Abstract
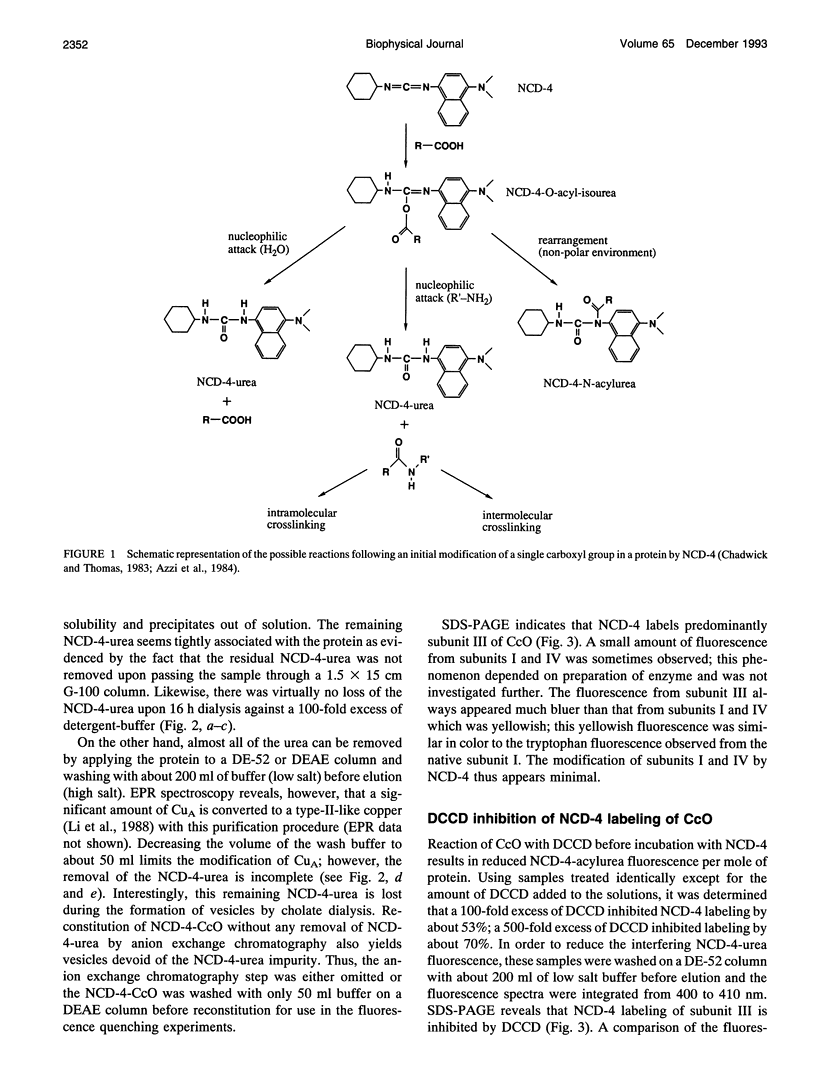
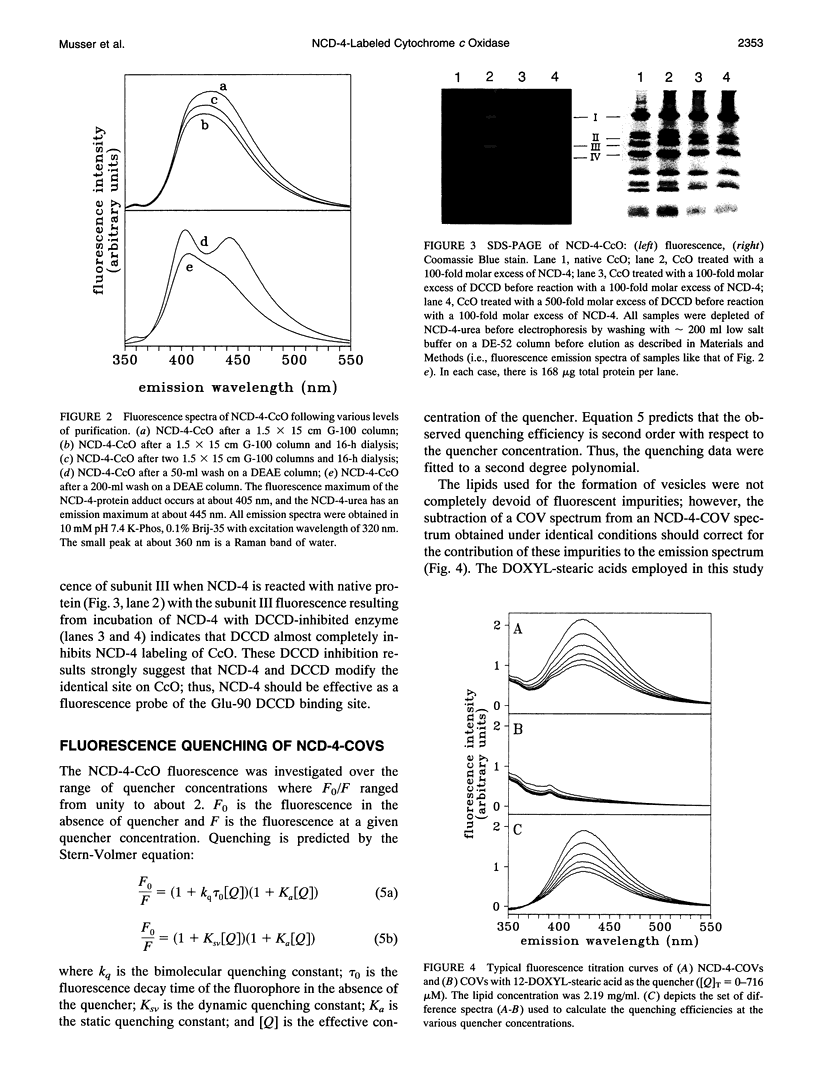
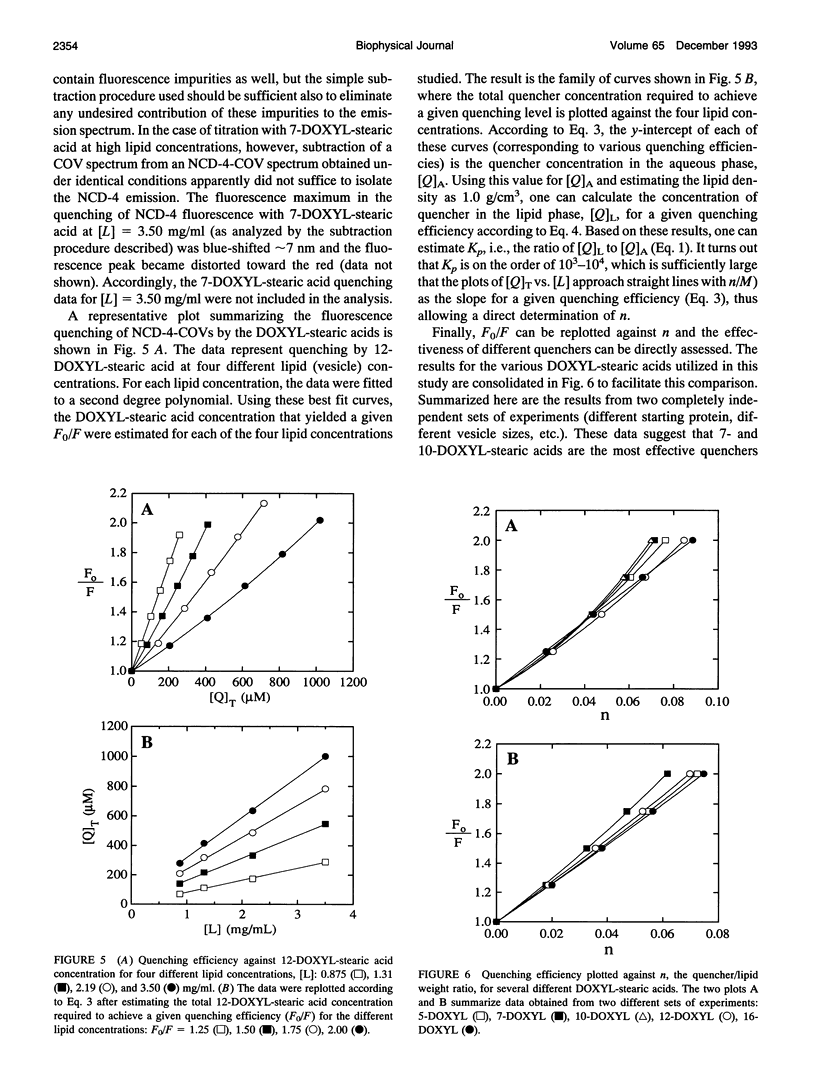
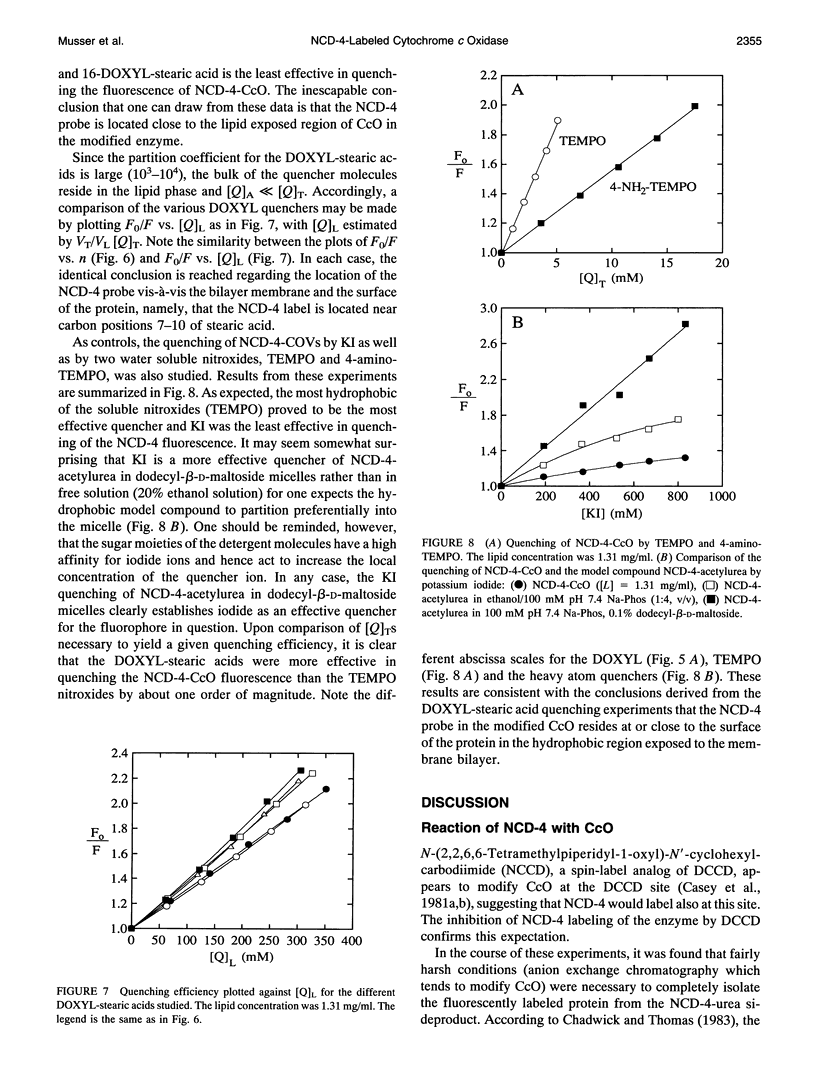
It has been known for some time that dicyclohexylcarbodiimide (DCCD) inhibits the proton translocation function of the cytochrome c oxidase complex (CcO) and that there is one major site in subunit III which is modified upon reaction with DCCD (Glu-90 for the bovine enzyme). We have examined the reaction of bovine CcO with N-cyclohexyl-N'-(4-dimethylamino-alpha-napthyl)carbodiimide (NCD-4), a fluorescent analog of DCCD. NCD-4 labeling of CcO is strongly inhibited by DCCD implicating Glu-90 of subunit III as the site of chemical modification by NCD-4. The fluorescence of reconstituted NCD-4-labeled bovine CcO is strongly quenched by hydrophobic nitroxides, whereas hydrophilic nitroxides and iodide ions have a reduced quenching ability. It is concluded that the Glu-90 of subunit III resides near the protein-lipid interface of the membrane spanning region of the enzyme. Different quenching abilities of 5-, 7-, 10-, 12-, and 16-4,4-dimethyl-3-oxazolinyloxy-stearic acids suggest that the NCD-4 label is located in the membrane bilayer in the region near the middle of the hydrocarbon tail of stearic acid. In light of these results, it is unlikely that Glu-90 is part of a proton channel that is associated with the proton pumping machinery of the enzyme but the outcome of this study does not eliminate an allosteric regulatory role for this residue.
Full text
PDF




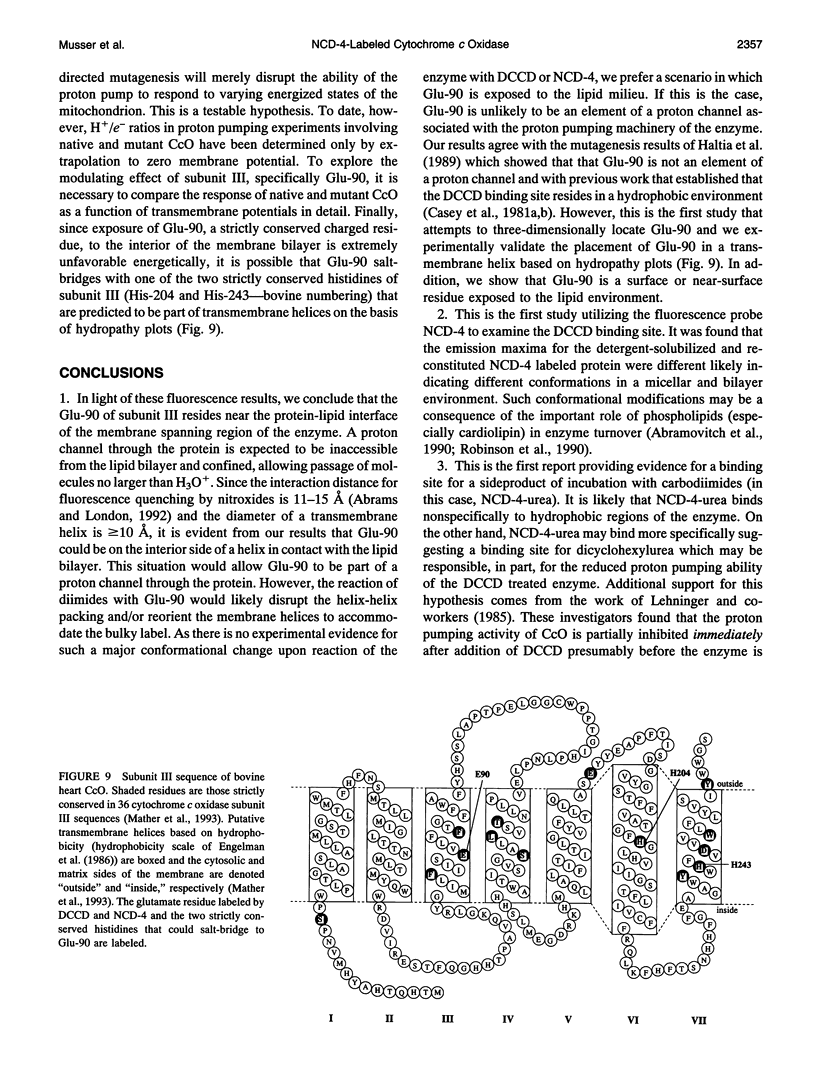


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitch D. A., Marsh D., Powell G. L. Activation of beef-heart cytochrome c oxidase by cardiolipin and analogues of cardiolipin. Biochim Biophys Acta. 1990 Oct 24;1020(1):34–42. doi: 10.1016/0005-2728(90)90090-q. [DOI] [PubMed] [Google Scholar]
- Abrams F. S., London E. Calibration of the parallax fluorescence quenching method for determination of membrane penetration depth: refinement and comparison of quenching by spin-labeled and brominated lipids. Biochemistry. 1992 Jun 16;31(23):5312–5322. doi: 10.1021/bi00138a010. [DOI] [PubMed] [Google Scholar]
- Azzi A., Casey R. P., Nałecz M. J. The effect of N,N'-dicyclohexylcarbodiimide on enzymes of bioenergetic relevance. Biochim Biophys Acta. 1984 Dec 17;768(3-4):209–226. doi: 10.1016/0304-4173(84)90017-x. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Callahan P. M. Redox-linked hydrogen bond strength changes in cytochrome a: implications for a cytochrome oxidase proton pump. Biochemistry. 1983 May 10;22(10):2314–2319. doi: 10.1021/bi00279a002. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Wikström M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992 Mar 26;356(6367):301–309. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- Brunori M., Antonini G., Colosimo A., Malatesta F., Sarti P., Jones M. G., Wilson M. T. Stopped-flow studies of cytochrome oxidase reconstituted into liposomes: proton pumping and control of activity. J Inorg Biochem. 1985 Mar-Apr;23(3-4):373–379. doi: 10.1016/0162-0134(85)85048-0. [DOI] [PubMed] [Google Scholar]
- Brunori M., Antonini G., Malatesta F., Sarti P., Wilson M. T. Cytochrome-c oxidase. Subunit structure and proton pumping. Eur J Biochem. 1987 Nov 16;169(1):1–8. doi: 10.1111/j.1432-1033.1987.tb13572.x. [DOI] [PubMed] [Google Scholar]
- Buse G., Steffens G. C. Cytochrome c oxidase in Paracoccus denitrificans. Protein, chemical, structural, and evolutionary aspects. J Bioenerg Biomembr. 1991 Apr;23(2):269–289. doi: 10.1007/BF00762222. [DOI] [PubMed] [Google Scholar]
- Casey R. P., Broger C., Azzi A. Structural studies on the cytochrome c oxidase proton pump using a spin-label probe. Biochim Biophys Acta. 1981 Nov 12;638(1):86–93. doi: 10.1016/0005-2728(81)90189-4. [DOI] [PubMed] [Google Scholar]
- Casey R. P., Broger C., Thelen M., Azzi A. Studies on the molecular basis of H+ translocation by cytochrome c oxidase. J Bioenerg Biomembr. 1981 Dec;13(5-6):219–228. doi: 10.1007/BF00743201. [DOI] [PubMed] [Google Scholar]
- Casey R. P., Chappell J. B., Azzi A. Limited-turnover studies on proton translocation in reconstituted cytochrome c oxidase-containing vesicles. Biochem J. 1979 Jul 15;182(1):149–156. doi: 10.1042/bj1820149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey R. P., Thelen M., Azzi A. Dicyclohexylcarbodiimide binds specifically and covalently to cytochrome c oxidase while inhibiting its H+-translocating activity. J Biol Chem. 1980 May 10;255(9):3994–4000. [PubMed] [Google Scholar]
- Casey R. P., Thelen M., Azzi A. Dicyclohexylcarbodiimide inhibits proton translocation by cytochrome c oxidase. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1044–1051. doi: 10.1016/s0006-291x(79)80013-3. [DOI] [PubMed] [Google Scholar]
- Chadwick C. C., Thomas E. W. Inactivation of sarcoplasmic reticulum (Ca2+ + Mg2+)-ATPase by N-cyclohexyl-N'-(4-dimethylamino-alpha-naphthyl)carbodiimide. Biochim Biophys Acta. 1983 May 5;730(2):201–206. doi: 10.1016/0005-2736(83)90334-6. [DOI] [PubMed] [Google Scholar]
- Chan S. I., Li P. M. Cytochrome c oxidase: understanding nature's design of a proton pump. Biochemistry. 1990 Jan 9;29(1):1–12. doi: 10.1021/bi00453a001. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Finel M., Wikström M. Studies on the role of the oligomeric state and subunit III of cytochrome oxidase in proton translocation. Biochim Biophys Acta. 1986 Aug 13;851(1):99–108. doi: 10.1016/0005-2728(86)90253-7. [DOI] [PubMed] [Google Scholar]
- Gelles J., Blair D. F., Chan S. I. The proton-pumping site of cytochrome c oxidase: a model of its structure and mechanism. Biochim Biophys Acta. 1986;853(3-4):205–236. doi: 10.1016/0304-4173(87)90002-4. [DOI] [PubMed] [Google Scholar]
- Haltia T., Finel M., Harms N., Nakari T., Raitio M., Wikström M., Saraste M. Deletion of the gene for subunit III leads to defective assembly of bacterial cytochrome oxidase. EMBO J. 1989 Dec 1;8(12):3571–3579. doi: 10.1002/j.1460-2075.1989.tb08529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltia T., Saraste M., Wikström M. Subunit III of cytochrome c oxidase is not involved in proton translocation: a site-directed mutagenesis study. EMBO J. 1991 Aug;10(8):2015–2021. doi: 10.1002/j.1460-2075.1991.tb07731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell C. R., Beinert H. Components of cytochrome c oxidase detectable by EPR spectroscopy. Biochim Biophys Acta. 1974 Dec 19;368(3):318–338. doi: 10.1016/0005-2728(74)90178-9. [DOI] [PubMed] [Google Scholar]
- Hinkle P. C., Kim J. J., Racker E. Ion transport and respiratory control in vesicles formed from cytochrome oxidase and phospholipids. J Biol Chem. 1972 Feb 25;247(4):1338–1339. [PubMed] [Google Scholar]
- Kadenbach B., Stroh A., Hüther F. J., Reimann A., Steverding D. Evolutionary aspects of cytochrome c oxidase. J Bioenerg Biomembr. 1991 Apr;23(2):321–334. doi: 10.1007/BF00762225. [DOI] [PubMed] [Google Scholar]
- Larsen R. W., Pan L. P., Musser S. M., Li Z. Y., Chan S. I. Could CuB be the site of redox linkage in cytochrome c oxidase? Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):723–727. doi: 10.1073/pnas.89.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger A. L., Reynafarje B., Costa L. Action of DCCD on the H+/O stoichiometry of mitoplast cytochrome c oxidase. J Inorg Biochem. 1985 Mar-Apr;23(3-4):335–340. doi: 10.1016/0162-0134(85)85043-1. [DOI] [PubMed] [Google Scholar]
- Li P. M., Morgan J. E., Nilsson T., Ma M., Chan S. I. Heat treatment of cytochrome c oxidase perturbs the CuA site and affects proton pumping behavior. Biochemistry. 1988 Sep 20;27(19):7538–7546. doi: 10.1021/bi00419a054. [DOI] [PubMed] [Google Scholar]
- Mather M. W., Springer P., Hensel S., Buse G., Fee J. A. Cytochrome oxidase genes from Thermus thermophilus. Nucleotide sequence of the fused gene and analysis of the deduced primary structures for subunits I and III of cytochrome caa3. J Biol Chem. 1993 Mar 15;268(8):5395–5408. [PubMed] [Google Scholar]
- Matko J., Ohki K., Edidin M. Luminescence quenching by nitroxide spin labels in aqueous solution: studies on the mechanism of quenching. Biochemistry. 1992 Jan 28;31(3):703–711. doi: 10.1021/bi00118a010. [DOI] [PubMed] [Google Scholar]
- Mitchell P. A new redox loop formality involving metal-catalysed hydroxide-ion translocation. A hypothetical Cu loop mechanism for cytochrome oxidase. FEBS Lett. 1987 Oct 5;222(2):235–245. doi: 10.1016/0014-5793(87)80378-2. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Mitchell R., Moody A. J., West I. C., Baum H., Wrigglesworth J. M. Chemiosmotic coupling in cytochrome oxidase. Possible protonmotive O loop and O cycle mechanisms. FEBS Lett. 1985 Aug 19;188(1):1–7. doi: 10.1016/0014-5793(85)80863-2. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Possible protonmotive osmochemistry in cytochrome oxidase. Ann N Y Acad Sci. 1988;550:185–198. doi: 10.1111/j.1749-6632.1988.tb35335.x. [DOI] [PubMed] [Google Scholar]
- Müller M., Azzi A. Cytochrome c oxidase metal centers: location and function. J Bioenerg Biomembr. 1991 Apr;23(2):291–302. doi: 10.1007/BF00762223. [DOI] [PubMed] [Google Scholar]
- Pringle M. J., Taber M. Fluorescent analogues of N,N'-dicyclohexylcarbodiimide as structural probes of the bovine mitochondrial proton channel. Biochemistry. 1985 Dec 3;24(25):7366–7371. doi: 10.1021/bi00346a051. [DOI] [PubMed] [Google Scholar]
- Prochaska L. J., Bisson R., Capaldi R. A., Steffens G. C., Buse G. Inhibition of cytochrome c oxidase function by dicyclohexylcarbodiimide. Biochim Biophys Acta. 1981 Sep 14;637(2):360–373. doi: 10.1016/0005-2728(81)90175-4. [DOI] [PubMed] [Google Scholar]
- Prochaska L. J., Fink P. S. On the role of subunit III in proton translocation in cytochrome c oxidase. J Bioenerg Biomembr. 1987 Apr;19(2):143–166. doi: 10.1007/BF00762722. [DOI] [PubMed] [Google Scholar]
- Prochaska L. J., Reynolds K. A. Characterization of electron-transfer and proton-translocation activities in bovine heart mitochondrial cytochrome c oxidase deficient in subunit III. Biochemistry. 1986 Feb 25;25(4):781–787. doi: 10.1021/bi00352a007. [DOI] [PubMed] [Google Scholar]
- Proteau G., Wrigglesworth J. M., Nicholls P. Protonmotive functions of cytochrome c oxidase in reconstituted vesicles. Influence of turnover rate on 'proton translocation'. Biochem J. 1983 Jan 15;210(1):199–205. doi: 10.1042/bj2100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puettner I., Carafoli E., Malatesta F. Spectroscopic and functional properties of subunit III-depleted cytochrome oxidase. J Biol Chem. 1985 Mar 25;260(6):3719–3723. [PubMed] [Google Scholar]
- Robinson N. C., Zborowski J., Talbert L. H. Cardiolipin-depleted bovine heart cytochrome c oxidase: binding stoichiometry and affinity for cardiolipin derivatives. Biochemistry. 1990 Sep 25;29(38):8962–8969. doi: 10.1021/bi00490a012. [DOI] [PubMed] [Google Scholar]
- Saraste M. Structural features of cytochrome oxidase. Q Rev Biophys. 1990 Nov;23(4):331–366. doi: 10.1017/s0033583500005588. [DOI] [PubMed] [Google Scholar]
- Sarti P., Jones M. G., Antonini G., Malatesta F., Colosimo A., Wilson M. T., Brunori M. Kinetics of redox-linked proton pumping activity of native and subunit III-depleted cytochrome c oxidase: a stopped-flow investigation. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4876–4880. doi: 10.1073/pnas.82.15.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E., Carafoli E. Quantitative analysis of the proton and charge stoichiometry of cytochrome c oxidase from beef heart reconstituted into phospholipid vesicles. Eur J Biochem. 1980 Oct;111(2):299–306. doi: 10.1111/j.1432-1033.1980.tb04942.x. [DOI] [PubMed] [Google Scholar]
- Thelen M., O'Shea P. S., Petrone G., Azzi A. Proton translocation by a native and subunit III-depleted cytochrome c oxidase reconstituted into phospholipid vesicles. Use of fluorescein-phosphatidylethanolamine as an intravesicular pH indicator. J Biol Chem. 1985 Mar 25;260(6):3626–3631. [PubMed] [Google Scholar]
- Thompson D. A., Gregory L., Ferguson-Miller S. Cytochrome c oxidase depleted of subunit III: proton-pumping, respiratory control, and pH dependence of the midpoint potential of cytochrome a. J Inorg Biochem. 1985 Mar-Apr;23(3-4):357–364. doi: 10.1016/0162-0134(85)85046-7. [DOI] [PubMed] [Google Scholar]
- Wikstrom M. K. Proton pump coupled to cytochrome c oxidase in mitochondria. Nature. 1977 Mar 17;266(5599):271–273. doi: 10.1038/266271a0. [DOI] [PubMed] [Google Scholar]
- Wikström M., Casey R. P. What is the essential proton-translocating molecular machinery in cytochrome oxidase? J Inorg Biochem. 1985 Mar-Apr;23(3-4):327–334. doi: 10.1016/0162-0134(85)85042-x. [DOI] [PubMed] [Google Scholar]
- Wikström M. How does cytochrome oxidase pump protons? A "cooperative proton pump" model. Ann N Y Acad Sci. 1988;550:199–206. doi: 10.1111/j.1749-6632.1988.tb35336.x. [DOI] [PubMed] [Google Scholar]
- Wikström M. Identification of the electron transfers in cytochrome oxidase that are coupled to proton-pumping. Nature. 1989 Apr 27;338(6218):776–778. doi: 10.1038/338776a0. [DOI] [PubMed] [Google Scholar]
- Woodruff W. H., Einarsdóttir O., Dyer R. B., Bagley K. A., Palmer G., Atherton S. J., Goldbeck R. A., Dawes T. D., Kliger D. S. Nature and functional implications of the cytochrome a3 transients after photodissociation of CO-cytochrome oxidase. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2588–2592. doi: 10.1073/pnas.88.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Verseveld H. W., Krab K., Stouthamer A. H. Proton pump coupled to cytochrome c oxidase in Paracoccus denitrificans. Biochim Biophys Acta. 1981 May 13;635(3):525–534. doi: 10.1016/0005-2728(81)90111-0. [DOI] [PubMed] [Google Scholar]



