Abstract
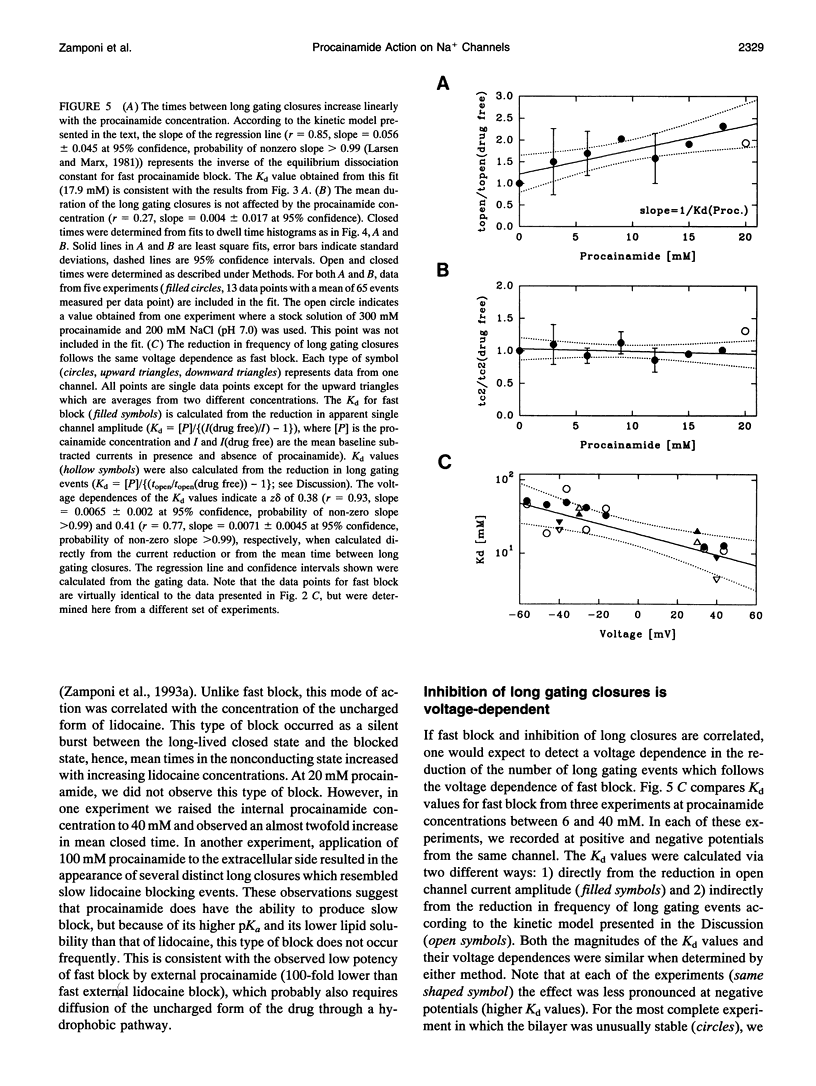
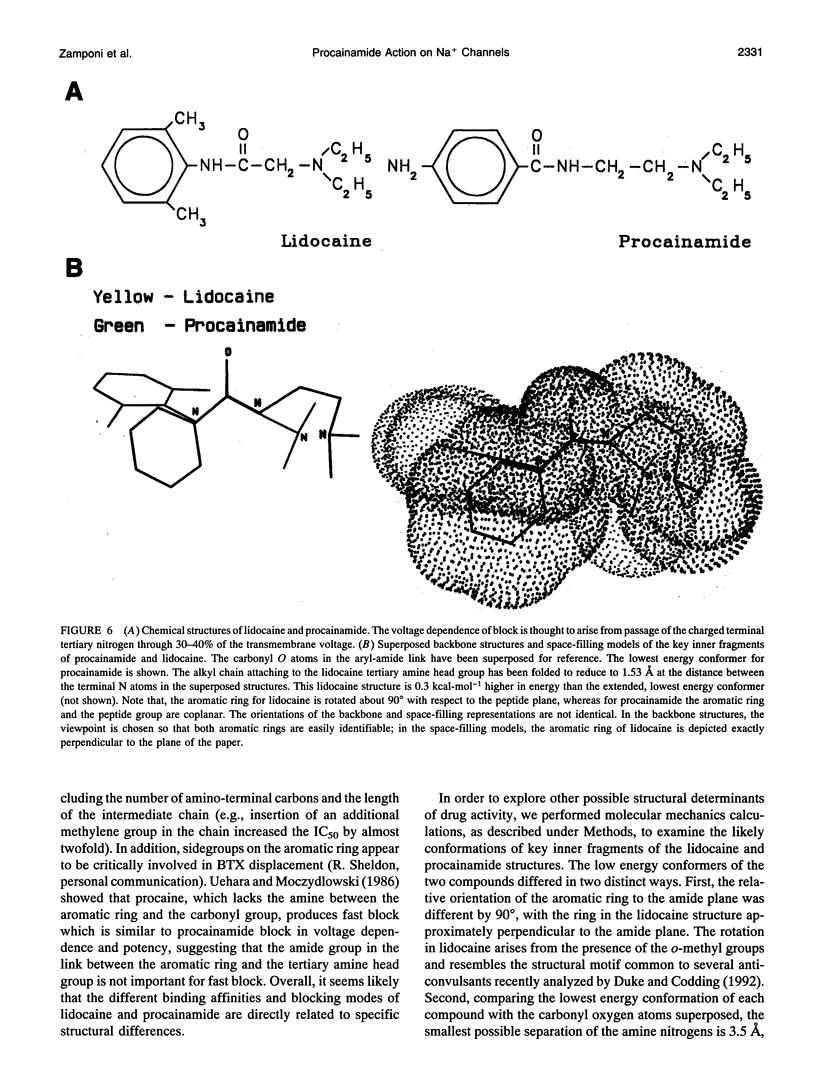
We have investigated the action of procainamide on batrachotoxin (BTX)-activated sodium channels from bovine heart and rat skeletal muscle. When applied to the intracellular side, procainamide induced rapid, open-channel block. We estimated rate constants using amplitude distribution analysis (Yellen, G. 1984. J. Gen. Physiol. 84:157). Membrane depolarization increased the blocking rate and slowed unblock. The rate constants were similar in both magnitude and voltage dependence for cardiac and skeletal muscle channels. Qualitatively, this block resembled the fast open-channel block by lidocaine (Zamponi, G. W., D. D. Doyle, and R. J. French. 1993. Biophys. J. 65:80), but procainamide was about sevenfold less potent. Molecular modeling suggests that the difference in potency between procainamide and lidocaine might arise from the relative orientation of their aromatic rings, or from differences in the structure of the aryl-amine link. For the cardiac channels, procainamide reduced the frequency of transitions to a long-lived closed state which shows features characteristic of inactivation (Zamponi, G. W., D. D. Doyle, and R. J. French. 1993. Biophys J. 65:91). Mean durations of kinetically identified closed states were not affected. The degree of fast block and of inhibition of the slow closures were correlated. Internally applied QX-314, a lidocaine derivative and also a fast blocker, produced a similar effect. Thus, drug binding to the fast blocking site appears to inhibit inactivation in BTX-activated cardiac channels.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert L. A., Fozzard H. A., Hanck D. A., Makielski J. C. Is there a second external lidocaine binding site on mammalian cardiac cells? Am J Physiol. 1989 Jul;257(1 Pt 2):H79–H84. doi: 10.1152/ajpheart.1989.257.1.H79. [DOI] [PubMed] [Google Scholar]
- Arnsdorf M. F., Bigger J. T., Jr The effect of procaine amide on components of excitability in long mammalian cardiac Purkinje fibers. Circ Res. 1976 Feb;38(2):115–122. doi: 10.1161/01.res.38.2.115. [DOI] [PubMed] [Google Scholar]
- Baumgarten C. M., Makielski J. C., Fozzard H. A. External site for local anesthetic block of cardiac Na+ channels. J Mol Cell Cardiol. 1991 Feb;23 (Suppl 1):85–93. doi: 10.1016/0022-2828(91)90027-j. [DOI] [PubMed] [Google Scholar]
- Carmeliet E., Saikawa T. Shortening of the action potential and reduction of pacemaker activity by lidocaine, quinidine, and procainamide in sheep cardiac purkinje fibers. An effect on Na or K currents? Circ Res. 1982 Feb;50(2):257–272. doi: 10.1161/01.res.50.2.257. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Ogden D. C. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988 Jan;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A. M., Latorre R., Bezanilla F. Ion permeation in normal and batrachotoxin-modified Na+ channels in the squid giant axon. J Gen Physiol. 1991 Mar;97(3):605–625. doi: 10.1085/jgp.97.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K. R. Comparative actions of mexiletine on sodium channels in nerve, skeletal and cardiac muscle. Eur J Pharmacol. 1981 Aug 27;74(1):9–18. doi: 10.1016/0014-2999(81)90317-4. [DOI] [PubMed] [Google Scholar]
- Courtney K. R. Quantifying antiarrhythmic drug blocking during action potentials in guinea-pig papillary muscle. J Mol Cell Cardiol. 1983 Nov;15(11):749–757. doi: 10.1016/0022-2828(83)90334-6. [DOI] [PubMed] [Google Scholar]
- Duke N. E., Codding P. W. Molecular modeling and crystallographic studies of 4-amino-N-phenylbenzamide anticonvulsants. J Med Chem. 1992 May 15;35(10):1806–1812. doi: 10.1021/jm00088a016. [DOI] [PubMed] [Google Scholar]
- Ehring G. R., Moyer J. W., Hondeghem L. M. Quantitative structure activity studies of antiarrhythmic properties in a series of lidocaine and procainamide derivatives. J Pharmacol Exp Ther. 1988 Feb;244(2):479–492. [PubMed] [Google Scholar]
- Garber S. S., Miller C. Single Na+ channels activated by veratridine and batrachotoxin. J Gen Physiol. 1987 Mar;89(3):459–480. doi: 10.1085/jgp.89.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. J., Duff H. J., Sheldon R. S. Determinants of stereospecific binding of type I antiarrhythmic drugs to cardiac sodium channels. Mol Pharmacol. 1988 Nov;34(5):659–663. [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The pH-dependent rate of action of local anesthetics on the node of Ranvier. J Gen Physiol. 1977 Apr;69(4):475–496. doi: 10.1085/jgp.69.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorov B. I., Peganov E. M., Revenko S. V., Shishkova L. D. Sodium currents in voltage clamped nerve fiber of frog under the combined action of batrachotoxin and procaine. Brain Res. 1975 Feb 14;84(3):541–546. doi: 10.1016/0006-8993(75)90771-4. [DOI] [PubMed] [Google Scholar]
- Krueger B. K., Worley J. F., 3rd, French R. J. Single sodium channels from rat brain incorporated into planar lipid bilayer membranes. Nature. 1983 May 12;303(5913):172–175. doi: 10.1038/303172a0. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- Postma S. W., Catterall W. A. Inhibition of binding of [3H]batrachotoxinin A 20-alpha-benzoate to sodium channels by local anesthetics. Mol Pharmacol. 1984 Mar;25(2):219–227. [PubMed] [Google Scholar]
- Quandt F. N., Narahashi T. Modification of single Na+ channels by batrachotoxin. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6732–6736. doi: 10.1073/pnas.79.21.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M. R., Gelband H., Hoffman B. F. Canine electrocardiographic and cardiac electrophysiologic changes induced by procainamide. Circulation. 1972 Sep;46(3):528–536. doi: 10.1161/01.cir.46.3.528. [DOI] [PubMed] [Google Scholar]
- Rosen M. R., Merker C., Gelband H., Hoffman B. F. Effects of procaine amide on the electrophysiologic properties of the canine ventricular conducting system. J Pharmacol Exp Ther. 1973 Jun;185(3):438–446. [PubMed] [Google Scholar]
- Sada H., Kojima M., Ban T. Effect of procainamide on transmembrane action potentials in guinea-pig papillary muscles as affected by external potassium concentration. Naunyn Schmiedebergs Arch Pharmacol. 1979 Nov;309(2):179–190. doi: 10.1007/BF00501227. [DOI] [PubMed] [Google Scholar]
- Sheldon R. S., Cannon N. J., Duff H. J. A receptor for type I antiarrhythmic drugs associated with rat cardiac sodium channels. Circ Res. 1987 Oct;61(4):492–497. doi: 10.1161/01.res.61.4.492. [DOI] [PubMed] [Google Scholar]
- Sheldon R. S., Hill R. J., Taouis M., Wilson L. M. Aminoalkyl structural requirements for interaction of lidocaine with the class I antiarrhythmic drug receptor on rat cardiac myocytes. Mol Pharmacol. 1991 May;39(5):609–614. [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G. R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973 Jul;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G. Molecular mechanisms of nerve block by local anesthetics. Anesthesiology. 1976 Oct;45(4):421–441. doi: 10.1097/00000542-197610000-00012. [DOI] [PubMed] [Google Scholar]
- Uehara A., Moczydlowski E. Blocking mechanisms of batrachotoxin-activated Na channels in artificial bilayers. Membr Biochem. 1986;6(2):111–147. doi: 10.3109/09687688609065446. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. Effects of calcium ions and local anesthetics on electrical properties of Purkinje fibres. J Physiol. 1955 Sep 28;129(3):568–582. doi: 10.1113/jphysiol.1955.sp005379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderlin W. F., Finkel A., French R. J. Optimizing planar lipid bilayer single-channel recordings for high resolution with rapid voltage steps. Biophys J. 1990 Aug;58(2):289–297. doi: 10.1016/S0006-3495(90)82376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G. W., Doyle D. D., French R. J. Fast lidocaine block of cardiac and skeletal muscle sodium channels: one site with two routes of access. Biophys J. 1993 Jul;65(1):80–90. doi: 10.1016/S0006-3495(93)81042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G. W., Doyle D. D., French R. J. State-dependent block underlies the tissue specificity of lidocaine action on batrachotoxin-activated cardiac sodium channels. Biophys J. 1993 Jul;65(1):91–100. doi: 10.1016/S0006-3495(93)81043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G. W., French R. J. Dissecting lidocaine action: diethylamide and phenol mimic separate modes of lidocaine block of sodium channels from heart and skeletal muscle. Biophys J. 1993 Dec;65(6):2335–2347. doi: 10.1016/S0006-3495(93)81292-X. [DOI] [PMC free article] [PubMed] [Google Scholar]