Abstract
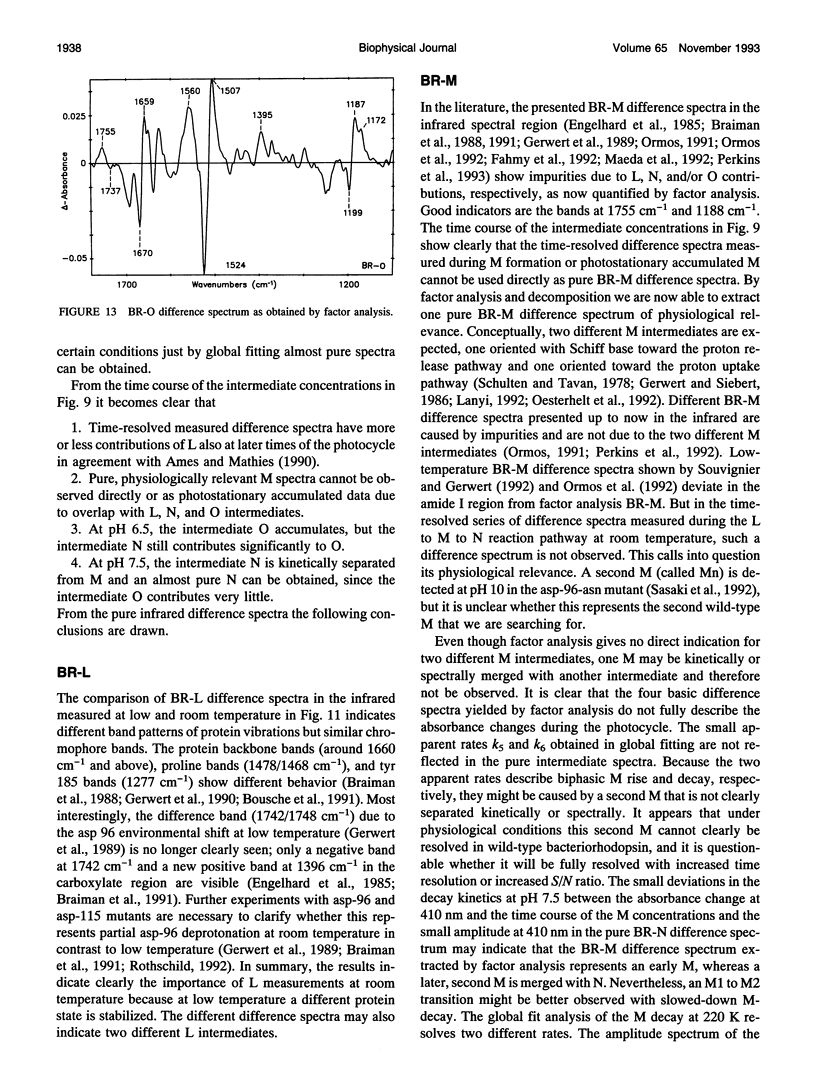
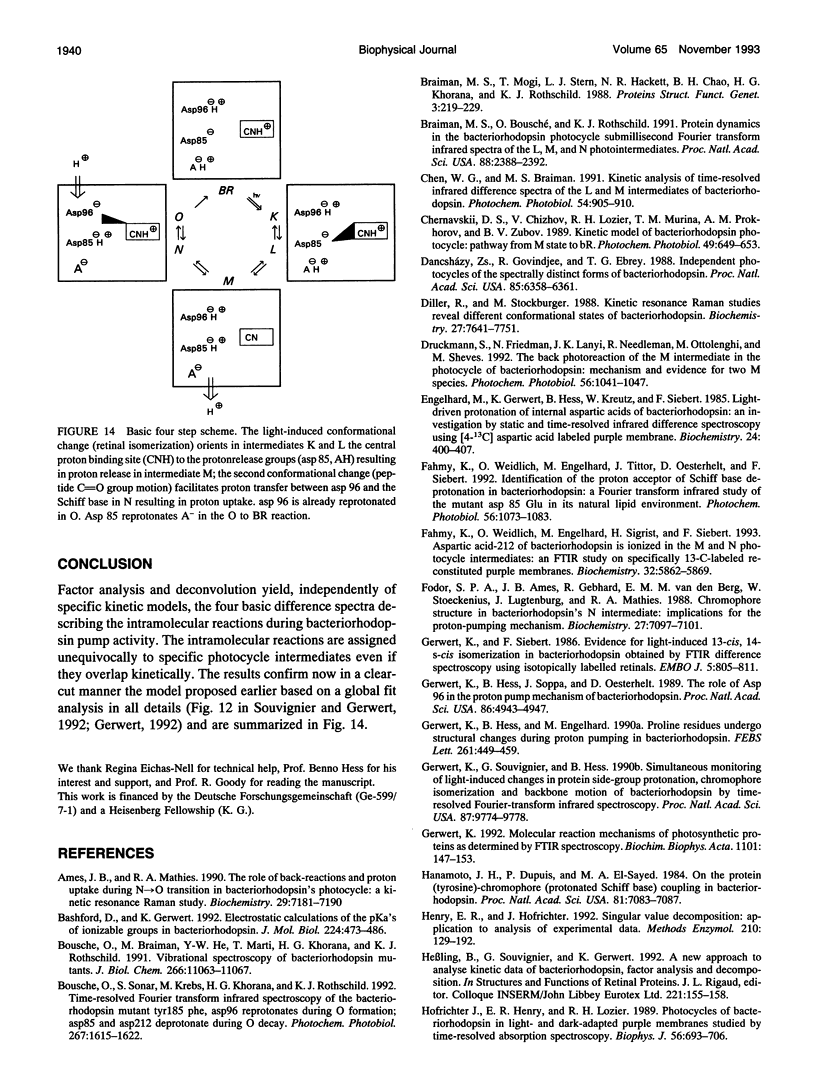
By using factor analysis and decomposition, bacteriorhodopsin's intramolecular reactions have been assigned to photocycle intermediates. Independent of specific kinetic models, the pure BR-L, BR-M, BR-N, and BR-O difference spectra were calculated by analyzing simultaneously two different measurements in the visible and infrared spectral region performed at pH 6.5, 298 K, 1 M KCl, and pH 7.5, 288 K, 1 M KCl. Even though after M formation L, M, N, and O intermediates kinetically overlap under physiological conditions, their pure spectra have been separated by this analysis in contrast to other approaches at which unphysiological conditions or mutants have been used or specific photocycle models have been assumed. The results now provide a set reference spectra for further studies. The following conclusions for physiologically relevant reactions are drawn: (a) the catalytic proton release binding site, asp 85, is protonated in the L to M transition and remains protonated in the intermediates N and O; (b) the catalytic proton uptake binding site asp 96 is deprotonated in the M to N transition and already reprotonated in the N to O transition; (c) proton transfer between asp 96 and the Schiff base is facilitated by backbone movements of a few peptide carbonyl groups in the M to N transition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames J. B., Mathies R. A. The role of back-reactions and proton uptake during the N----O transition in bacteriorhodopsin's photocycle: a kinetic resonance Raman study. Biochemistry. 1990 Aug 7;29(31):7181–7190. doi: 10.1021/bi00483a005. [DOI] [PubMed] [Google Scholar]
- Bashford D., Gerwert K. Electrostatic calculations of the pKa values of ionizable groups in bacteriorhodopsin. J Mol Biol. 1992 Mar 20;224(2):473–486. doi: 10.1016/0022-2836(92)91009-e. [DOI] [PubMed] [Google Scholar]
- Bousché O., Braiman M., He Y. W., Marti T., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants. Evidence that ASP-96 deprotonates during the M----N transition. J Biol Chem. 1991 Jun 15;266(17):11063–11067. [PubMed] [Google Scholar]
- Braiman M. S., Bousché O., Rothschild K. J. Protein dynamics in the bacteriorhodopsin photocycle: submillisecond Fourier transform infrared spectra of the L, M, and N photointermediates. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2388–2392. doi: 10.1073/pnas.88.6.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Stern L. J., Hackett N. R., Chao B. H., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: I. Tyrosine-185 protonates and deprotonates during the photocycle. Proteins. 1988;3(4):219–229. doi: 10.1002/prot.340030403. [DOI] [PubMed] [Google Scholar]
- Chernavskii D. S., Chizhov I. V., Lozier R. H., Murina T. M., Prokhorov A. M., Zubov B. V. Kinetic model of bacteriorhodopsin photocycle: pathway from M state to bR. Photochem Photobiol. 1989 May;49(5):649–653. doi: 10.1111/j.1751-1097.1989.tb08437.x. [DOI] [PubMed] [Google Scholar]
- Dancsházy Z., Govindjee R., Ebrey T. G. Independent photocycles of the spectrally distinct forms of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6358–6361. doi: 10.1073/pnas.85.17.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann S., Friedman N., Lanyi J. K., Needleman R., Ottolenghi M., Sheves M. The back photoreaction of the M intermediate in the photocycle of bacteriorhodopsin: mechanism and evidence for two M species. Photochem Photobiol. 1992;56(6):1041–1047. doi: 10.1111/j.1751-1097.1992.tb09727.x. [DOI] [PubMed] [Google Scholar]
- Engelhard M., Gerwert K., Hess B., Kreutz W., Siebert F. Light-driven protonation changes of internal aspartic acids of bacteriorhodopsin: an investigation by static and time-resolved infrared difference spectroscopy using [4-13C]aspartic acid labeled purple membrane. Biochemistry. 1985 Jan 15;24(2):400–407. doi: 10.1021/bi00323a024. [DOI] [PubMed] [Google Scholar]
- Fahmy K., Weidlich O., Engelhard M., Sigrist H., Siebert F. Aspartic acid-212 of bacteriorhodopsin is ionized in the M and N photocycle intermediates: an FTIR study on specifically 13C-labeled reconstituted purple membranes. Biochemistry. 1993 Jun 8;32(22):5862–5869. doi: 10.1021/bi00073a020. [DOI] [PubMed] [Google Scholar]
- Fodor S. P., Ames J. B., Gebhard R., van den Berg E. M., Stoeckenius W., Lugtenburg J., Mathies R. A. Chromophore structure in bacteriorhodopsin's N intermediate: implications for the proton-pumping mechanism. Biochemistry. 1988 Sep 6;27(18):7097–7101. doi: 10.1021/bi00418a064. [DOI] [PubMed] [Google Scholar]
- Gerwert K., Hess B., Soppa J., Oesterhelt D. Role of aspartate-96 in proton translocation by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4943–4947. doi: 10.1073/pnas.86.13.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwert K., Siebert F. Evidence for light-induced 13-cis, 14-s-cis isomerization in bacteriorhodopsin obtained by FTIR difference spectroscopy using isotopically labelled retinals. EMBO J. 1986 Apr;5(4):805–811. doi: 10.1002/j.1460-2075.1986.tb04285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
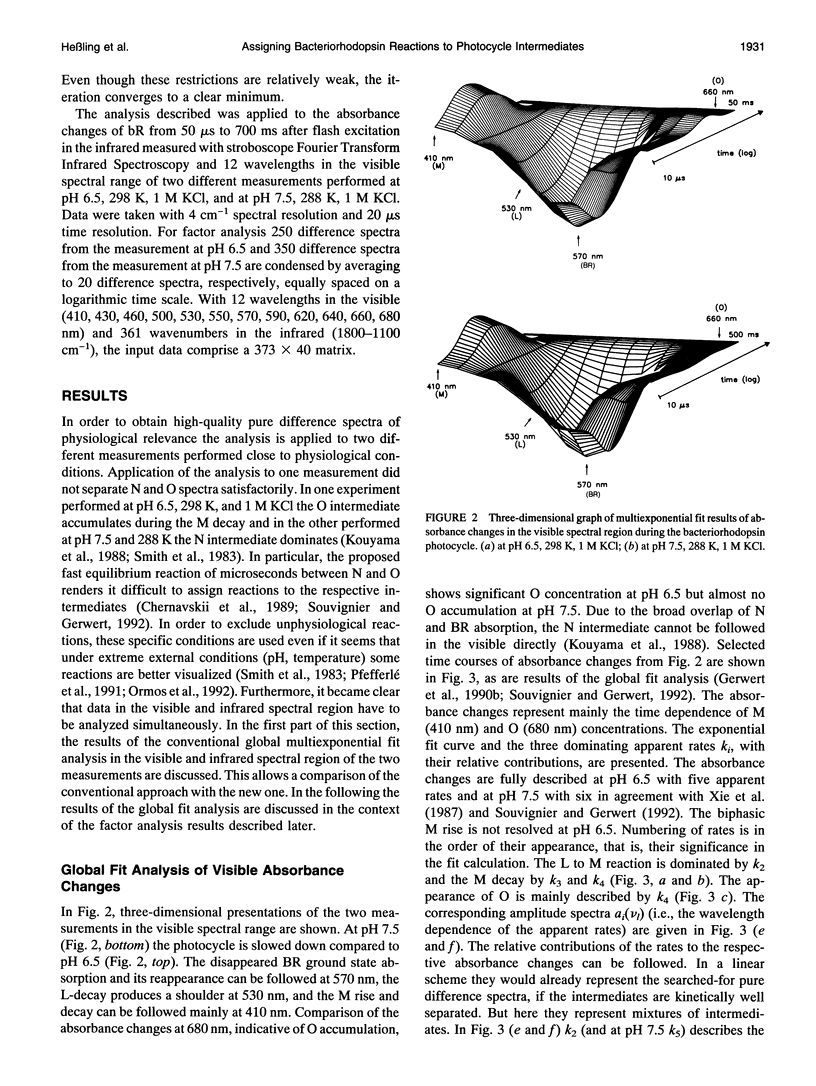
- Gerwert K., Souvignier G., Hess B. Simultaneous monitoring of light-induced changes in protein side-group protonation, chromophore isomerization, and backbone motion of bacteriorhodopsin by time-resolved Fourier-transform infrared spectroscopy. Proc Natl Acad Sci U S A. 1990 Dec 15;87(24):9774–9778. doi: 10.1073/pnas.87.24.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamoto J. H., Dupuis P., El-Sayed M. A. On the protein (tyrosine)-chromophore (protonated Schiff base) coupling in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7083–7087. doi: 10.1073/pnas.81.22.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Henry E. R., Lozier R. H. Photocycles of bacteriorhodopsin in light- and dark-adapted purple membrane studied by time-resolved absorption spectroscopy. Biophys J. 1989 Oct;56(4):693–706. doi: 10.1016/S0006-3495(89)82716-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. H., Dencher N. A., Oesterhelt D., Plöhn H. J., Rapp G., Büldt G. Time-resolved X-ray diffraction study of structural changes associated with the photocycle of bacteriorhodopsin. EMBO J. 1991 Mar;10(3):521–526. doi: 10.1002/j.1460-2075.1991.tb07978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyama T., Nasuda-Kouyama A., Ikegami A., Mathew M. K., Stoeckenius W. Bacteriorhodopsin photoreaction: identification of a long-lived intermediate N (P,R350) at high pH and its M-like photoproduct. Biochemistry. 1988 Aug 9;27(16):5855–5863. doi: 10.1021/bi00416a006. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Proton transfer and energy coupling in the bacteriorhodopsin photocycle. J Bioenerg Biomembr. 1992 Apr;24(2):169–179. doi: 10.1007/BF00762675. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Xie A., Hofrichter J., Clore G. M. Reversible steps in the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3610–3614. doi: 10.1073/pnas.89.8.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
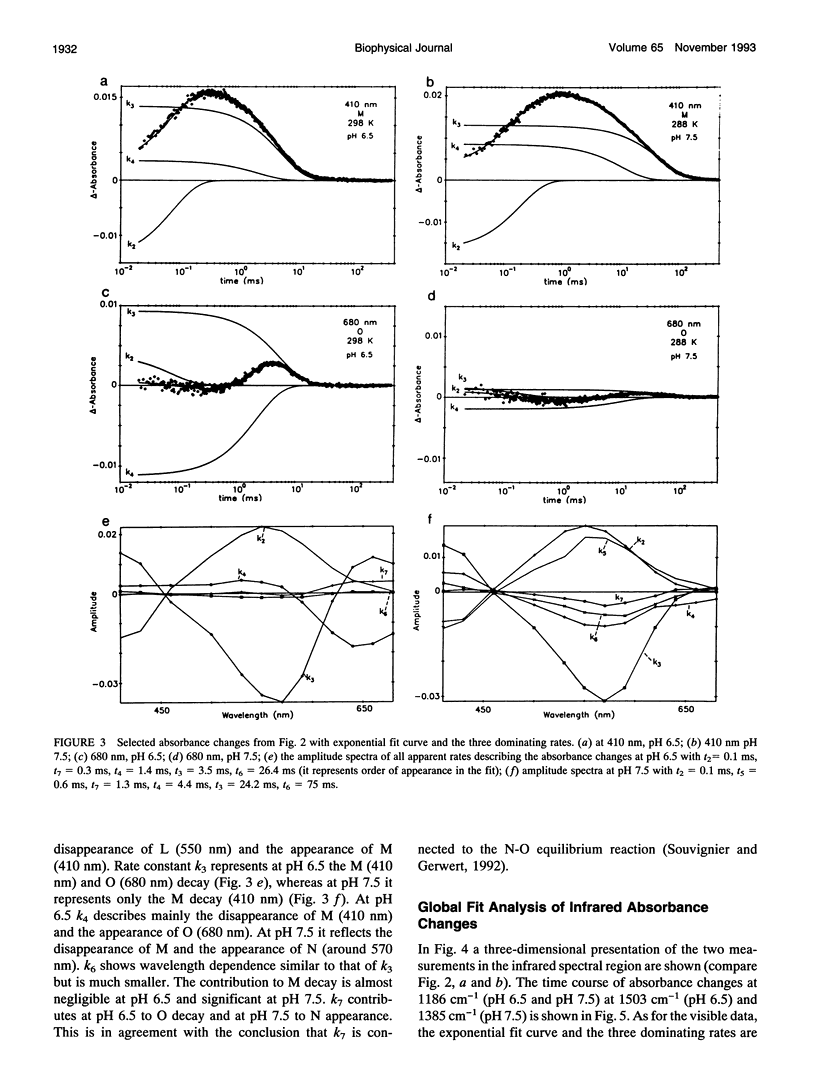
- Maeda A., Sasaki J., Shichida Y., Yoshizawa T., Chang M., Ni B., Needleman R., Lanyi J. K. Structures of aspartic acid-96 in the L and N intermediates of bacteriorhodopsin: analysis by Fourier transform infrared spectroscopy. Biochemistry. 1992 May 19;31(19):4684–4690. doi: 10.1021/bi00134a022. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Tittor J., Bamberg E. A unifying concept for ion translocation by retinal proteins. J Bioenerg Biomembr. 1992 Apr;24(2):181–191. doi: 10.1007/BF00762676. [DOI] [PubMed] [Google Scholar]
- Ormos P., Chu K., Mourant J. Infrared study of the L, M, and N intermediates of bacteriorhodopsin using the photoreaction of M. Biochemistry. 1992 Aug 4;31(30):6933–6937. doi: 10.1021/bi00145a010. [DOI] [PubMed] [Google Scholar]
- Ormos P. Infrared spectroscopic demonstration of a conformational change in bacteriorhodopsin involved in proton pumping. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):473–477. doi: 10.1073/pnas.88.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins G. A., Liu E., Burkard F., Berry E. A., Glaeser R. M. Characterization of the conformational change in the M1 and M2 substates of bacteriorhodopsin by the combined use of visible and infrared spectroscopy. J Struct Biol. 1992 Sep-Oct;109(2):142–151. doi: 10.1016/1047-8477(92)90045-c. [DOI] [PubMed] [Google Scholar]
- Pfefferlé J. M., Maeda A., Sasaki J., Yoshizawa T. Fourier transform infrared study of the N intermediate of bacteriorhodopsin. Biochemistry. 1991 Jul 2;30(26):6548–6556. doi: 10.1021/bi00240a027. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J. FTIR difference spectroscopy of bacteriorhodopsin: toward a molecular model. J Bioenerg Biomembr. 1992 Apr;24(2):147–167. doi: 10.1007/BF00762674. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., He Y. W., Mogi T., Marti T., Stern L. J., Khorana H. G. Vibrational spectroscopy of bacteriorhodopsin mutants: evidence for the interaction of proline-186 with the retinylidene chromophore. Biochemistry. 1990 Jun 26;29(25):5954–5960. doi: 10.1021/bi00477a011. [DOI] [PubMed] [Google Scholar]
- Sasaki J., Shichida Y., Lanyi J. K., Maeda A. Protein changes associated with reprotonation of the Schiff base in the photocycle of Asp96-->Asn bacteriorhodopsin. The MN intermediate with unprotonated Schiff base but N-like protein structure. J Biol Chem. 1992 Oct 15;267(29):20782–20786. [PubMed] [Google Scholar]
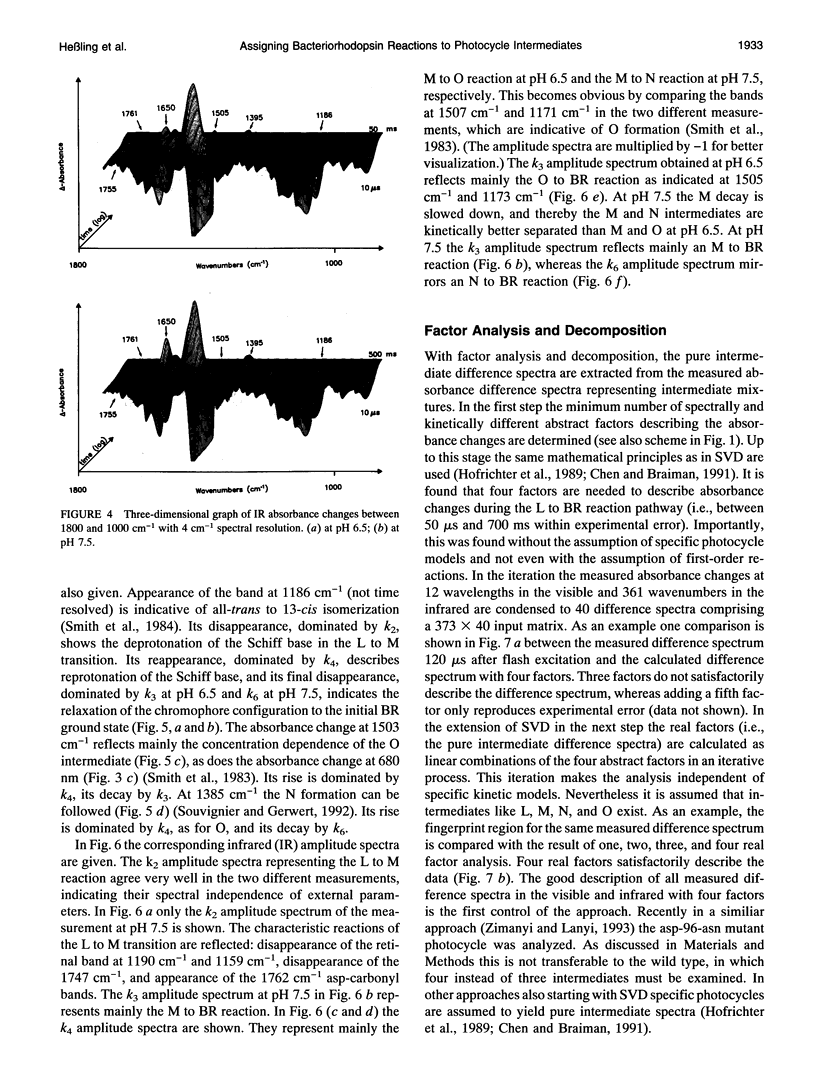
- Schulten K., Tavan P. A mechanism for the light-driven proton pump of Halobacterium halobium. Nature. 1978 Mar 2;272(5648):85–86. doi: 10.1038/272085a0. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Myers A. B., Pardoen J. A., Winkel C., Mulder P. P., Lugtenburg J., Mathies R. Determination of retinal Schiff base configuration in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2055–2059. doi: 10.1073/pnas.81.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souvignier G., Gerwert K. Proton uptake mechanism of bacteriorhodopsin as determined by time-resolved stroboscopic-FTIR-spectroscopy. Biophys J. 1992 Nov;63(5):1393–1405. doi: 10.1016/S0006-3495(92)81722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S., Gerstein M., Oesterhelt D., Henderson R. Electron diffraction analysis of structural changes in the photocycle of bacteriorhodopsin. EMBO J. 1993 Jan;12(1):1–8. doi: 10.1002/j.1460-2075.1993.tb05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Distortions in the photocycle of bacteriorhodopsin at moderate dehydration. Biophys J. 1991 Feb;59(2):313–322. doi: 10.1016/S0006-3495(91)82225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Kinetic and spectroscopic evidence for an irreversible step between deprotonation and reprotonation of the Schiff base in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5008–5015. doi: 10.1021/bi00234a024. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Pathways of the rise and decay of the M photointermediate(s) of bacteriorhodopsin. Biochemistry. 1990 Mar 6;29(9):2241–2250. doi: 10.1021/bi00461a006. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Thermodynamics and energy coupling in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5016–5022. doi: 10.1021/bi00234a025. [DOI] [PubMed] [Google Scholar]
- Xie A. H., Nagle J. F., Lozier R. H. Flash spectroscopy of purple membrane. Biophys J. 1987 Apr;51(4):627–635. doi: 10.1016/S0006-3495(87)83387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimányi L., Lanyi J. K. Deriving the intermediate spectra and photocycle kinetics from time-resolved difference spectra of bacteriorhodopsin. The simpler case of the recombinant D96N protein. Biophys J. 1993 Jan;64(1):240–251. doi: 10.1016/S0006-3495(93)81360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]