Abstract
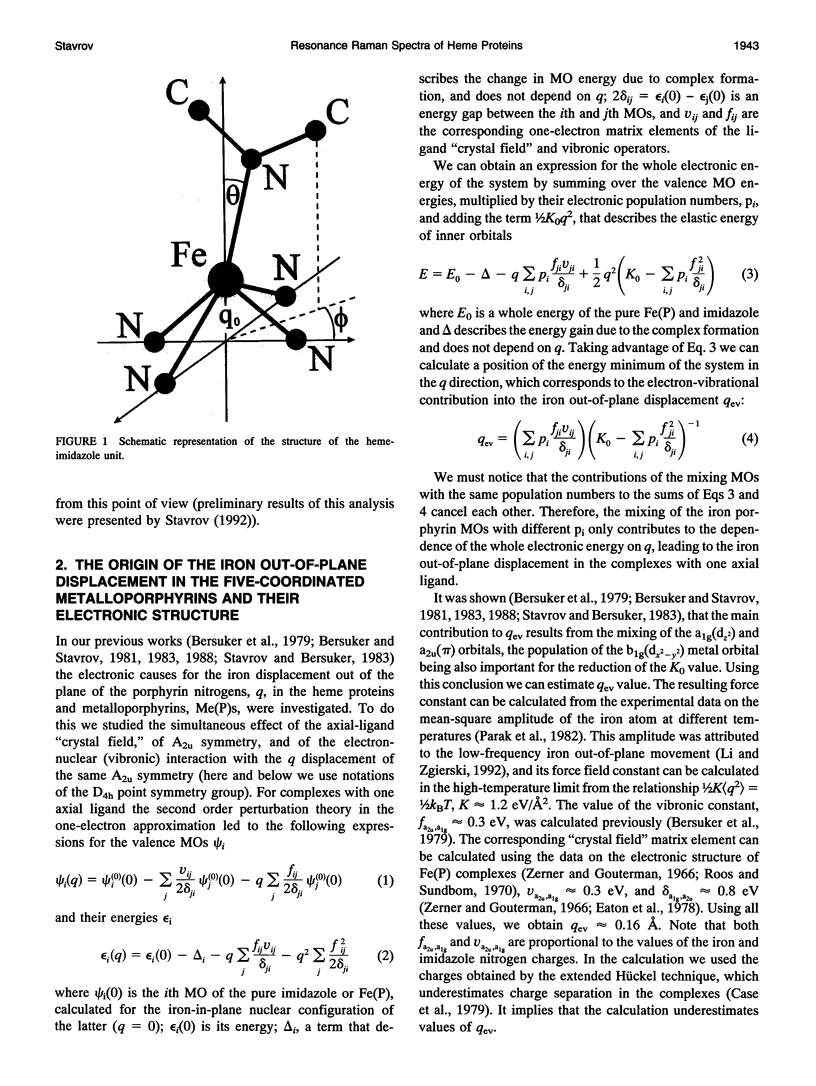
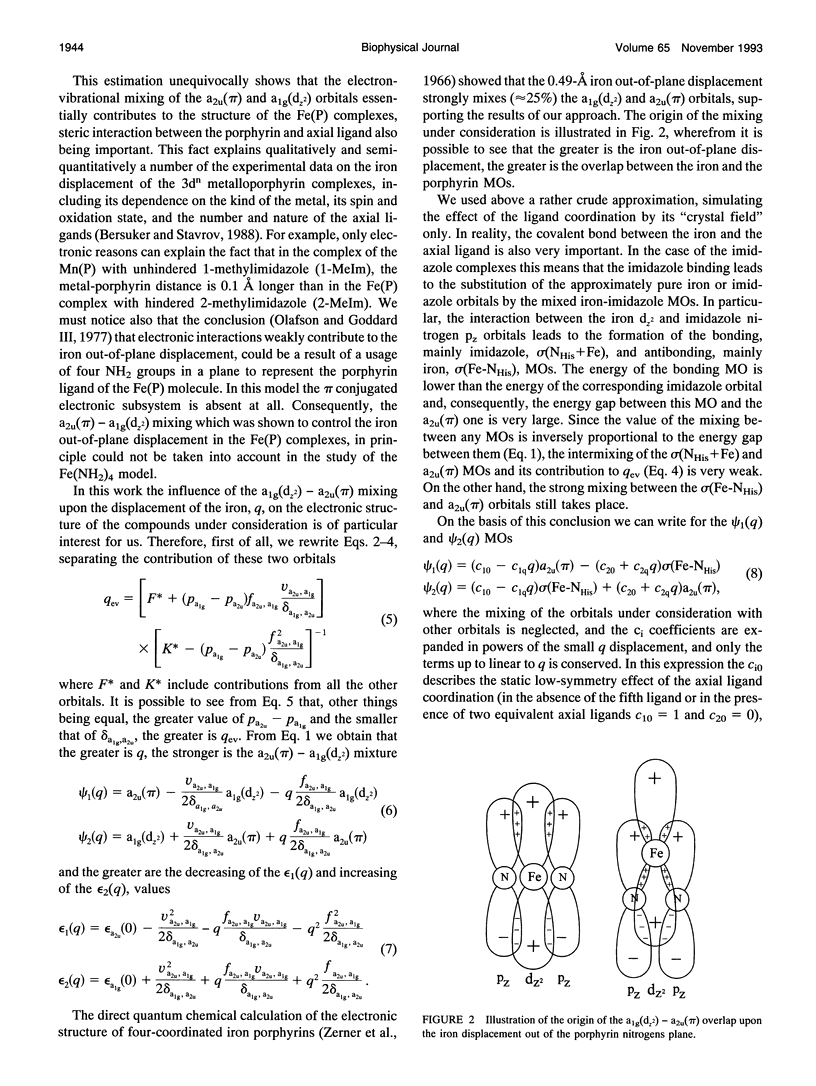
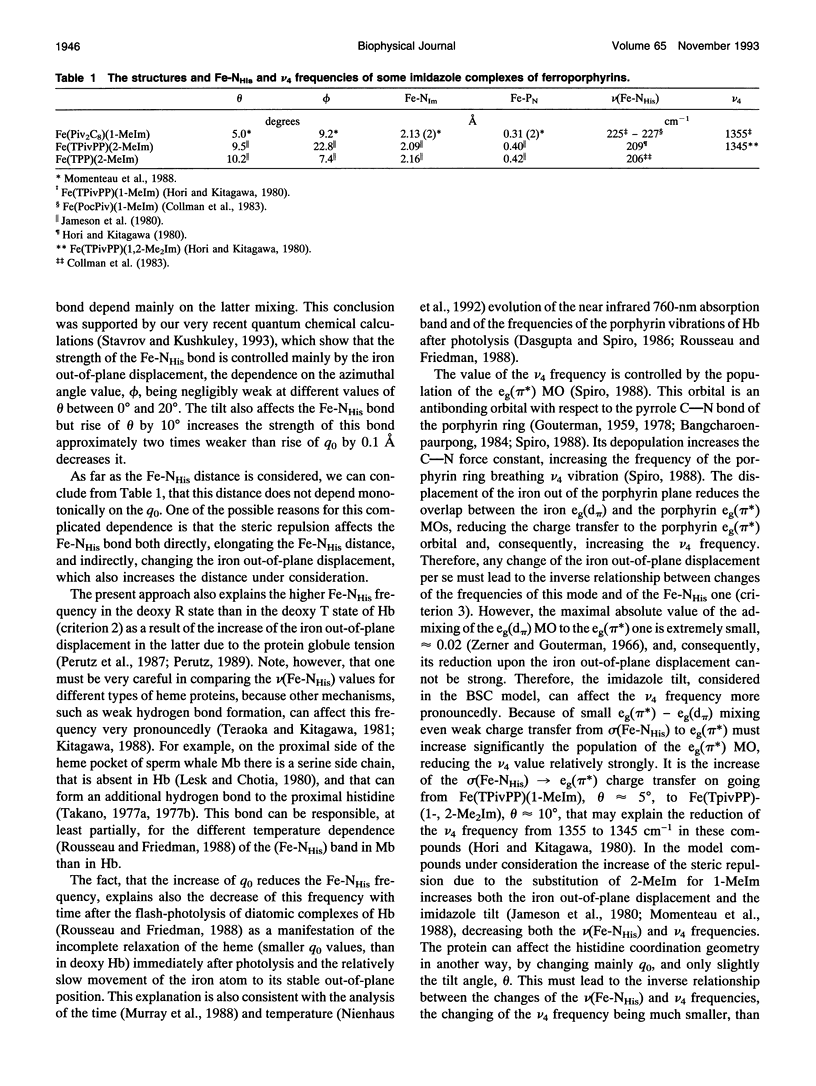
The causes of the strong coupling of the iron-histidine vibration to the Soret resonance in the resonance Raman spectra of deoxyhemoglobin, myoglobin, and peroxidase are explored, using the vibronic theory. It is shown that the extent of the iron displacement out of the plane of the porphyrin nitrogens is the main structural parameter controlling the Fe-NHis band features, such as the dependence of its frequency and intensity on the protein conformation and number of the axial ligands, time evolution after the photolysis of the diatomic complexes of the proteins under consideration, and inverse relationship between the changes Fe-NHis and v4 porphyrin breathing mode frequencies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosenbeck M., Schweitzer-Stenner R., Dreybrodt W. pH-induced conformational changes of the Fe(2+)-N epsilon (His F8) linkage in deoxyhemoglobin trout IV detected by the Raman active Fe(2+)-N epsilon (His F8) stretching mode. Biophys J. 1992 Jan;61(1):31–41. doi: 10.1016/S0006-3495(92)81813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S., Spiro T. G. Resonance Raman characterization of the 7-ns photoproduct of (carbonmonoxy)hemoglobin: implications for hemoglobin dynamics. Biochemistry. 1986 Oct 7;25(20):5941–5948. doi: 10.1021/bi00368a016. [DOI] [PubMed] [Google Scholar]
- Desbois A., Lutz M., Banerjee R. Protoheme conformations in low-spin ferrohemoproteins. Resonance Raman spectroscopy. Biochim Biophys Acta. 1981 Dec 29;671(2):184–192. doi: 10.1016/0005-2795(81)90133-1. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Campbell B. F., Noble R. W. A possible new control mechanism suggested by resonance Raman spectra from a deep ocean fish hemoglobin. Biophys Chem. 1990 Aug 31;37(1-3):43–59. doi: 10.1016/0301-4622(90)88006-e. [DOI] [PubMed] [Google Scholar]
- Gelin B. R., Lee A. W., Karplus M. Hemoglobin tertiary structural change on ligand binding. Its role in the co-operative mechanism. J Mol Biol. 1983 Dec 25;171(4):489–559. doi: 10.1016/0022-2836(83)90042-6. [DOI] [PubMed] [Google Scholar]
- Lesk A. M., Chothia C. How different amino acid sequences determine similar protein structures: the structure and evolutionary dynamics of the globins. J Mol Biol. 1980 Jan 25;136(3):225–270. doi: 10.1016/0022-2836(80)90373-3. [DOI] [PubMed] [Google Scholar]
- Liddington R., Derewenda Z., Dodson G., Harris D. Structure of the liganded T state of haemoglobin identifies the origin of cooperative oxygen binding. Nature. 1988 Feb 25;331(6158):725–728. doi: 10.1038/331725a0. [DOI] [PubMed] [Google Scholar]
- Murray L. P., Hofrichter J., Henry E. R., Eaton W. A. Time-resolved optical spectroscopy and structural dynamics following photodissociation of carbonmonoxyhemoglobin. Biophys Chem. 1988 Feb;29(1-2):63–76. doi: 10.1016/0301-4622(88)87025-x. [DOI] [PubMed] [Google Scholar]
- Nagai K., Kitagawa T. Differences in Fe(II)-N epsilon(His-F8) stretching frequencies between deoxyhemoglobins in the two alternative quaternary structures. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2033–2037. doi: 10.1073/pnas.77.4.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Kitagawa T., Morimoto H. Quaternary structures and low frequency molecular vibrations of haems of deoxy and oxyhaemoglobin studied by resonance raman scattering. J Mol Biol. 1980 Jan 25;136(3):271–289. doi: 10.1016/0022-2836(80)90374-5. [DOI] [PubMed] [Google Scholar]
- Nienhaus G. U., Mourant J. R., Frauenfelder H. Spectroscopic evidence for conformational relaxation in myoglobin. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2902–2906. doi: 10.1073/pnas.89.7.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafson B. D., Goddard W. A., 3rd Molecular description of dioxygen bonding in hemoglobin. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1315–1319. doi: 10.1073/pnas.74.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parak F., Knapp E. W., Kucheida D. Protein dynamics. Mössbauer spectroscopy on deoxymyoglobin crystals. J Mol Biol. 1982 Oct 15;161(1):177–194. doi: 10.1016/0022-2836(82)90285-6. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Ladner J. E., Simon S. R., Ho C. Influence of globin structure on the state of the heme. I. Human deoxyhemoglobin. Biochemistry. 1974 May 7;13(10):2163–2173. doi: 10.1021/bi00707a026. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Mechanisms of cooperativity and allosteric regulation in proteins. Q Rev Biophys. 1989 May;22(2):139–237. doi: 10.1017/s0033583500003826. [DOI] [PubMed] [Google Scholar]
- Sassaroli M., Dasgupta S., Rousseau D. L. Cryogenic stabilization of myoglobin photoproducts. J Biol Chem. 1986 Oct 15;261(29):13704–13713. [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. I. Crystallographic refinement of metmyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):537–568. doi: 10.1016/s0022-2836(77)80111-3. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):569–584. doi: 10.1016/s0022-2836(77)80112-5. [DOI] [PubMed] [Google Scholar]
- Teraoka J., Kitagawa T. Structural implication of the heme-linked ionization of horseradish peroxidase probed by the Fe-histidine stretching Raman line. J Biol Chem. 1981 Apr 25;256(8):3969–3977. [PubMed] [Google Scholar]



