156250 |
| 229.6 | 146630 | 510.08 | 394230 |
| 230.48 | 117790 | 532 | 192310 |
| 237.12 | 1334100 | 547.92 | 117790 |
| 238.16 | 353370 | 570 | 331730 |
| 239.12 | 257210 | 650 | 514420 |
| 239.68 | 125000 | 651.04 | 149040 |
| 239.92 | 110580 | 705.92 | 100960 |
| 241.2 | 115380 | 712 | 278850 |
| 255.2 | 562500 | 714.96 | 637020 |
| 256.24 | 189900 | 715.92 | 153850 |
| 265.12 | 262020 | 728 | 360580 |
| 274.96 | 132210 | 728.88 | 158650 |
| 283.28 | 1180300 | 737.04 | 235580 |
| 284.24 | 312500 | 743.92 | 180290 |
| 295.12 | 310100 | 752.88 | 312500 |
| 297.2 | 168270 | 758.96 | 144230 |
| 311.2 | 230770 | 774.96 | 300480 |
| 325.2 | 266830 | 790.96 | 173080 |
| 339.2 | 158650 | 796.88 | 158650 |
1H nuclear magnetic resonance spectroscopy (NMR).
Samples were lyophilized overnight using a FreeZone 2.5 Freeze Dryer (Labconoco, Kansas City, MO) and resuspended in D2O at 5–10 mM. Powdered Na2CO3 was added to increase sample pH. Data were acquired using an Avance III 400 MHz NMR (Bruker; Billerica MA) and TopSpin software. Peaks and integrals are reported in Table 2. Peak assignments were based on a published 1H-NMR spectrum of TNP-ATP (Stephen et al., 2016).
Table 2. 1H-NMR peaks and integrals.
Boxes are used to group multiplets. Peak f (4.7289 ppm) was not integrated as it was on the shoulder of the H2O/HOD peak. We did not integrate the impurity peak around 0 ppm.
| h | 8.8499 | 2092430 | 1 |
| h | 8.7465 | 2014814 | 1.004234 |
| 8.4301 | 3183596 | ||
| 8.2042 | 3107256 | ||
| c | 6.4821 | 2389398 | 1.129836 |
| 5.5229 | 1482018 | ||
| 5.4534 | 727046 | ||
| f | 4.7289 | 1577966 | |
| 4.1985 | 2497092 | ||
| 2.0978 | 1464712 | ||
| 0.0663 | 1039234 |
Molecular biology.
Homo sapiens Kir6.2 was previously subcloned into pcDNA4/TO using standard techniques. Homo sapiens SUR1 was cloned into the mOrange-N1 vector using standard PCR/subcloning techniques, which allows for the expression of protein with a carboxy terminal mOrange tag. Mutagenesis was performed using the QuickChange kit (Agilent; Santa Clara, CA). All clones were verified by direct sequencing by Azenta-Genewiz (South Plainfield, NJ).
Cell culture.
HEK-293T cells were obtained from and verified by ATCC (Manassas, VA). Frozen stocks were prepared from these cells and stored in liquid nitrogen. We performed no additional verification or mycoplasma testing. Cells were grown in T-75 flasks (Grenier Bio-One; Monroe, NC) in Dulbecco’s Modified Eagle Medium (DMEM; Gibco; Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Corning; Tewksbury, MA), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco). Cultures were maintained at 37 °C in a 5% CO2/95% air atmosphere. Cells were plated in 35 mm dishes (Thermo Scientific; Waltham, MA) on glass coverslips (#1 thickness; Chemglass Life Sciences; Vineland, NJ) a day before transfection.
Expression of ANAP-tagged proteins in HEK293T cells.
ANAP-tagged proteins were obtained as previously described (Chatterjee et al., 2013; Puljung et al., 2019; Puljung, 2021). Briefly, cells were co-transfected with 0.5 μg of Kir6.2 plasmid and 1–1.5 μg of SUR1 plasmid with an amber stop codon (TAG) at the position corresponding to the site at which ANAP was to be inserted. Two additional plasmids were transfected: 1) pANAP, which encodes a tRNA with the appropriate anticodon (CUA) to recognize the amber stop codon and a synthetase enzyme capable of charging the tRNA with ANAP, and 2) peRF1-E55D, encoding a dominant negative eukaryotic exchange factor. We used TransIT transfection reagent (Mirus; Madison, WI) at a ratio of 3 μL/μg of DNA. Just prior to transfection, the medium was replaced with DMEM supplemented with FBS, penicillin/streptomycin, and 20 μM ANAP methyl ester (AsisChem Inc.; Waltham, MA). Experiments were performed 3–5 days post-transfection.
Unroofing HEK cells for imaging.
To gain access to the intracellular face of the plasma membrane, cells were unroofed (Usher et al., 2020a; Heuser, 2000). Briefly, a small fragment of glass coverslip with adherent cells was broken off using jeweler’s forceps. The fragment was dipped in a solution of 0.1% poly-L-lysine for 3×10 s. Cells were unroofed by briefly blotting the coverslip fragment (cell-side down) on a clean piece of filter paper (Cytiva Whatman Grade 1; Wilmington, DE), leaving behind adherent plasma membrane fragments.
Microscopy/spectroscopy of unroofed HEK cell fragments expressing fluorescently tagged channels.
Coverslip fragments were imaged in FluoroDishes (World Precision Instruments; Sarasota FL) filled with bath solution (140 mM KCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM ethylene glycol-bis(2-aminoethyleether)-N,N,N’,N’-tetraacetic acid (EGTA), 10 mM HEPES, pH7.4). Unroofed membranes were imaged using a Nikon TE2000-U (Melville, NY) or Ti2E microscope equipped with a 60X water-immersion objective (Nikon Plan Apo VC, 1.20 NA). ANAP was excited using a ThorLabs LED source (LED4D067; Newton, NJ) with a center wavelength of 385 nm. For imaging ANAP, the filter set contained a 390/18 nm band-pass filter (MF390–18, ThorLabs), an MD416 dichroic mirror (ThorLabs) and a 470/40 nm band-pass filter (MF479–40, ThorLabs). To obtain spectra, the emission filter was replaced with a 400 nm long-pass filter (FEL0400, ThorLabs). To image the mOrange tag, the same light source was used with a broad LED centered at 565 nm. A 530–30 band-pass filter was used for excitation (Chroma; Bellows Falls, VT) with a T550lpxr dichroic mirror (Chroma) and 575/50 band pass emission filter (Chroma).
Images and spectra were collected using an IsoPlane81 camera/spectrograph (Teledyne Princeton Instruments; Princeton, NJ). Acquisition was automated using trigger pulses sent from a Sutter IPA amplifier controlled by SutterPatch software (Sutter Instruments; Novato, CA). Exposure times were typically 10 s. Photobleaching artifacts were corrected as previously described (Puljung et al., 2019; Usher et al., 2020a). Briefly, we acquired five images in the absence of nucleotide, plotted the peak intensity of the ANAP spectrum as a function of exposure time, fit the intensity decay with a single exponential, and used the fit curve to correct subsequent exposures.
Measuring nucleotide binding.
The FluoroDish containing the coverslip with cell fragments was constantly perfused with bath solution using a MiniStar peristaltic pump (World Precision Instruments). Solutions of AMP-PCP, TNP-ATP, and TNP-AMP-PCP were prepared from frozen stocks in bath solution. Stocks and dilutions were stored at −20 °C until use and kept in a covered ice bucket during experiments. Nucleotides were applied directly to the cell fragments using a pressure-driven SmartSquirt Micro-Perfusion System (AutoMate Scientific; Berkeley, CA). The Smart Squirt was controlled using a Sutter IPA amplifier and SutterPatch software. For AMP-PCP/TNP-ATP competition assays, a solution of AMP-PCP alone was applied for 30 s prior to co-application of AMP-PCP and TNP-ATP.
Data analysis.
Spectra and chromatograms were analyzed and plotted using custom code written in R version 4.5.0, with plotly, hyperSpec, beepr, ggplot2, gridExtra, and xlsx packages (R Core Team; Beleites and Sergo, 2024; Bååth, 2024; Dragulescu and Arendt, 2020; Wickham, 2016; Auguie, 2017; Sievert, 2020). Images were analyzed and displayed using Fiji (Schindelin et al., 2012). Additional plots, hypothesis testing, and curve fits were carried out using GraphPad Prism (Graphpad Software, Inc.; La Jolla, CA).
For concentration-response relationships, spectra were acquired at each concentration of nucleotide. ANAP fluorescence was averaged between 460 nm and 470 nm at each concentration and plotted as a function of nucleotide concentration. Fluorescence data were normalized to the lowest concentration. The data were fit with curve of the form
| (equation 1), |
where F is fluorescence, Fmax is the fluorescence intensity at the lowest nucleotide concentration (where we observed no appreciable quenching), [TNP] is the concentration of applied nucleotide, EC50 is the concentration that produced half-maximal quenching, and min refers to the residual fluorescence at saturating [TNP]. This equation assumes a single binding site with no cooperativity. We obtained better fits when using an equation that incorporated a slope factor. However, in the absence of Mg2+, we do not expect nucleotide binding to induce a conformational change in SUR1 that would result in cooperative binding (Puljung et al., 2019). Thus, we did not believe that the use of an additional free parameter was justified.
In our previous work, we fit the curve for TNP-ATP binding to Kir6.2/SUR1-T1397ANAP channels in the absence of Mg2+ with the following equation
| (equation 2), |
where Emax is the FRET efficiency at saturating TNP-ATP concentrations, h is the Hill slope and the other variables have the same meaning as for equation 1 (Puljung et al., 2019). Our fits yielded values of Emax = 0.94 ±0.01, EC50 = 4.7 μM ± 0.8 μM, and h =0.83. For competition assays between TNP-ATP and unlabeled AMP-PCP, we assumed that both ligands bound with the same cooperativity. We also assumed that the EC50 value from previous fits reflects a true dissociation constant. Thus, the ratio of ANAP quenching by TNP-ATP in the presence of AMP-PCP to the quenching by TNP-ATP in the absence of AMP-PCP is given by the following equation:
| (equation 3) |
where Kd is the equilibrium dissociation constant for TNP-ATP binding, Ki is the equilibrium dissociation constant for AMP-PCP binding, [AMP-PCP] is the concentration of AMP-PCP, and the other variables have the same meaning as in previous equations. We solved for Ki using the measured quenching ratio, the known concentrations of TNP-ATP and AMP-PCP and the fits from previous TNP-ATP only data.
Data presentation and statistics.
All error bars represent ±SEM. Wherever possible, we display all of the individual data points. n values and fit parameters are included in the figure legends. We did not perform any power analysis prior to conducting our experiments to determine the number of experiments. Means comparisons were conducted using a two-tailed Student’s t-test with the threshold of statistical significance at p < 0.01. Protein structures were displayed using ChimeraX developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco with support from National Institutes of Health RO1-GM129325 and the Office of Cyber Infrastructure and Computational Biology, National Institute of Allergy and Infectious Disease (Pettersen et al., 2021). Chemical structures were created and formula masses predicted using ChemDraw (Revvity Signals Software; Waltham, MA).
Materials and chemicals.
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO), unless otherwise noted. pcDNA4/TO was obtained from Invitrogen (Carlsbad, CA). mOrange-N1 was a gift from Michael Davidson (Addgene plasmid # 54499; http://n2t.net/addgene:54499; RRID:Addgene_54499) (Kremers et al., 2009). pANAP was a gift from Peter Schultz (Addgene plasmid # 48696; http://n2t.net/addgene:48696; RRID:Addgene_48696) (Chatterjee et al., 2013). peRF1-E55D (Homo sapiens) was a kind gift from the Chin Laboratory (MRC Laboratory of Molecular Biology, Cambridge UK) (Schmied et al., 2014).
RESULTS
Synthesis and characterization of TNP-AMP-PCP.
We adapted an existing protocol for synthesis of trinitrophenyl adenosine and adenosine phosphates to synthesize TNP-AMP-PCP (Figure 2A) (Azegami and Iwai, 1975; Hiratsuka and Uchida, 1973). AMP-PCP was reacted with a four-fold molar excess of TNBS under basic conditions (pH adjusted with LiOH) for four days. We precipitated the resulting orange product with ethanol/sodium acetate and stored it at −20 °C. After centrifugation and washing with ethanol, we dissolved the product in water for further analysis.
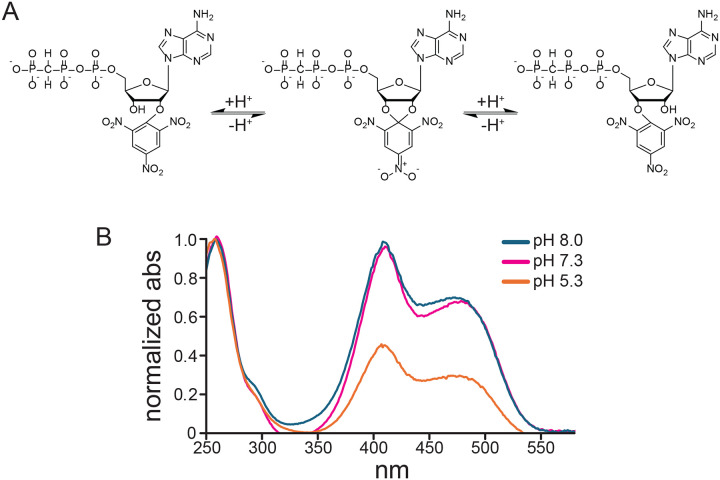
The UV/Vis spectrum of our product was consistent with our expectations based on published spectra of TNP-ATP (Figure 2B) (Hiratsuka and Uchida, 1973). When attached to the ribose of AMP-PCP, the trinitrophenyl group formed a Meisenheimer complex, which was apparent from the appearance of two peaks at 408 nm and 420 nm in addition to the peak at 259 nm, corresponding to the adenine ring (Azegami and Iwai, 1975; Hiratsuka and Uchida, 1973). Reversible protonation of the Meisenheimer complex results in a loss of these peaks as well as a loss of the orange color (Figure 3A,B) (Azegami and Iwai, 1975; Hiratsuka and Uchida, 1973). The pKa for this effect in TNP adenosine is around 4.5, so we expect this complex and the color to both be present at physiological pH. Consistent with this, the measured UV/Vis spectra in Tris buffer at pH 8.0 and HEPES buffer at pH 7.3 were nearly identical (Figure 3B). Based on published results with TNP-ATP, the ratio of A259 nm:A408 nm should be 1:1.06. The A259 nm:A408 nm for our product at pH 8.0 was 1:0.99. Thus, we conclude that our product had little contamination from unreacted AMP-PCP (which would only absorb at 259 nm). We also measured the fluorescence emission spectrum of our product (Figure 2B). Excitation at 400 nm produced a single emission peak at 551 nm, similar to the value of 561 nm measured for TNP-ATP in water (Hiratsuka, 2003).
Figure 3. Protonation of TNP-AMP-PCP.
A. Schematic depicting reversible protonation of the fluorescent Meisenheimer complex of TNP-AMP-PCP. B. UV/Vis spectrum of 42.15 μM TNP-AMP-PCP in Tris buffer (pH 8.0), HEPES buffer (pH 7.3), and MES buffer (pH 5.3). Data were normalized to the peak at 259 nm.
We assessed the purity of our product using reversed-phase HPLC (Figure 2C,D). Samples were run on a C18 column on a linear gradient from 0–60% acetonitrile in aqueous 0.1 M triethylammonium bicarbonate buffer (pH 7.5). Using a diode-array detector, we obtained a complete UV/Vis spectrum at every time point. Our chromatograms showed one major peak with an absorbance spectrum identical to that expected from TNP-AMP-PCP. We note a drift in the baseline of the chromatogram at 260 nm, which we observed in blank runs as well and attribute to the absorbance of acetonitrile.
Mass spectrometry of our product with electrospray ionization in negative-ion mode showed prominent peaks at m/z ratios of 714.96 Da and 237.12 Da, consistent with the expected values for [TNP-AMP-PCP]− (715 Da) and [TNP-AMP-PCP]3− (237.66 Da) (Figure 4A–C, Table 1). The ratio of intensities of isotope peaks at 716 Da and 717 Da to the intensity of the 715 Da peak was consistent with predictions based on the natural abundances of the constituent elements in TNP-AMP-PCP (Figure 4C). We observed several peaks at m/z ratios above 715 Da. We take these to be various salt adducts of TNP-AMP-PCP and have assigned them where possible. Two of these peaks correspond to the expected mass of TNP-AMP-PCP plus 13 Da and 29 Da, respectively. We could not unequivocally assign these peaks based on their masses, but believe them to be Li+ adducts as we observed them in our samples of TNP-AMP-PCP (where LiOH was used in the preparation), but did not observe mass + 13 Da or mass + 29 Da peaks in samples of commercially prepared TNP-ATP, which did not contain Li+.
Figure 4. Mass spectrometry of TNP-AMP-PCP.
A. Mass spectrum for TNP-AMP-PCP with electrospray ionization in negative-ion mode, showing a peak at the expected charge-to-mass (m/z) ratio (715 Da) for [TNP-AMP-PCP]− as well as several peaks corresponding to likely salt adducts (labeled). B. Zoomed in spectrum showing the three peaks corresponding to isotopic variants of [TNP-AMP-PCP] − and predictions of the expected isotope ratios (dashed lines). C. Mass spectrum showing the complete range examined. Peaks that are also present in the blank samples are marked with “*”. D. Mass spectrum of our blank solution (0.1% triethylamine in 50:50 acetonitrile:water).
We observed additional peaks between 200–300 Da. Several of these reflected impurities in our solvent, as they showed up in blank measurements (Figure 4C,D; marked by “*”). The substantial peak at 228 Da is likely picrate. Picrate could either be formed by hydrolysis of TNBS, one of our starting materials, or could result from fragmentation of TNP-AMP-PCP, either in solution or in our mass spec. We did not observe a peak for picrate in our HPLC chromatograms, implying it may be a product of fragmentation in the mass spectrometer. Whereas picrate may represent an impurity in our samples, based on its structure and spectral properties, we do not expect it to interfere with subsequent measurements on KATP.
Finally, we used 1H-NMR to verify our product (Figure 5A). The spectrum of our sample, obtained in D2O showed peaks consistent with the published structure of TNP-ATP (Stephen et al., 2016). Of note, the peaks at 8.75 and 8.85 ppm correspond to the two protons on the trinitrophenyl group. The triplet centered at 2.15 ppm represents the methylene group between the β and γ phosphates. We noticed some fine splitting of the trinitrophenyl peaks in our original spectra, which we hypothesized could arise from the protonation of the Meisenheimer complex, which would be expected to produce additional structures (Figure 3A). Addition of Na2CO3 to our NMR samples to increase the pH resolved this issue, consistent with this hypothesis (Figure 5B). There was some deviation from expected values in our peak integrals (Table 2). This was likely the result of the large H2O/H-OD peak present in our spectra (Figure 5A).
Figure 5. 1H-NMR spectrum of TNP-AMP-PCP.
A. 1H-NMR spectrum of TNP-AMP-PCP in D2O. Peak assignments are based on the published spectrum of TNP-ATP (Stephen et al., 2016). B. pH dependence of the peaks at 8.7 ppm and 8.8 ppm. The sample was maintained at a basic pH through the addition of solid Na2CO3.
Measuring nucleotide binding to NBS1 of SUR1.
In our previous work, we measured nucleotide binding to Kir6.2 and NBS2 of SUR1 (Usher et al., 2020b; Puljung et al., 2019). However, we made no measurements of nucleotide binding to NBS1. Therefore, we first sought to measure TNP-ATP binding to NBS1. We chose to measure binding in the absence of Mg2+ (i.e. in the presence of 1 mM EDTA and no added Mg2+). Based on our results at NBS2, we do not expect that Mg2+ will be required for binding at either NBS of SUR1. Indeed, it has long been suggested that ATP binding to NBS1 does not require Mg2+ (Matsuo et al., 1999). Furthermore, we do not expect TNP-ATP binding to affect the conformation of SUR, as Mg2+ is required for SUR1 to change conformation subsequent to nucleotide binding (Puljung et al., 2019).
We introduced an amber (TAG) stop codon into the SUR1 gene at a position corresponding to W688 in the amino acid sequence (SUR1-W688stop, Figure 1C). In the cryo-EM structure of KATP, determined in the presence of MgATP, the tryptophan residue normally occupying this position forms a π-stacking interaction with the adenine ring of ATP (Lee et al., 2017). Therefore, we reasoned that ANAP incorporated at this position would be ideally positioned for FRET with bound TNP-ATP. In our previous work, a similar substitution in NBS2 produced robust FRET with TNP-ATP and TNP-ADP (Puljung et al., 2019).
Expressing SUR1-W688stop alone would result in the translation of SUR1 protein truncated after position 687. To express full-length, ANAP-labeled SUR1 proteins, we co-expressed SUR1-W688stop with Kir6.2 and two additional plasmids. The first, pANAP, encodes several copies of a tRNA with the appropriate anticodon (CUA) to recognize the amber stop codon along with a tRNA synthetase capable of charging this tRNA with ANAP (Chatterjee et al., 2013). The second plasmid encodes a dominant negative eukaryotic exchange factor, which helps to increase the yield of full-length, ANAP-tagged channels (Schmied et al., 2014). ANAP was introduced to the cell culture medium before transfection. In our past work, we obtained 92% full-length, ANAP labeled channels under similar conditions (Puljung et al., 2019). To ensure ANAP incorporation, we tagged the carboxy terminus of SUR1 with mOrange. The presence of orange fluorescence along with ANAP fluorescence indicates the production of full-length SUR1-W688ANAP.
To measure the binding of TNP-ATP to our labeled SUR1, we first needed to expose the cytoplasmic face of the channel. We did this by preparing unroofed membrane fragments by blotting cells grown on coverslips with filter paper (Heuser, 2000; Usher et al., 2020a). This process removes the cells, leaving behind adherent fragments of plasma membrane with the intracellular face exposed to bulk solution. We identified unroofed membrane fragments expressing Kir6.2/SUR1-W688ANAP by the presence of fluorescence for both mOrange and ANAP tags (Figure 6). We assumed the presence of Kir6.2, as Kir6.2 and SUR1 must first co-assemble before exiting the endoplasmic reticulum (Zerangue et al., 1999). However, tagging the individual subunits with GFP does allow some degree of independent trafficking of the subunits to the plasma membrane, so it remains possible that some of our signals arise from SUR1 expressed by itself (Makhina and Nichols, 1998).
Figure 6. Unroofed membrane fragments expressing fluorescently tagged KATP.
The top panels show mOrange and ANAP fluorescence from an unroofed HEK293T cell expressing Kir6.2/SUR1-W688ANAP channels with a carboxy terminal mOrange tag. In the bottom panel, the emitted ANAP fluorescence is reflected off a grating to produce spectra. Note that the x-axis now represents wavelength, but the y-axis still represents spatial information (same scale bar as in the top panels).
We used FRET between ANAP and TNP-ATP to measure binding to Kir6.2/SUR1-W688ANAP in unroofed membranes. Fluorescence emission spectra were obtained by passing the emitted light from our samples through the slit of a spectrograph mounted on our microscope (Figure 7A,B). This allowed for easy separation of the emission peaks corresponding to ANAP (peak emission around 485 nm) and TNP-ATP (peak emission around 551 nm). Theoretically, we could measure FRET from the quenching of the donor (ANAP) peak or the sensitization of the acceptor (TNP-ATP peak). However, we chose to quantify binding from the reduction in ANAP fluorescence for two reasons. Firstly, the quenching of the donor peak is directly proportional to the FRET efficiency. Secondly, whereas the ANAP peak is specific to labeled KATP channels, some fraction of the TNP-ATP fluorescence can be attributed to non-specific TNP-ATP binding to the plasma membrane or incomplete background subtraction (Puljung et al., 2019; Usher et al., 2020a).
Figure 7. Measuring TNP-ATP binding to NBS1 of SUR1.

A. Spectra acquired from an unroofed membrane expressing Kir6.2/SUR1-W688 ANAP acquired at increasing concentrations of TNP-ATP. The peak around 485 nm corresponds to ANAP and the peak around 551 nm is from TNP-ATP. B. Zoomed in spectra from panel A. The gray bar denotes the wavelength range used to quantify ANAP fluorescence (460–470 nm). C. Concentration-response relationship for ANAP quenching as a function of TNP-ATP concentration. ANAP quenching was quantified by a reduction in fluorescence. Fluorescence intensities were normalized to the ANAP fluorescence at 10 nM TNP-ATP. Data were fit to equation 1. logEC50 = −5.3; min = 0.34. Dashed lines indicate 95% confidence intervals for the fit. Individual data points are shown in gray. n = 5 for each concentration.
We measured the emission spectra of Kir6.2/SUR1-W688ANAP channels at concentrations of TNP-ATP ranging from 10 nM to 1 mM (Figure 7A–C). On our current optical setup, there was some overlap evident between the ANAP peak and the TNP-ATP peaks (Figure 7A). Therefore, we quantified quenching of ANAP by measuring the average fluorescence emission over a range from 460 nm to 470 nm (Figure 7B, gray bar). Figure 7C shows the average Kir6.2/SUR1-W688ANAP fluorescence normalized to the fluorescence at 10 nM TNP-ATP, where no quenching was observed. The data were fit with a single-site binding curve (equation 1) with an EC50 value of 4.9 μM and a minimum normalized fluorescence of 0.34 at saturating concentrations, indicating 66% quenching. We expected a greater degree of quenching between ANAP at position 688 and TNP-ATP bound directly to NBS1, given the proximity and the calculated R0 (distance where FRET efficiency is half-maximal) for ANAP/TNP-ATP (about 43 Å) (Puljung et al., 2019). However, the value we measured is much greater than the value predicted if TNP-ATP were bound to NBS2 (about 20% FRET) based on the inhibited and apo structures of KATP (Martin et al., 2017, 2019). The smaller than expected value may reflect TNP-ATP binding in an unfavorable orientation relative to ANAP for FRET to occur or simply insufficient background subtraction in our experiments, which could make the baseline fluorescence between 460 nm and 470 nm appear higher.
Binding of TNP-AMP-PCP to NBS1 and NBS2.
We next tested the ability of our newly synthesized ATP derivative to bind to both NBSs of SUR1 (Figure 8). We again labeled NBS1 of SUR1 at position W688. To measure binding to NBS2, we replaced a threonine residue at position 1397 with ANAP (SUR1-T1397ANAP, Figure 1C). In previous experiments, we measured TNP-ATP and TNP-ADP binding at this site in the presence and absence of Mg2+ (Puljung et al., 2019). Furthermore, Kir6.2-G334D/SUR1-T1397ANAP channels (in which the inhibitory site on Kir6.2 was mutated) were functional and showed robust activation by MgADP (Puljung et al., 2019).
Figure 8. Binding of TNP-AMP-PCP to NBS1 and NBS2 of SUR1.
Curves showing concentration-dependent quenching of Kir6.2/SUR1-W688ANAP (NBS1 labeled) and Kir6.2/SUR1-T1397ANAP (NBS2 labeled) by TNP-AMP-PCP. Data were fit with equation 1. Dashed lines indicate 95% confidence intervals for the fit. Individual data points are shown in gray. Kir6.2/SUR1-W688ANAP: logEC50 = −4.3; min = 0.26. A total of 7 membranes were used in these experiments. The n values at each concentration are as follows: 83 nM, n = 7; 830 nM, n = 4; 8.3 μM, n = 4; 83 μM, n = 4, 830 μM, n = 2; 2 mM, n = 3. Kir6.2/SUR1-T1397ANAP: logEC50 = −4.9; min = 0.29. A total of 6 membranes were used in these experiments. n = 6 for each concentration.
We measured the binding of TNP-AMP-PCP to both NBS1 and NBS2 of SUR1 (Figure 8A,B). Concentration-response relationships for each were fit to single-site binding curves. For NBS1 (Kir6.2/SUR1-W688ANAP), half-maximal quenching occurred at a concentration of 52.8 μM TNP-AMP-PCP. ANAP fluorescence was quenched by 73% at saturating TNP-AMP-PCP, again consistent with direct binding to NBS1. For NBS2 (Kir6.2/SUR1-T1397ANAP), we observed half-maximal quenching at 13.5 μM TNP-AMP-PCP. ANAP fluorescence was quenched by 70% at saturating concentrations. By comparison, TNP-ATP quenching of Kir6.2/SUR1-T1397ANAP had an EC50 value of 4.7 μM, with 94% quenching at saturating concentrations (Puljung et al., 2019). Based on these data, we conclude that TNP-AMP-PCP was able to bind both NBSs of SUR1.
However, the ability of TNP-AMP-PCP to bind SUR1 does not guarantee that non-fluorescent AMP-PCP is also able to bind. For example, it is possible that the trinitrophenyl moiety was able to stabilize binding of the TNP-AMP-PCP to SUR1, and that AMP-PCP would not appreciably bind without it. As a complementary means of assessing AMP-PCP binding to SUR1, we tested its ability to compete with TNP-ATP for binding at NBS2 of SUR1 (Figure 9A–C). In the absence of AMP-PCP, 5 μM TNP-ATP quenched the fluorescence of Kir6.2/SUR1-T1397ANAP by 44% ± 9%, close to the expected value of 48% based on our fits to published experimental data (Puljung et al., 2019). However, when the unroofed membrane fragments were perfused with 100 μM AMP-PCP before co-application of 100 μM AMP-PCP and 5 μM TNP-ATP, the quenching was reduced to 22% ± 1%. The difference in quenching was statistically significant (p < 0.01) and implies direct binding of AMP-PCP to NBS2 of SUR1. We performed a back-of-the-envelope calculation using equation 3 (see Materials and Methods for equation and assumptions) and estimated the affinity of NBS2 for AMP-PCP to be around 42 μM.
Figure 9. AMP-PCP binds to NBS2 of SUR1.

A. Fluorescence of Kir6.2/SUR1-T1397ANAP in the presence and absence of 5 μM TNP-ATP. B. Fluorescence of Kir6.2/SUR1-T1397ANAP in the presence and absence of 5 μM TNP-ATP plus 100 μM AMP-PCP. C. Bar chart summarizing the reduction in Kir6.2/SUR1-T1397ANAP fluorescence in 5 μM TNP-ATP in the absence and presence of 100 μM AMP-PCP. n = 6 for 5 μM TNP-ATP; n = 3 for 5 μM TNP-ATP plus 100 μM TNP-ATP. Individual data points are superimposed on the graph. Data sets were compared using a two-tailed Student’s t-test. * indicates p < 0.01.
DISCUSSION
We have successfully synthesized and purified TNP-AMP-PCP, a fluorescent, non-hydrolyzable ATP analog. Using our established, FRET-based approach, we measured binding of this analog to both NBSs of SUR1. We also measured the binding of TNP-ATP to NBS1, which had not been examined previously. Finally, we demonstrated that AMP-PCP can bind to NBS2 of SUR1 using a competition assay.
ATP binding to NBS1 was previously observed in cryo-EM structures and in equilibrium binding experiments performed in the presence and absence of Mg2+ (Matsuo et al., 1999; Lee et al., 2017). Whereas the presence of TNP-ATP/TNP-ADP at NBS1 could be inferred from our previous studies on NBS2, we did not previously make any direct measurements of nucleotide binding to NBS1. In this study, we used our assay to measure direct TNP-ATP binding to NBS1 in the absence of Mg2+ with apparent affinity of 5.0 μM (Figure 7). This is similar to the apparent affinity we previously reported for TNP-ATP binding to NBS2 (Kir6.2/T1397ANAP, 4.7 μM) (Puljung et al., 2019). It has been hypothesized that, for asymmetric ABC transporters like CFTR and TM287/288, the degenerate NBS (NBS1 here) binds nucleotides with a very high affinity and that bound nucleotides may act as a structural support, anchoring the NBDs together (Stockner et al., 2020; Hohl et al., 2012). Based on these results, it appears that this may not be true for KATP channels. This is consistent with existing cryo-EM structures of KATP that show the NBDs either tightly bundled in the presence of Mg2+ and nucleotides or completely separated (Driggers and Shyng, 2023). However, it should be noted that our experiments were performed using a nucleotide derivative and the affinity of this site for unlabeled ATP was not directly tested. Indeed, equilibrium binding studies suggest that ATP can reside at NBS1 of SUR1 for long periods of time (Matsuo et al., 1999).
We report here the first synthesis of TNP-AMP-PCP, a fluorescent non-hydrolyzable ATP analog. Based on our UV/Vis and HPLC analysis, we obtained a relatively pure sample of TNP-AMP-PCP (Figure 2). Mass spectrometry showed the presence of some impurities that were not obvious from the HPLC traces (Figure 4). We ascribe the peak at 228 Da to picrate, which could be a product of the hydrolysis of TNP-AMP-PCP to picrate and AMP-PCP. However, based on the ratio of our peaks in the UV/Vis spectra, we do not believe that there was any significant loss of the trinitrophenyl group from our TNP-AMP-PCP samples prior to mass spectrometry. Another possible source of picrate is the hydrolysis of TNBS, which is present in a four-fold excess in our synthesis, but we observed no picrate peak in our HPLC traces. Regardless, if picrate were a substantial contaminant in our purified TNP-AMP-PCP samples, we would not expect it to interfere with our measurements, as 1) it is unlikely to bind to SUR1 and 2) if it were to bind, it would not FRET with ANAP. Likewise, whereas a substantial AMP-PCP contamination is unlikely, such an impurity would compete with TNP-AMP-PCP for binding to SUR1. Whereas this would affect our estimated EC50 values, it would not undermine our ultimate conclusion that TNP-AMP-PCP can bind to both NBSs.
Importantly, use of TNP-AMP-PCP allowed us to measure binding in real time to KATP channels in the plasma membrane. We conclude that non-hydrolyzable ATP analogs can bind both NBS1 and NBS2 of SUR1, albeit at relatively low apparent affinity. Our experiments produced estimates for TNP-AMP-PCP apparent affinity of around 50 μM for NBS1 and 13 μM for NBS2. Furthermore, we calculated the affinity of AMP-PCP binding to NBS2 to be 42 μM, based on competition with TNP-ATP binding. Thus, their inability to activate KATP is not due to a failure to bind.
Ortiz et al. (Ortiz et al., 2013) previously examined the ability of non-hydrolyzable nucleotides to bind SUR1. In their experiments, binding was assessed by the ability of nucleotides to displace tritiated glibenclamide from isolated SUR1 subunits expressed in Pichia membranes in equilibrium binding assays. ATP-γ-S was able to displace glibenclamide binding, implying that it not only bound to SUR1, but induced it to adopt an “active” conformation. In contrast to this, AMP-PNP and AMP-PCP did not displace glibenclamide, but did reduce the ability of ATP to displace glibenclamide, implying that they compete with ATP for binding. Indeed, the authors further demonstrated that AMP-PCP could displace 8-azido-[α−32P]ATP from NBS1. Bernardi et al. also showed that AMP-PNP and ATP-γ-S can both complete for α-[32P]Ox-ATP binding to SUR subunits purified from pig brain (Bernardi et al., 1988).
The most direct readout of nucleotide effects on KATP activation comes from inside-out patch-clamp experiments in which the experimenter can easily change the contents of the “intracellular” bathing solution. For the majority of such experiments, authors report only inhibition by AMP-PCP, AMP-PNP, and ATP-γ-S in the presence of Mg2+, which is mediated by binding to Kir6.2 (Ashcroft and Kakei, 1989; Treherne and Ashford, 1992; Jiang and Haddad, 1997; Schwanstecher and Panten, 1994; Schwanstecher et al., 1994, 1992). However, it can be difficult to isolate stimulatory nucleotide effects in wild-type channels with intact inhibition at Kir6.2. For native Kir6.2/SUR1 channels in β-cells and heterologously expressed in Xenopus oocytes, the stimulatory effects of SUR1 in the presence of ATP manifest as a shift of the inhibition curve to higher concentrations of ATP in the presence of Mg2+ (Ashcroft and Kakei, 1989; Proks et al., 2010). Hehl and Numcke reported a similar rightward shift in the concentration response curve for AMP-PNP in the presence of Mg2+ for KATP channels in inside-out patches from mouse skeletal muscle, consistent with mixed activation and inhibition by AMP-PNP (Hehl and Neumcke, 1994). However, no such Mg2+ effect was observed in inside-out patches from pancreatic β-cells (Schwanstecher et al., 1992). This may reflect differences in KATP subtypes between the two tissues (Kir6.2/SUR1 in β-cells and Kir6.2/SUR2A in muscle) (Ashcroft, 2023). In each case, concentrations ranged from tens of μM to low mM, the range over which we observed TNP-AMP-PCP binding to both NBSs of SUR1 (Figure 8).
The most direct way to measure nucleotide activation is to measure the response of channels bearing a point mutation that disrupts the inhibitory NBS on Kir6.2 (Kir6.2-G334D) in inside-out patches (Drain et al., 1998; Li et al., 2002; Proks et al., 2010, 2014). Proks et al. measured robust activation by MgATP-γ-S of Kir6.2-G334D/SUR1 in outside-out patches from Xenopus oocytes channels, but observed no such activation by MgAMP-PNP (Proks et al., 2010). To our knowledge, no such experiments have been performed with AMP-PCP.
Our experiments build on previous results in significant ways. First of all, our approach allows us to measure nucleotide binding in real time in intact, functional KATP channel complexes in the plasma membrane of mammalian cells. As such, our method is fully compatible with patch-clamp, allowing binding and channel activity to be measured simultaneously (Usher et al., 2020b). Finally, the short distance dependence of FRET allows us to measure binding in a site-specific fashion so that the effects of the NBSs can be examined independently.
Our conclusion, that non-hydrolyzable ATP analogs bind both NBS1 and NBS2 of SUR1 represents only the first step in understanding the requirement, or lack thereof, for SUR1 to hydrolyze ATP to ADP in order to activate KATP. At least two possibilities remain to explain the inability of non-hydrolyzable ATP analogs to support channel activation. The first possibility is that, while able to bind, non-hydrolyzable ATPs do not stabilize a conformation in SUR1 that promotes activation (NBD dimerization). In our previous work, we noted that nucleotide dissociation from NBS2 was greatly slowed by the presence of Mg2+ (Puljung et al., 2019). We interpreted this result to mean that NBD dimerization in the presence of Mg2+ impeded nucleotide dissociation. In future work, we will use this metric as an indicator of the ability of TNP-AMP-PCP to promote an activating conformational change in SUR1. A key advantage of our technique is that we can also perform such measurements using patch-clamp fluorometry, which will allow us to monitor changes in channel activity while we monitor the conformational change in SUR1. We aim to combine this approach with more traditional structure-function approaches to delineate the role of ATP hydrolysis in the gating cycle of KATP. We also hope to extend this approach to study the enzymatic activity of other ABC transporters, P-type ATPases, and kinases.
ACKNOWLEDGMENTS
We would like to thank Dr. Ysobel Baker, Dr. Cheyenne Brindle and Dr. Timothy Curran for helpful discussions and Jazmin Johnson and Christina Alcaro for additional technical support. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R15GM155848.
DATA AVAILABITY STATEMENT
All data for this work are available on the Dryad repository (XX URL) and all custom code is available on GitHub (XX URL).
REFERENCES
- Aguilar-Bryan L., Nichols C., Wechsler S., Clement J., Boyd A., Gonzalez G., Herrera-Sosa H., Nguy K., Bryan J., and Nelson D.. 1995. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Aleksandrov L., Aleksandrov A.A., Chang X.-B., and Riordan J.R.. 2002. The First Nucleotide Binding Domain of Cystic Fibrosis Transmembrane Conductance Regulator Is a Site of Stable Nucleotide Interaction, whereas the Second Is a Site of Rapid Turnover. J Biol Chem. 277:15419–15425. doi: 10.1074/jbc.M111713200. [DOI] [PubMed] [Google Scholar]
- Ashcroft F.M. 2023. KATP Channels and the Metabolic Regulation of Insulin Secretion in Health and Disease: The 2022 Banting Medal for Scientific Achievement Award Lecture. Diabetes. 72:693–702. doi: 10.2337/dbi22-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F.M., and Kakei M.. 1989. ATP-sensitive K+ channels in rat pancreatic beta-cells: modulation by ATP and Mg2+ ions. J Physiol. 416:349–367. doi: 10.1113/jphysiol.1989.sp017765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F.M., Puljung M.C., and Vedovato N.. 2017. Neonatal Diabetes and the K ATP Channel: From Mutation to Therapy. Trends in Endocrinology & Metabolism. 28:377–387. doi: 10.1016/j.tem.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auguie B. 2017. _gridExtra: Miscellaneous Functions for “Grid” Graphics_. R package version 2.3.
- Azegami M., and Iwai K.. 1975. Trinitrophenylation of Nucleic Acids and Their Constituents. The Journal of Biochemistry. 78:409–420. doi: 10.1093/oxfordjournals.jbchem.a130921. [DOI] [PubMed] [Google Scholar]
- Bååth R. 2024. _beepr: Easily Play Notification Sounds on any Platform_. R package version 2.0,.
- Beleites C., and Sergo V.. 2024. hyperSpec: a package to handle hyperspectral data sets in R’, R package version 0.100.2.
- Bernardi H., Fosset M., and Lazdunski M.. 1988. Characterization, purification, and affinity labeling of the brain [3H]glibenclamide-binding protein, a putative neuronal ATP-regulated K+ channel. Proceedings of the National Academy of Sciences. 85:9816–9820. doi: 10.1073/pnas.85.24.9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A., Guo J., Lee H.S., and Schultz P.G.. 2013. A Genetically Encoded Fluorescent Probe in Mammalian Cells. J. Am. Chem. Soc. 135:12540–12543. doi: 10.1021/ja4059553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.-H., Tantama M., and Licht S.. 2008. Testing for Violations of Microscopic Reversibility in ATP-Sensitive Potassium Channel Gating. J. Phys. Chem. B. 112:10314–10321. doi: 10.1021/jp712088v. [DOI] [PubMed] [Google Scholar]
- Csanady L., Vergani P., and Gadsby D.C.. 2010. Strict coupling between CFTR’s catalytic cycle and gating of its Cl- ion pore revealed by distributions of open channel burst durations. Proceedings of the National Academy of Sciences. 107:1241–1246. doi: 10.1073/pnas.0911061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragulescu A., and Arendt C.. 2020. _xlsx: Read, Write, Format Excel 2007 and Excel 97/2000/XP/2003 Files_. R package version 0.6.5.
- Drain P., Li L., and Wang J.. 1998. KATP channel inhibition by ATP requires distinct functional domains of the cytoplasmic C terminus of the pore-forming subunit. Proc Natl Acad Sci U S A. 95:13953–13958. doi: 10.1073/pnas.95.23.13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driggers C.M., and Shyng S.-L.. 2023. Mechanistic insights on KATP channel regulation from cryo-EM structures. J Gen Physiol. 155:e202113046. doi: 10.1085/jgp.202113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne M.J. 1989. Protein phosphorylation is required for diazoxide to open ATP-sensitive potassium channels in insulin (RINm5F) secreting cells. FEBS Letters. 250:262–266. doi: 10.1016/0014-5793(89)80734-3. [DOI] [PubMed] [Google Scholar]
- Findlay I. 1987. ATP-sensitive K+ channels in rat ventricular myocytes are blocked and inactivated by internal divalent cations. Pflugers Arch. 410:313–320. doi: 10.1007/BF00580282. [DOI] [PubMed] [Google Scholar]
- Gribble F.M., Tucker S.J., Haug T., and Ashcroft F.M.. 1998. MgATP activates the cell KATP channel by interaction with its SUR1 subunit. Proceedings of the National Academy of Sciences. 95:7185–7190. doi: 10.1073/pnas.95.12.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehl S., and Neumcke B.. 1994. KATP channels of mouse skeletal muscle: mechanism of channel blockage by AMP-PNP. Eur Biophys J. 23:231–237. doi: 10.1007/BF00213573. [DOI] [PubMed] [Google Scholar]
- Heuser J. 2000. The Production of ‘Cell Cortices’ for Light and Electron Microscopy. Traffic. 1:545–552. doi: 10.1034/j.1600-0854.2000.010704.x. [DOI] [PubMed] [Google Scholar]
- Hiratsuka T. 2003. Fluorescent and colored trinitrophenylated analogs of ATP and GTP. European Journal of Biochemistry. 270:3479–3485. doi: 10.1046/j.1432-1033.2003.03748.x. [DOI] [PubMed] [Google Scholar]
- Hiratsuka T., and Uchida K.. 1973. PREPARATION AND PROPERTIES OF 2’(or Y)-O-(2,4,6-TRINITROPHENYL) ADENOSINE 5’-TRIPHOSPHATE, AN ANALOG OF ADENOSINE TRIPHOSPHATE. Biochimica et Biophysical Acta (BBA) - General Subjects. 320:635–647. [DOI] [PubMed] [Google Scholar]
- Hohl M., Briand C., Grütter M.G., and Seeger M.A.. 2012. Crystal structure of a heterodimeric ABC transporter in its inward-facing conformation. Nat Struct Mol Biol. 19:395–402. doi: 10.1038/nsmb.2267. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi T., Clement J.P., Namba N., Inazawa J., Gonzalez G., Aguilar-Bryan L., Seino S., and Bryan J.. 1995. Reconstitution of IKATP : An Inward Rectifier Subunit Plus the Sulfonylurea Receptor. Science. 270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi T., and Seino S.. 1997. Subunit stoichiometry of the pancreatic β-cell ATP-sensitive K + channel. FEBS Letters. 409:232–236. doi: 10.1016/S0014-5793(97)00488-2. [DOI] [PubMed] [Google Scholar]
- Jiang C., and Haddad G.G.. 1997. Modulation of K+ channels by intracellular ATP in human neocortical neurons. J Neurophysiol. 77:93–102. doi: 10.1152/jn.1997.77.1.93. [DOI] [PubMed] [Google Scholar]
- Kozlowski R.Z., and Ashford M.L.. 1992. Nucleotide-dependent activation of KATP channels by diazoxide in CRI-G1 insulin-secreting cells. Br J Pharmacol. 107:34–43. doi: 10.1111/j.1476-5381.1992.tb14460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremers G.-J., Hazelwood K.L., Murphy C.S., Davidson M.W., and Piston D.W.. 2009. Photoconversion in orange and red fluorescent proteins. Nat Methods. 6:355–358. doi: 10.1038/nmeth.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.P.K., Chen J., and MacKinnon R.. 2017. Molecular structure of human KATP in complex with ATP and ADP. eLife. 6:e32481. doi: 10.7554/eLife.32481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Geng X., and Drain P.. 2002. Open State Destabilization by Atp Occupancy Is Mechanism Speeding Burst Exit Underlying KATP Channel Inhibition by Atp. The Journal of General Physiology. 119:105–116. doi: 10.1085/jgp.119.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhina E.N., and Nichols C.G.. 1998. Independent trafficking of KATP channel subunits to the plasma membrane. J Biol Chem. 273:3369–3374. doi: 10.1074/jbc.273.6.3369. [DOI] [PubMed] [Google Scholar]
- Martin G.M., Kandasamy B., DiMaio F., Yoshioka C., and Shyng S.-L.. 2017. Anti-diabetic drug binding site in a mammalian KATP channel revealed by Cryo-EM. eLife. 6:e31054. doi: 10.7554/eLife.31054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.M., Sung M.W., Yang Z., Innes L.M., Kandasamy B., David L.L., Yoshioka C., and Shyng S.-L.. 2019. Mechanism of pharmacochaperoning in a mammalian KATP channel revealed by cryo-EM. eLife. 8:e46417. doi: 10.7554/eLife.46417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo M., Kioka N., Amachi T., and Ueda K.. 1999. ATP Binding Properties of the Nucleotide-binding Folds of SUR1. Journal of Biological Chemistry. 274:37479–37482. doi: 10.1074/jbc.274.52.37479. [DOI] [PubMed] [Google Scholar]
- Ortiz D., Gossack L., Quast U., and Bryan J.. 2013. Reinterpreting the Action of ATP Analogs on KATP Channels. Journal of Biological Chemistry. 288:18894–18902. doi: 10.1074/jbc.M113.476887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz D., Voyvodic P., Gossack L., Quast U., and Bryan J.. 2012. Two Neonatal Diabetes Mutations on Transmembrane Helix 15 of SUR1 Increase Affinity for ATP and ADP at Nucleotide Binding Domain 2. Journal of Biological Chemistry. 287:17985–17995. doi: 10.1074/jbc.M112.349019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Meng E.C., Couch G.S., Croll T.I., Morris J.H., and Ferrin T.E.. 2021. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 30:70–82. doi: 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks P., de Wet H., and Ashcroft F.M.. 2010. Activation of the KATP channel by Mg-nucleotide interaction with SUR1. Journal of General Physiology. 136:389–405. doi: 10.1085/jgp.201010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks P., de Wet H., and Ashcroft F.M.. 2014. Sulfonylureas suppress the stimulatory action of Mg-nucleotides on Kir6.2/SUR1 but not Kir6.2/SUR2A KATP channels: A mechanistic study. Journal of General Physiology. 144:469–486. doi: 10.1085/jgp.201411222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puljung M. 2023. KATP channels and the regulation of insulin secretion. In TEXTBOOK OF ION CHANNELS: three volume set. Zheng J. and Trudeau M.C., editors. ROUTLEDGE, S.l. [Google Scholar]
- Puljung M., Vedovato N., Usher S., and Ashcroft F.. 2019. Activation mechanism of ATP-sensitive K+ channels explored with real-time nucleotide binding. eLife. 8:e41103. doi: 10.7554/eLife.41103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puljung M.C. 2021. ANAP: A versatile, fluorescent probe of ion channel gating and regulation. In Methods in Enzymology. Elsevier. 49–84. [DOI] [PubMed] [Google Scholar]
- R Core Team. _R: A Language and Environment for Statistical Computing_.
- Sakura H., Ammälä C., Smith P.A., Gribble F.M., and Ashcroft F.M.. 1995. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D.J., Hartenstein V., Eliceiri K., Tomancak P., and Cardona A.. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods. 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmied W.H., Elsässer S.J., Uttamapinant C., and Chin J.W.. 2014. Efficient Multisite Unnatural Amino Acid Incorporation in Mammalian Cells via Optimized Pyrrolysyl tRNA Synthetase/tRNA Expression and Engineered eRF1. J. Am. Chem. Soc. 136:15577–15583. doi: 10.1021/ja5069728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanstecher C., Dickel C., and Panten U.. 1992. Cytosolic nucleotides enhance the tolbutamide sensitivity of the ATP-dependent K+ channel in mouse pancreatic B cells by their combined actions at inhibitory and stimulatory receptors. Mol Pharmacol. 41:480–486. [PubMed] [Google Scholar]
- Schwanstecher C., Dickel C., and Panten U.. 1994. Interaction of tolbutamide and cytosolic nucleotides in controlling the ATP-sensitive K+ channel in mouse beta-cells. Br J Pharmacol. 111:302–310. doi: 10.1111/j.1476-5381.1994.tb14060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanstecher C., and Panten U.. 1994. Identification of an ATP-sensitive K+ channel in spiny neurons of rat caudate nucleus. Pflugers Arch. 427:187–189. doi: 10.1007/BF00585961. [DOI] [PubMed] [Google Scholar]
- Shyng S.-L., and Nichols C.G.. 1997. Octameric Stoichiometry of the KATP Channel Complex. Journal of General Physiology. 110:655–664. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert C. 2020. Interactive Web-Based Data Visualization with R, plotly, and shiny.
- Stephen T.K.L., Guillemette K.L., and Green T.K.. 2016. Analysis of Trinitrophenylated Adenosine and Inosine by Capillary Electrophoresis and γ-Cyclodextrin-Enhanced Fluorescence Detection. Anal Chem. 88:7777–7785. doi: 10.1021/acs.analchem.6b01796. [DOI] [PubMed] [Google Scholar]
- Stockner T., Gradisch R., and Schmitt L.. 2020. The role of the degenerate nucleotide binding site in type I ABC exporters. FEBS Lett. 594:3815–3838. doi: 10.1002/1873-3468.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treherne J.M., and Ashford M.L.. 1992. Extracellular cations modulate the ATP sensitivity of ATP-K+ channels in rat ventromedial hypothalamic neurons. Proc Biol Sci. 247:121–124. doi: 10.1098/rspb.1992.0017. [DOI] [PubMed] [Google Scholar]
- Tucker S.J., Gribble F.M., Zhao C., Trapp S., and Ashcroft F.M.. 1997. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- Tusnády G.E., Bakos É., Váradi A., and Sarkadi B.. 1997. Membrane topology distinguishes a subfamily of the ATP-binding cassette (ABC) transporters. FEBS Letters. 402:1–3. doi: 10.1016/S0014-5793(96)01478-0. [DOI] [PubMed] [Google Scholar]
- Usher S.G., Ashcroft F.M., and Puljung M.C.. 2020a. Measuring Nucleotide Binding to Intact, Functional Membrane Proteins in Real Time. Journal of Visualized Experiments. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Usher S.G., Ashcroft F.M., and Puljung M.C.. 2020b. Nucleotide inhibition of the pancreatic ATP-sensitive K+ channel explored with patch-clamp fluorometry. eLife. 9:e52775. doi: 10.7554/eLife.52775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedovato N., Ashcroft F.M., and Puljung M.C.. 2015. The Nucleotide-Binding Sites of SUR1: A Mechanistic Model. Biophysical Journal. 109:2452–2460. doi: 10.1016/j.bpj.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet H., Mikhailov M.V., Fotinou C., Dreger M., Craig T.J., Vénien-Bryan C., and Ashcroft F.M.. 2007. Studies of the ATPase activity of the ABC protein SUR1. The FEBS Journal. 274:3532–3544. doi: 10.1111/j.1742-4658.2007.05879.x. [DOI] [PubMed] [Google Scholar]
- Wickham H. 2016. ggplot2: Elegant Graphics for Data Analysis.
- Zerangue N., Schwappach B., Jan Y.N., and Jan L.Y.. 1999. A New ER Trafficking Signal Regulates the Subunit Stoichiometry of Plasma Membrane KATP Channels. Neuron. 22:537–548. doi: 10.1016/S0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- Zingman L.V., Alekseev A.E., Bienengraeber M., Hodgson D., Karger A.B., Dzeja P.P., and Terzic A.. Signaling in Channel/Enzyme Multimers: ATPase Transitions in SUR Module Gate ATP-Sensitive K؉Conductance. 13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data for this work are available on the Dryad repository (XX URL) and all custom code is available on GitHub (XX URL).