Abstract
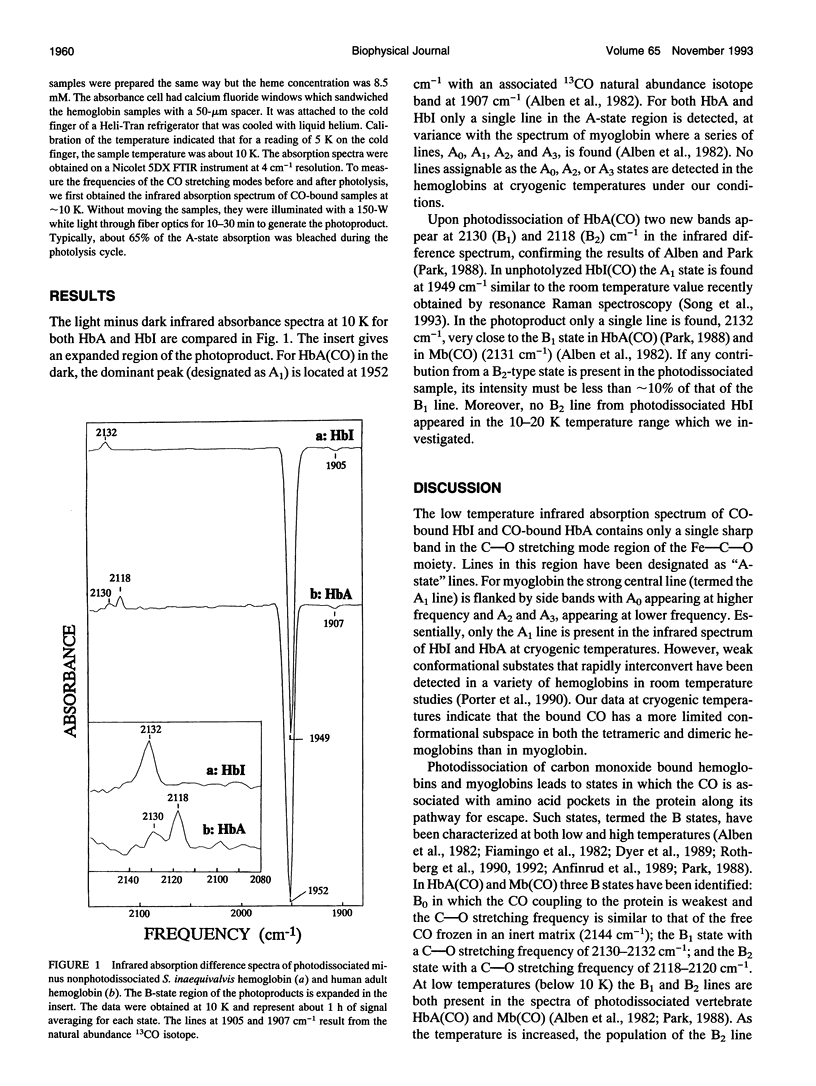
The infrared absorption spectrum of the CO-photoproduct from Scapharca inaequivalvis hemoglobin (Hbl) at 10 K yields only a single line in the "B" state region at 2132 cm-1. This is the same frequency as the B1 line observed in photodissociated vertebrate HbCO and MbCO. No evidence was found for the B2 line detected in vertebrate hemoglobins and myoglobin in the 2118-2120 cm-1 region. These data demonstrate that the protein does not have the same conformationally accessible ligand-binding sites as do vertebrate hemoglobins and myoglobins. The absence of the B2 line indicates that only a single weak site is accessible to the photolyzed CO molecule. These results are in accord with geminate rebinding experiments and ligand escape pathway calculations which have shown that the distal properties of Hbl are distinct from those of tetrameric hemoglobins and vertebrate myoglobins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfinrud P. A., Han C., Hochstrasser R. M. Direct observations of ligand dynamics in hemoglobin by subpicosecond infrared spectroscopy. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8387–8391. doi: 10.1073/pnas.86.21.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., DiIorio E. E., Dlott D. D., Frauenfelder H., Iben I. E., Langer P., Roder H., Sauke T. B., Shyamsunder E. Ligand binding to heme proteins: relevance of low-temperature data. Biochemistry. 1986 Jun 3;25(11):3139–3146. doi: 10.1021/bi00359a011. [DOI] [PubMed] [Google Scholar]
- Antonini E., Ascoli F., Brunori M., Chiancone E., Verzili D., Morris R. J., Gibson Q. H. Kinetics of ligand binding and quaternary conformational change in the homodimeric hemoglobin from Scapharca inaequivalvis. J Biol Chem. 1984 Jun 10;259(11):6730–6738. [PubMed] [Google Scholar]
- Case D. A., Karplus M. Dynamics of ligand binding to heme proteins. J Mol Biol. 1979 Aug 15;132(3):343–368. doi: 10.1016/0022-2836(79)90265-1. [DOI] [PubMed] [Google Scholar]
- Chiancone E., Elber R., Royer W. E., Jr, Regan R., Gibson Q. H. Ligand binding and conformation change in the dimeric hemoglobin of the clam Scapharca inaequivalvis. J Biol Chem. 1993 Mar 15;268(8):5711–5718. [PubMed] [Google Scholar]
- Chiancone E., Gibson Q. H. Ligand binding to the dimeric hemoglobin from Scapharca inaequivalvis, a hemoglobin with a novel mechanism for cooperativity. J Biol Chem. 1989 Dec 15;264(35):21062–21065. [PubMed] [Google Scholar]
- Chiancone E., Vecchini P., Verzili D., Ascoli F., Antonini E. Dimeric and tetrameric hemoglobins from the mollusc Scapharca inaequivalvis. Structural and functional properties. J Mol Biol. 1981 Nov 5;152(3):577–592. doi: 10.1016/0022-2836(81)90270-9. [DOI] [PubMed] [Google Scholar]
- Chiancone E., Verzili D., Boffi A., Royer W. E., Jr, Hendrickson W. A. A cooperative hemoglobin with directly communicating hemes. The Scapharca inaequivalvis homodimer. Biophys Chem. 1990 Aug 31;37(1-3):287–292. doi: 10.1016/0301-4622(90)88028-q. [DOI] [PubMed] [Google Scholar]
- Coletta M., Boffi A., Ascenzi P., Brunori M., Chiancone E. A novel mechanism of heme-heme interaction in the homodimeric hemoglobin from Scapharca inaequivalvis as manifested upon cleavage of the proximal Fe-N epsilon bond at low pH. J Biol Chem. 1990 Mar 25;265(9):4828–4830. [PubMed] [Google Scholar]
- Fiamingo F. G., Altschuld R. A., Moh P. P., Alben J. O. Dynamic interactions of CO with a3Fe and CuB in cytochrome c oxidase in beef heart mitochondria studied by Fourier transform infrared spectroscopy at low temperatures. J Biol Chem. 1982 Feb 25;257(4):1639–1650. [PubMed] [Google Scholar]
- Hofrichter J., Sommer J. H., Henry E. R., Eaton W. A. Nanosecond absorption spectroscopy of hemoglobin: elementary processes in kinetic cooperativity. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2235–2239. doi: 10.1073/pnas.80.8.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inubushi T., Yonetani T., Chiancone E. Proton nuclear magnetic resonance study of the ferrous derivatives of the dimeric and tetrameric hemoglobin from the mollusc Scapharca inaequivalvis. FEBS Lett. 1988 Aug 1;235(1-2):87–92. doi: 10.1016/0014-5793(88)81239-0. [DOI] [PubMed] [Google Scholar]
- McGourty J. L., La Mar G. N., Smith K. M., Ascoli F., Chiancone E., Pandey R. K., Singh J. P. Nuclear-magnetic-resonance investigation of the cooperative homodimeric hemoglobin from the mollusc Scapharca inaequivalvis. Molecular and electronic structure of the cyano-met derivative. Eur J Biochem. 1989 Sep 1;184(1):53–61. doi: 10.1111/j.1432-1033.1989.tb14989.x. [DOI] [PubMed] [Google Scholar]
- Potter W. T., Hazzard J. H., Choc M. G., Tucker M. P., Caughey W. S. Infrared spectra of carbonyl hemoglobins: characterization of dynamic heme pocket conformers. Biochemistry. 1990 Jul 3;29(26):6283–6295. doi: 10.1021/bi00478a025. [DOI] [PubMed] [Google Scholar]
- Rothberg L., Jedju T. M., Austin R. H. Ligand dynamics in the photodissociation of carboxyhemoglobin by subpicosecond transient infrared spectroscopy. Biophys J. 1990 Feb;57(2):369–373. doi: 10.1016/S0006-3495(90)82538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau D. L., Song S., Friedman J. M., Boffi A., Chiancone E. Heme-heme interactions in a homodimeric cooperative hemoglobin. Evidence from transient Raman scattering. J Biol Chem. 1993 Mar 15;268(8):5719–5723. [PubMed] [Google Scholar]
- Royer W. E., Jr, Hendrickson W. A., Chiancone E. Structural transitions upon ligand binding in a cooperative dimeric hemoglobin. Science. 1990 Aug 3;249(4968):518–521. doi: 10.1126/science.2382132. [DOI] [PubMed] [Google Scholar]
- Royer W. E., Jr, Hendrickson W. A., Chiancone E. The 2.4-A crystal structure of Scapharca dimeric hemoglobin. Cooperativity based on directly communicating hemes at a novel subunit interface. J Biol Chem. 1989 Dec 15;264(35):21052–21061. [PubMed] [Google Scholar]
- Song S., Boffi A., Chiancone E., Rousseau D. L. Protein-heme interactions in hemoglobin from the mollusc Scapharca inaequivalvis: evidence from resonance Raman scattering. Biochemistry. 1993 Jun 29;32(25):6330–6336. doi: 10.1021/bi00076a005. [DOI] [PubMed] [Google Scholar]
- Spagnuolo C., Ascoli F., Chiancone E., Vecchini P., Antonini E. Dimeric and tetrameric hemoglobins from the mollusc Scapharca inaequivalvis. The oxidation reaction. J Mol Biol. 1983 Mar 15;164(4):627–644. doi: 10.1016/0022-2836(83)90054-2. [DOI] [PubMed] [Google Scholar]
- Yeh W. K., Shih C., Ornston L. N. Overlapping evolutionary affinities revealed by comparison of amino acid compositions. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3794–3797. doi: 10.1073/pnas.79.12.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]


