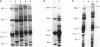Abstract
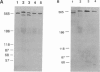
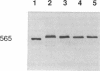
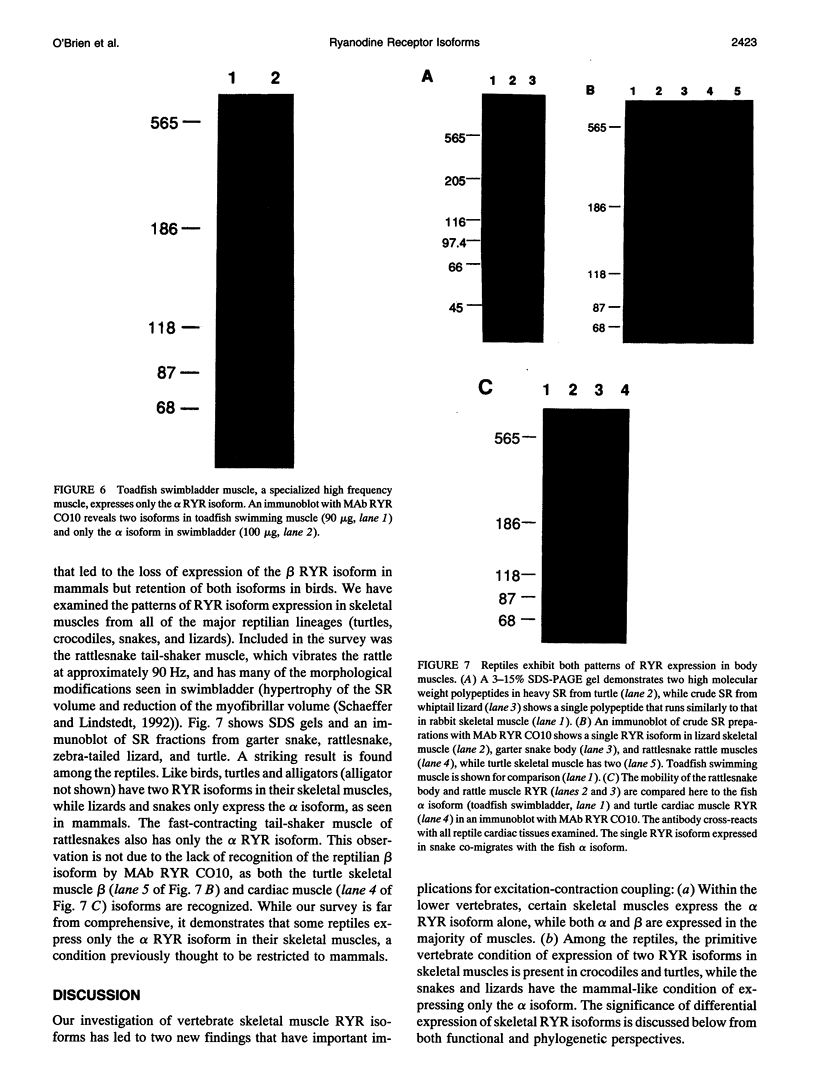
The skeletal muscles of chickens, frogs, and fish have been reported to express two isoforms (alpha and beta) of the sarcoplasmic reticulum calcium release channel (ryanodine receptor or RYR), while mammals express only one. We have studied patterns of RYR isoform expression in skeletal muscles from a variety of fish, reptiles, and birds with immunological techniques. Immunoblot analysis with a monoclonal antibody that recognizes both nonmammalian RYR isoforms and a polyclonal antibody specific to the alpha isoform show two key results: (a) two reptilian orders share with mammals the pattern of expressing only the alpha (skeletal) RYR isoform in skeletal muscle; and (b) certain functionally specialized muscles of fish and birds express only the alpha RYR isoforms. While both isoforms are expressed in the body musculature of fish and birds, the alpha isoform is expressed alone in extraocular muscles and swimbladder muscles. The appearance of the alpha RYR isoform alone in the extraocular muscles and a fast-contracting sonic muscle in fish (toadfish swimbladder muscle) provides evidence that this isoform is selectively expressed when rapid contraction is required. The functional and phylogenetic implications of expression of the alpha isoform alone are discussed in the context of the mechanism and evolution of excitation-contraction coupling.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. A., Tanabe T., Mikami A., Numa S., Beam K. G. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature. 1990 Aug 9;346(6284):569–572. doi: 10.1038/346569a0. [DOI] [PubMed] [Google Scholar]
- Airey J. A., Beck C. F., Murakami K., Tanksley S. J., Deerinck T. J., Ellisman M. H., Sutko J. L. Identification and localization of two triad junctional foot protein isoforms in mature avian fast twitch skeletal muscle. J Biol Chem. 1990 Aug 25;265(24):14187–14194. [PubMed] [Google Scholar]
- Airey J. A., Rogers M. J., Sutko J. L. Use of a reversible polyacrylamide gel cross-linker in western blotting for rapid transfer of a wide size range of polypeptides. Biotechniques. 1991 May;10(5):605–608. [PubMed] [Google Scholar]
- Anderson K., Lai F. A., Liu Q. Y., Rousseau E., Erickson H. P., Meissner G. Structural and functional characterization of the purified cardiac ryanodine receptor-Ca2+ release channel complex. J Biol Chem. 1989 Jan 15;264(2):1329–1335. [PubMed] [Google Scholar]
- Bach-y-Rita P., Ito F. In vivo studies on fast and slow muscle fibers in cat extraocular muscles. J Gen Physiol. 1966 Jul;49(6):1177–1198. doi: 10.1085/jgp.0491177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Knudson C. M., Powell J. A. A lethal mutation in mice eliminates the slow calcium current in skeletal muscle cells. Nature. 1986 Mar 13;320(6058):168–170. doi: 10.1038/320168a0. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Block B. A., Imagawa T., Campbell K. P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988 Dec;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Chu A., Díaz-Muñoz M., Hawkes M. J., Brush K., Hamilton S. L. Ryanodine as a probe for the functional state of the skeletal muscle sarcoplasmic reticulum calcium release channel. Mol Pharmacol. 1990 May;37(5):735–741. [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formelová J., Hurnák O., Novotová M., Zachar J. Ryanodine receptor purified from crayfish skeletal muscle. Gen Physiol Biophys. 1990 Oct;9(5):445–453. [PubMed] [Google Scholar]
- GORDON G., PHILLIPS C. G. Slow and rapid components in a flexor muscle. Q J Exp Physiol Cogn Med Sci. 1953;38(1):35–45. doi: 10.1113/expphysiol.1953.sp001005. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- Györke S., Palade P. Calcium-induced calcium release in crayfish skeletal muscle. J Physiol. 1992 Nov;457:195–210. doi: 10.1113/jphysiol.1992.sp019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN E., OLSON C. B. RELATIONS BETWEEN STRUCTURE AND FUNCTION IN THE DESIGN OF SKELETAL MUSCLES. J Neurophysiol. 1965 May;28:581–598. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]
- Hakamata Y., Nakai J., Takeshima H., Imoto K. Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett. 1992 Nov 9;312(2-3):229–235. doi: 10.1016/0014-5793(92)80941-9. [DOI] [PubMed] [Google Scholar]
- Herrmann-Frank A., Darling E., Meissner G. Functional characterization of the Ca(2+)-gated Ca2+ release channel of vascular smooth muscle sarcoplasmic reticulum. Pflugers Arch. 1991 May;418(4):353–359. doi: 10.1007/BF00550873. [DOI] [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- Jacquemond V., Csernoch L., Klein M. G., Schneider M. F. Voltage-gated and calcium-gated calcium release during depolarization of skeletal muscle fibers. Biophys J. 1991 Oct;60(4):867–873. doi: 10.1016/S0006-3495(91)82120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. K., Valdivia H. H., Maryon E. B., Anderson P., Coronado R. High molecular weight proteins in the nematode C. elegans bind [3H]ryanodine and form a large conductance channel. Biophys J. 1992 Nov;63(5):1379–1384. doi: 10.1016/S0006-3495(92)81702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Liu Q. Y., Xu L., el-Hashem A., Kramarcy N. R., Sealock R., Meissner G. Amphibian ryanodine receptor isoforms are related to those of mammalian skeletal or cardiac muscle. Am J Physiol. 1992 Aug;263(2 Pt 1):C365–C372. doi: 10.1152/ajpcell.1992.263.2.C365. [DOI] [PubMed] [Google Scholar]
- Lennerstrand G., Baker R. Motoneuronal innervation and mechanical properties of extraocular muscles in the catfish, (Ictalurus punctatus). Acta Physiol Scand. 1987 Nov;131(3):361–369. doi: 10.1111/j.1748-1716.1987.tb08251.x. [DOI] [PubMed] [Google Scholar]
- Marks A. R., Tempst P., Hwang K. S., Taubman M. B., Inui M., Chadwick C., Fleischer S., Nadal-Ginard B. Molecular cloning and characterization of the ryanodine receptor/junctional channel complex cDNA from skeletal muscle sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8683–8687. doi: 10.1073/pnas.86.22.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G., Rousseau E., Lai F. A. Structural and functional correlation of the trypsin-digested Ca2+ release channel of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1989 Jan 25;264(3):1715–1722. [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Murayama T., Ogawa Y. Purification and characterization of two ryanodine-binding protein isoforms from sarcoplasmic reticulum of bullfrog skeletal muscle. J Biochem. 1992 Oct;112(4):514–522. doi: 10.1093/oxfordjournals.jbchem.a123931. [DOI] [PubMed] [Google Scholar]
- Nakai J., Imagawa T., Hakamat Y., Shigekawa M., Takeshima H., Numa S. Primary structure and functional expression from cDNA of the cardiac ryanodine receptor/calcium release channel. FEBS Lett. 1990 Oct 1;271(1-2):169–177. doi: 10.1016/0014-5793(90)80399-4. [DOI] [PubMed] [Google Scholar]
- Näbauer M., Callewaert G., Cleemann L., Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989 May 19;244(4906):800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- Olivares E. B., Tanksley S. J., Airey J. A., Beck C. F., Ouyang Y., Deerinck T. J., Ellisman M. H., Sutko J. L. Nonmammalian vertebrate skeletal muscles express two triad junctional foot protein isoforms. Biophys J. 1991 Jun;59(6):1153–1163. doi: 10.1016/S0006-3495(91)82331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu K., Willard H. F., Khanna V. K., Zorzato F., Green N. M., MacLennan D. H. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem. 1990 Aug 15;265(23):13472–13483. [PubMed] [Google Scholar]
- Pessah I. N., Stambuk R. A., Casida J. E. Ca2+-activated ryanodine binding: mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol Pharmacol. 1987 Mar;31(3):232–238. [PubMed] [Google Scholar]
- Putnam R. W., Bennett A. F. Histochemical, enzymatic, and contractile proper of skeletal muscles of three anuran amphibians. Am J Physiol. 1983 Apr;244(4):R558–R567. doi: 10.1152/ajpregu.1983.244.4.R558. [DOI] [PubMed] [Google Scholar]
- Ridge R. M. Different types of extrafusal muscle fibres in snake costocutaneous muscles. J Physiol. 1971 Sep;217(2):393–418. doi: 10.1113/jphysiol.1971.sp009578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol Rev. 1991 Jul;71(3):849–908. doi: 10.1152/physrev.1991.71.3.849. [DOI] [PubMed] [Google Scholar]
- Seok J. H., Xu L., Kramarcy N. R., Sealock R., Meissner G. The 30 S lobster skeletal muscle Ca2+ release channel (ryanodine receptor) has functional properties distinct from the mammalian channel proteins. J Biol Chem. 1992 Aug 5;267(22):15893–15901. [PubMed] [Google Scholar]
- Stewart C. B. The powers and pitfalls of parsimony. Nature. 1993 Feb 18;361(6413):603–607. doi: 10.1038/361603a0. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Airey J. A., Murakami K., Takeda M., Beck C., Deerinck T., Ellisman M. H. Foot protein isoforms are expressed at different times during embryonic chick skeletal muscle development. J Cell Biol. 1991 May;113(4):793–803. doi: 10.1083/jcb.113.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Adams B. A., Niidome T., Numa S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature. 1990 Aug 9;346(6284):567–569. doi: 10.1038/346567a0. [DOI] [PubMed] [Google Scholar]