Abstract
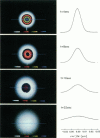
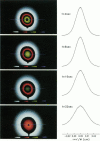
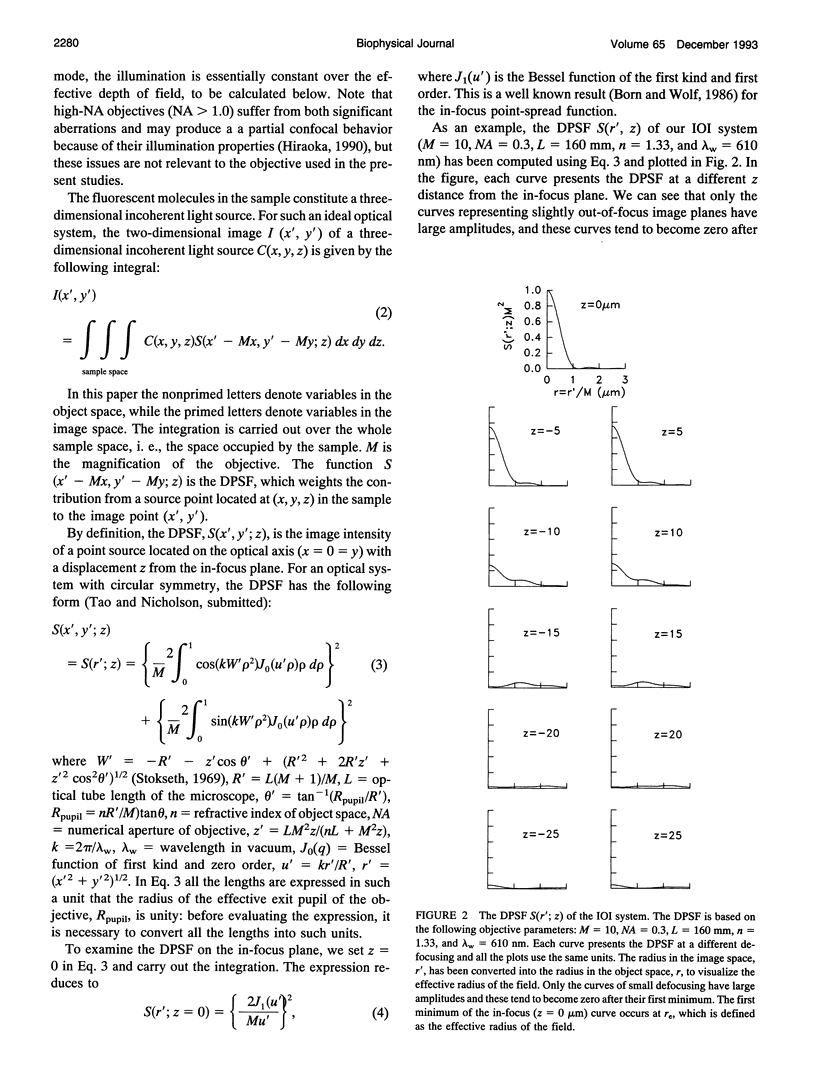
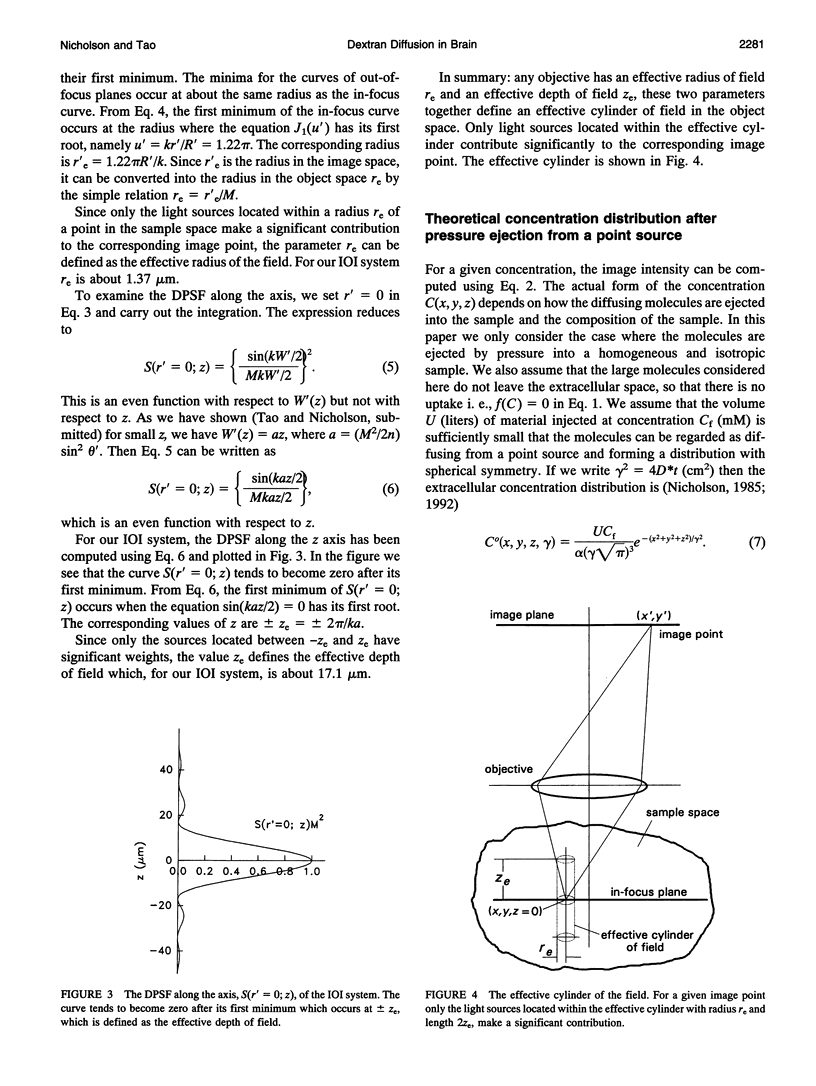
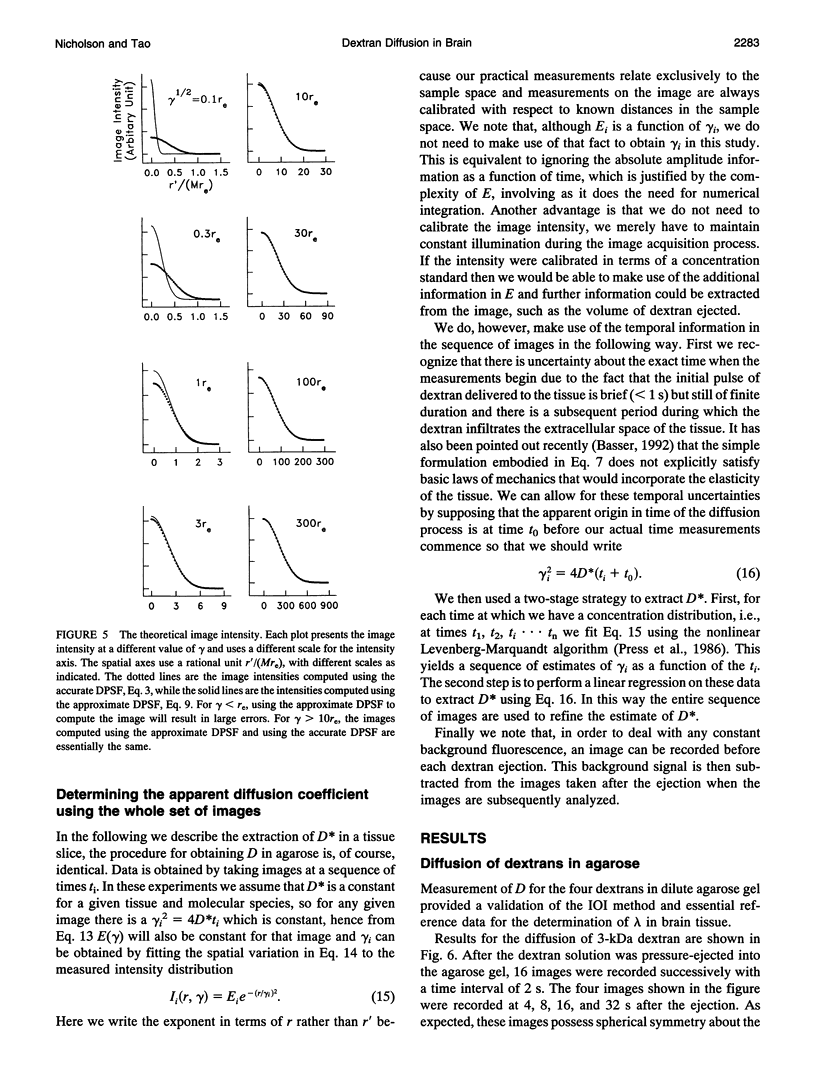
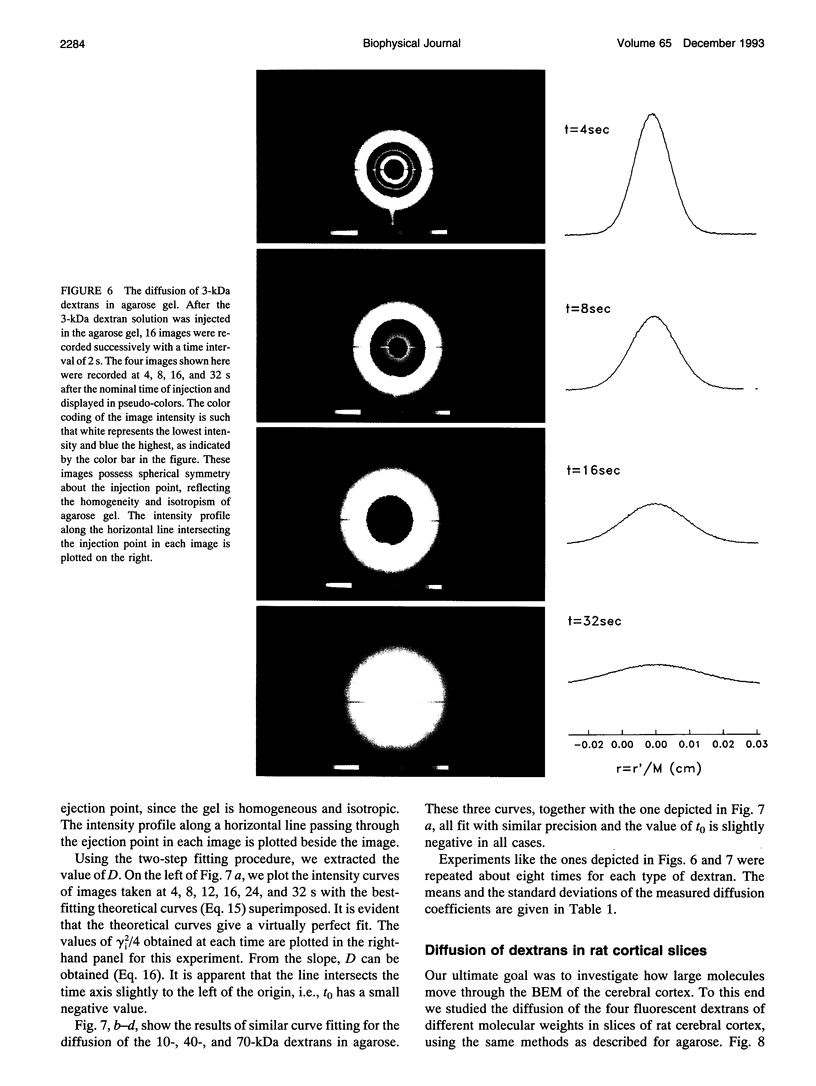
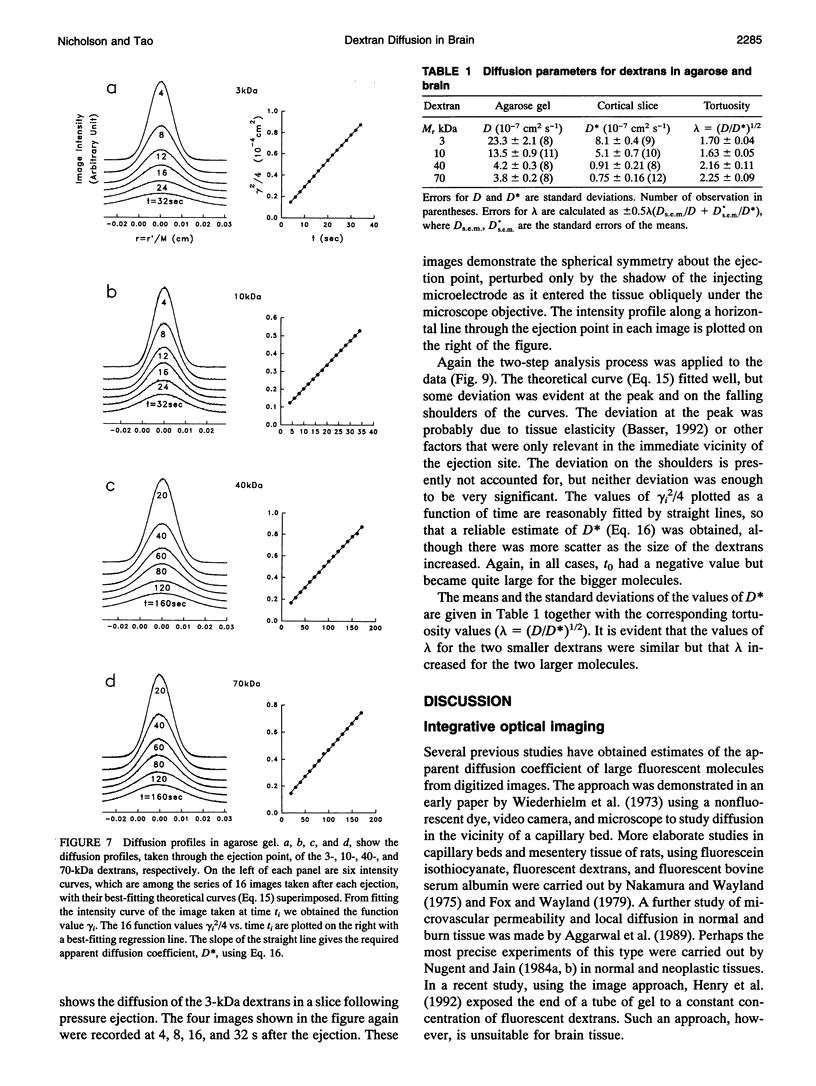
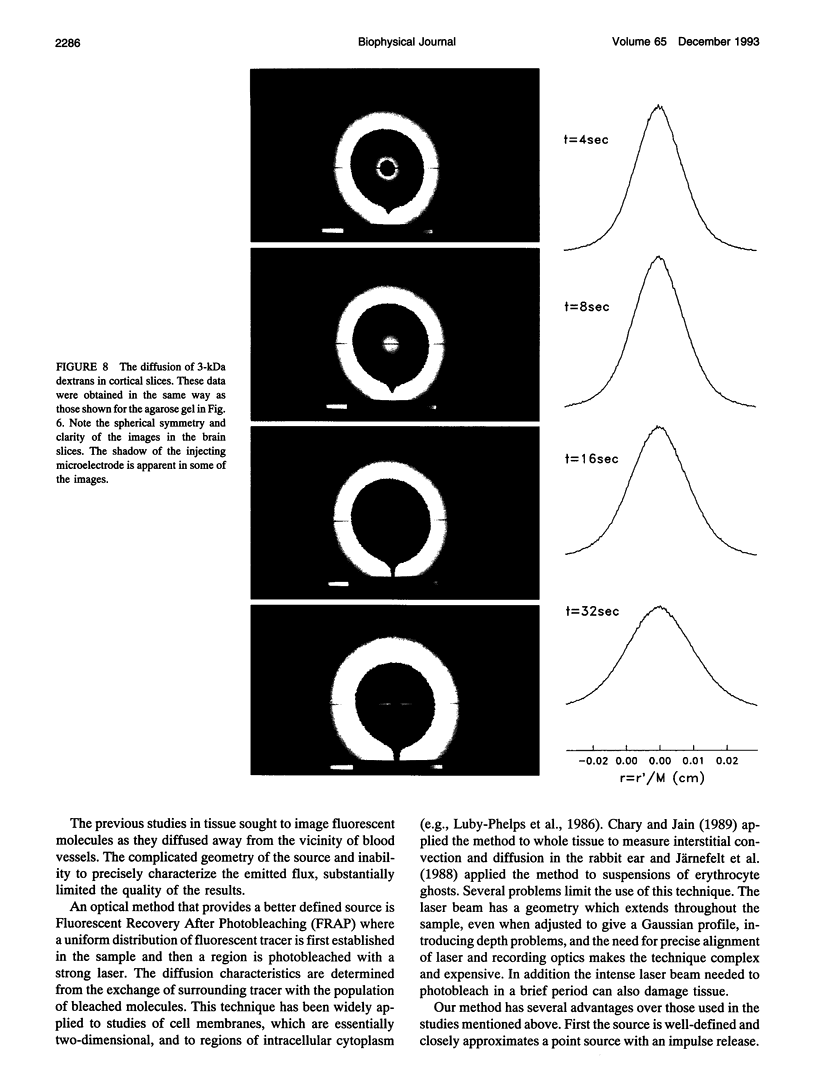
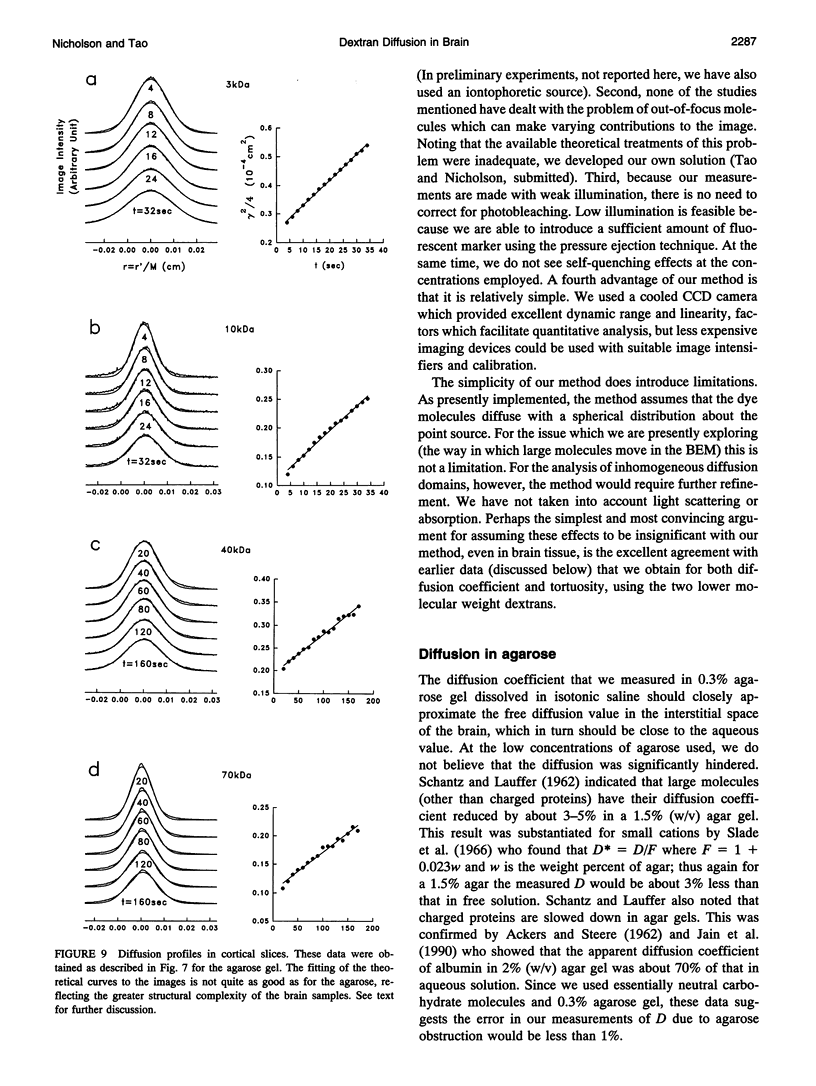
This paper describes the theory of an integrative optical imaging system and its application to the analysis of the diffusion of 3-, 10-, 40-, and 70-kDa fluorescent dextran molecules in agarose gel and brain extracellular microenvironment. The method uses a precisely defined source of fluorescent molecules pressure ejected from a micropipette, and a detailed theory of the intensity contributions from out-of-focus molecules in a three-dimensional medium to a two-dimensional image. Dextrans tagged with either tetramethylrhodamine or Texas Red were ejected into 0.3% agarose gel or rat cortical slices maintained in a perfused chamber at 34 degrees C and imaged using a compound epifluorescent microscope with a 10 x water-immersion objective. About 20 images were taken at 2-10-s intervals, recorded with a cooled CCD camera, then transferred to a 486 PC for quantitative analysis. The diffusion coefficient in agarose gel, D, and the apparent diffusion coefficient, D*, in brain tissue were determined by fitting an integral expression relating the measured two-dimensional image intensity to the theoretical three-dimensional dextran concentration. The measurements in dilute agarose gel provided a reference value of D and validated the method. Values of the tortuosity, lambda = (D/D*)1/2, for the 3- and 10-kDa dextrans were 1.70 and 1.63, respectively, which were consistent with previous values derived from tetramethylammonium measurements in cortex. Tortuosities for the 40- and 70-kDa dextrans had significantly larger values of 2.16 and 2.25, respectively. This suggests that the extracellular space may have local constrictions that hinder the diffusion of molecules above a critical size that lies in the range of many neurotrophic compounds.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K., STEERE R. L. Restricted diffusion of macromolecules through agar-gel membranes. Biochim Biophys Acta. 1962 May 7;59:137–149. doi: 10.1016/0006-3002(62)90704-7. [DOI] [PubMed] [Google Scholar]
- Agard D. A. Optical sectioning microscopy: cellular architecture in three dimensions. Annu Rev Biophys Bioeng. 1984;13:191–219. doi: 10.1146/annurev.bb.13.060184.001203. [DOI] [PubMed] [Google Scholar]
- Aggarwal S. J., Shah S. J., Diller K. R., Baxter C. R. Fluorescence digital microscopy of interstitial macromolecular diffusion in burn injury. Comput Biol Med. 1989;19(4):245–261. doi: 10.1016/0010-4825(89)90012-7. [DOI] [PubMed] [Google Scholar]
- Basser P. J. Interstitial pressure, volume, and flow during infusion into brain tissue. Microvasc Res. 1992 Sep;44(2):143–165. doi: 10.1016/0026-2862(92)90077-3. [DOI] [PubMed] [Google Scholar]
- Chary S. R., Jain R. K. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5385–5389. doi: 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr H. F., DePasquale M., Nicholson C., Patlak C. S., Pettigrew K. D., Rice M. E. Extracellular volume decreases while cell volume is maintained by ion uptake in rat brain during acute hypernatremia. J Physiol. 1991 Oct;442:277–295. doi: 10.1113/jphysiol.1991.sp018793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenstermacher J., Kaye T. Drug "diffusion" within the brain. Ann N Y Acad Sci. 1988;531:29–39. doi: 10.1111/j.1749-6632.1988.tb31809.x. [DOI] [PubMed] [Google Scholar]
- Fox J. R., Wayland H. Interstitial diffusion of macromolecules in the rat mesentery. Microvasc Res. 1979 Sep;18(2):255–276. doi: 10.1016/0026-2862(79)90033-5. [DOI] [PubMed] [Google Scholar]
- Granath K. A., Kvist B. E. Molecular weight distribution analysis by gel chromatography on Sephadex. J Chromatogr. 1967 May;28(1):69–81. doi: 10.1016/s0021-9673(01)85930-6. [DOI] [PubMed] [Google Scholar]
- Henry B. T., Adler J., Hibberd S., Cheema M. S., Davis S. S., Rogers T. G. Epi-fluorescence microscopy and image analysis used to measure diffusion coefficients in gel systems. J Pharm Pharmacol. 1992 Jul;44(7):543–549. doi: 10.1111/j.2042-7158.1992.tb05461.x. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y., Sedat J. W., Agard D. A. Determination of three-dimensional imaging properties of a light microscope system. Partial confocal behavior in epifluorescence microscopy. Biophys J. 1990 Feb;57(2):325–333. doi: 10.1016/S0006-3495(90)82534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R. K., Stock R. J., Chary S. R., Rueter M. Convection and diffusion measurements using fluorescence recovery after photobleaching and video image analysis: in vitro calibration and assessment. Microvasc Res. 1990 Jan;39(1):77–93. doi: 10.1016/0026-2862(90)90060-5. [DOI] [PubMed] [Google Scholar]
- Järnefelt J., Laurent T., Rigler R. Diffusion of fluorescein-labelled molecules in suspensions of erythrocyte ghosts. FEBS Lett. 1988 Dec 19;242(1):129–133. doi: 10.1016/0014-5793(88)81000-7. [DOI] [PubMed] [Google Scholar]
- Laurent T. C., Sundelöf L. O., Wik K. O., Wärmegård B. Diffusion of dextran in concentrated solutions. Eur J Biochem. 1976 Sep;68(1):95–102. doi: 10.1111/j.1432-1033.1976.tb10767.x. [DOI] [PubMed] [Google Scholar]
- Lehmenkühler A., Syková E., Svoboda J., Zilles K., Nicholson C. Extracellular space parameters in the rat neocortex and subcortical white matter during postnatal development determined by diffusion analysis. Neuroscience. 1993 Jul;55(2):339–351. doi: 10.1016/0306-4522(93)90503-8. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K., Castle P. E., Taylor D. L., Lanni F. Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4910–4913. doi: 10.1073/pnas.84.14.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K., Lanni F., Taylor D. L. The submicroscopic properties of cytoplasm as a determinant of cellular function. Annu Rev Biophys Biophys Chem. 1988;17:369–396. doi: 10.1146/annurev.bb.17.060188.002101. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K. Preparation of fluorescently labeled dextrans and ficolls. Methods Cell Biol. 1989;29:59–73. doi: 10.1016/s0091-679x(08)60187-9. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K., Taylor D. L., Lanni F. Probing the structure of cytoplasm. J Cell Biol. 1986 Jun;102(6):2015–2022. doi: 10.1083/jcb.102.6.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbaek J. A., Hansen A. J. Brain interstitial volume fraction and tortuosity in anoxia. Evaluation of the ion-selective micro-electrode method. Acta Physiol Scand. 1992 Dec;146(4):473–484. doi: 10.1111/j.1748-1716.1992.tb09449.x. [DOI] [PubMed] [Google Scholar]
- Margolis R. U., Aquino D. A., Klinger M. M., Ripellino J. A., Margolis R. K. Structure and localization of nervous tissue proteoglycans. Ann N Y Acad Sci. 1986;481:46–54. doi: 10.1111/j.1749-6632.1986.tb27138.x. [DOI] [PubMed] [Google Scholar]
- McBain C. J., Traynelis S. F., Dingledine R. Regional variation of extracellular space in the hippocampus. Science. 1990 Aug 10;249(4969):674–677. doi: 10.1126/science.2382142. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Wayland H. Macromolecular transport in the cat mesentery. Microvasc Res. 1975 Jan;9(1):1–21. doi: 10.1016/0026-2862(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985 May 6;333(2):325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Ion-selective microelectrodes and diffusion measurements as tools to explore the brain cell microenvironment. J Neurosci Methods. 1993 Jul;48(3):199–213. doi: 10.1016/0165-0270(93)90092-6. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Phillips J. M., Gardner-Medwin A. R. Diffusion from an iontophoretic point source in the brain: role of tortuosity and volume fraction. Brain Res. 1979 Jun 29;169(3):580–584. doi: 10.1016/0006-8993(79)90408-6. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Phillips J. M. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol. 1981 Dec;321:225–257. doi: 10.1113/jphysiol.1981.sp013981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C. Quantitative analysis of extracellular space using the method of TMA+ iontophoresis and the issue of TMA+ uptake. Can J Physiol Pharmacol. 1992;70 (Suppl):S314–S322. doi: 10.1139/y92-278. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Rice M. E. Calcium diffusion in the brain cell microenvironment. Can J Physiol Pharmacol. 1987 May;65(5):1086–1091. doi: 10.1139/y87-170. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Rice M. E. The migration of substances in the neuronal microenvironment. Ann N Y Acad Sci. 1986;481:55–71. doi: 10.1111/j.1749-6632.1986.tb27139.x. [DOI] [PubMed] [Google Scholar]
- Nugent L. J., Jain R. K. Extravascular diffusion in normal and neoplastic tissues. Cancer Res. 1984 Jan;44(1):238–244. [PubMed] [Google Scholar]
- Nugent L. J., Jain R. K. Plasma pharmacokinetics and interstitial diffusion of macromolecules in a capillary bed. Am J Physiol. 1984 Jan;246(1 Pt 2):H129–H137. doi: 10.1152/ajpheart.1984.246.1.H129. [DOI] [PubMed] [Google Scholar]
- OGSTON A. G., WOODS E. F. Molecular configuration of dextrans in aqueous solution. Nature. 1953 Jan 31;171(4344):221–222. doi: 10.1038/171221a0. [DOI] [PubMed] [Google Scholar]
- Rice M. E., Nicholson C. Diffusion characteristics and extracellular volume fraction during normoxia and hypoxia in slices of rat neostriatum. J Neurophysiol. 1991 Feb;65(2):264–272. doi: 10.1152/jn.1991.65.2.264. [DOI] [PubMed] [Google Scholar]
- SCHANTZ E. J., LAUFFER M. A. Diffusion measurements in agar gel. Biochemistry. 1962 Jul;1:658–663. doi: 10.1021/bi00910a019. [DOI] [PubMed] [Google Scholar]
- Schmitt F. O. Molecular regulators of brain function: a new view. Neuroscience. 1984 Dec;13(4):991–1001. doi: 10.1016/0306-4522(84)90283-5. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Syková E. Extracellular space volume changes in the rat spinal cord produced by nerve stimulation and peripheral injury. Brain Res. 1991 Sep 27;560(1-2):216–224. doi: 10.1016/0006-8993(91)91235-s. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Wiederhelm C. A., Shaw M. L., Kehl T. H., Fox J. R. A digital system for studying interstitial transport of dye molecules. Microvasc Res. 1973 May;5(3):243–250. doi: 10.1016/0026-2862(73)90033-2. [DOI] [PubMed] [Google Scholar]
- Yae H., Elias S. A., Ebner T. J. Deblurring of 3-dimensional patterns of evoked rat cerebellar cortical activity: a study using voltage-sensitive dyes and optical sectioning. J Neurosci Methods. 1992 May;42(3):195–209. doi: 10.1016/0165-0270(92)90099-y. [DOI] [PubMed] [Google Scholar]