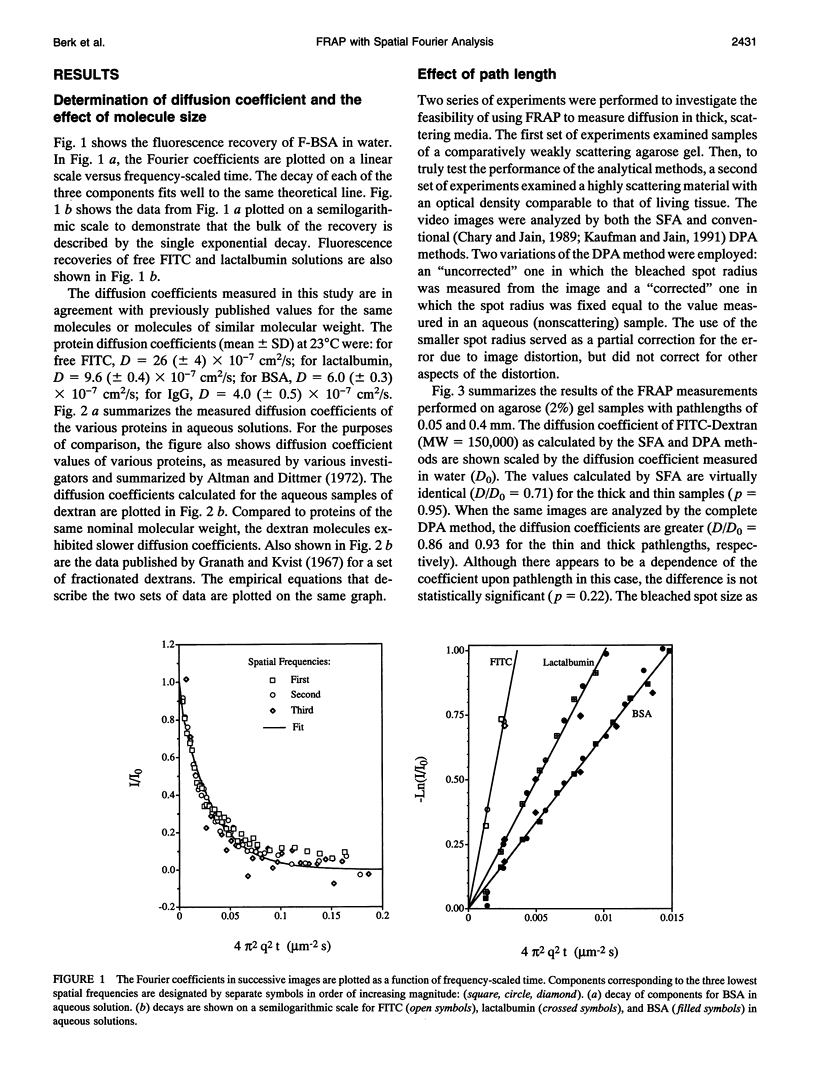
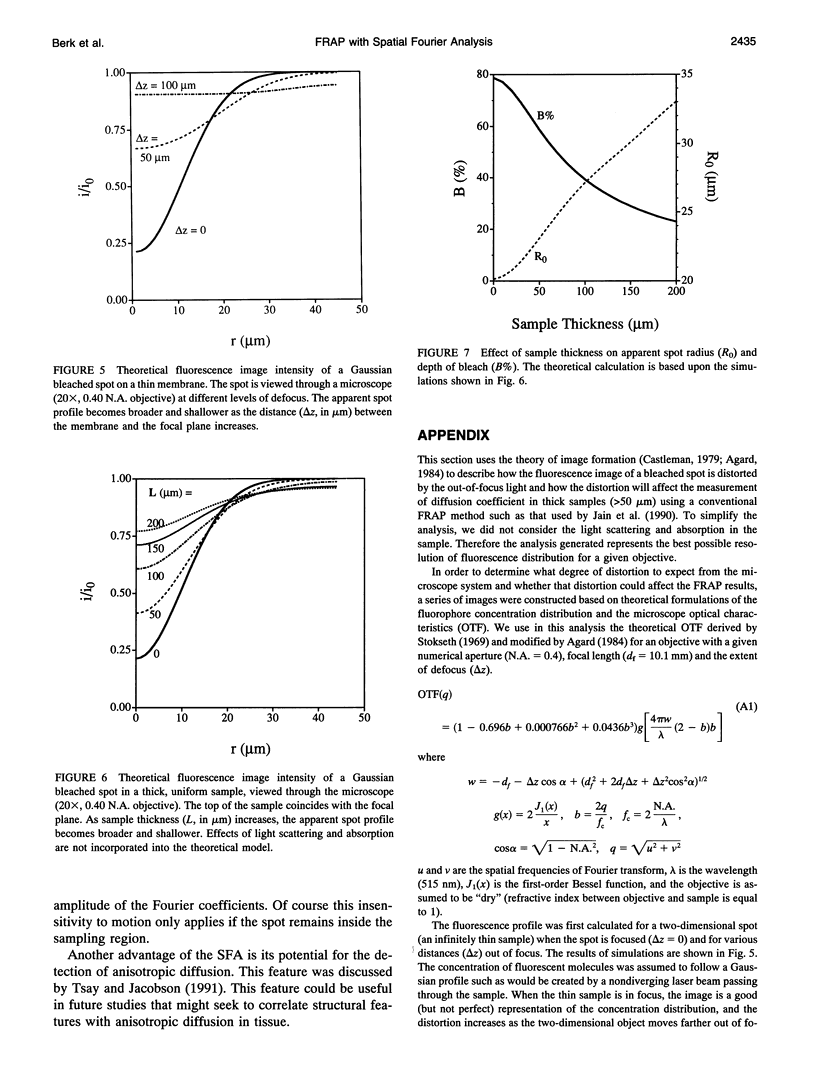
Abstract
A new method for the measurement of diffusion in thick samples is introduced, based upon the spatial Fourier analysis of Tsay and Jacobson (Biophys. J. 60: 360-368, 1991) for the video image analysis of fluorescence recovery after photobleaching (FRAP). In this approach, the diffusion coefficient is calculated from the decay of Fourier transform coefficients in successive fluorescence images. Previously, the application of FRAP in thick samples has been confounded by the optical effects of out-of-focus light and scattering and absorption by the sample. The theory of image formation is invoked to show that the decay rate is the same for both the observed fluorescence intensity and the true concentration distribution in the tissue. The method was tested in a series of macromolecular diffusion measurements in aqueous solution, in agarose gel, and in simulated tissue consisting of tumor cells (45% v/v) and blood cells (5% v/v) in an agarose gel. For a range of fluorescently labeled proteins (MW = 14 to 600 kD) and dextrans (MW = 4.4 to 147.8 kD), the diffusion coefficients in aqueous solution were comparable to previously published values. A comparison of the spatial Fourier analysis with a conventional direct photometric method revealed that even for the weakly scattering agarose sample, the conventional method gives a result that is inaccurate and dependent on sample thickness whereas the diffusion coefficient calculated by the spatial Fourier method agreed with published values and was independent of sample thickness. The diffusion coefficient of albumin in the simulated tissue samples, as determined by the spatial Fourier analysis, varied slightly with sample thickness. In contrast, when the same video images were analyzed by direct photometric analysis, the calculated diffusion coefficients were grossly inaccurate and highly dependent on sample thickness. No simple correction could be devised to ensure the accuracy of the direct photometric method of analysis.These in vitro experiments demonstrate the advantage of our new analysis for obtaining an accurate measure of the local diffusion coefficient in microscopic samples that are thick (thickness greater than the microscope depth of focus) and scatter light.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K., STEERE R. L. Restricted diffusion of macromolecules through agar-gel membranes. Biochim Biophys Acta. 1962 May 7;59:137–149. doi: 10.1016/0006-3002(62)90704-7. [DOI] [PubMed] [Google Scholar]
- Agard D. A. Optical sectioning microscopy: cellular architecture in three dimensions. Annu Rev Biophys Bioeng. 1984;13:191–219. doi: 10.1146/annurev.bb.13.060184.001203. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chary S. R., Jain R. K. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5385–5389. doi: 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss M. A., Jain R. K. Interstitial transport of rabbit and sheep antibodies in normal and neoplastic tissues. Cancer Res. 1990 Jun 15;50(12):3487–3492. [PubMed] [Google Scholar]
- Fox J. R., Wayland H. Interstitial diffusion of macromolecules in the rat mesentery. Microvasc Res. 1979 Sep;18(2):255–276. doi: 10.1016/0026-2862(79)90033-5. [DOI] [PubMed] [Google Scholar]
- Granath K. A., Kvist B. E. Molecular weight distribution analysis by gel chromatography on Sephadex. J Chromatogr. 1967 May;28(1):69–81. doi: 10.1016/s0021-9673(01)85930-6. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y., Sedat J. W., Agard D. A. Determination of three-dimensional imaging properties of a light microscope system. Partial confocal behavior in epifluorescence microscopy. Biophys J. 1990 Feb;57(2):325–333. doi: 10.1016/S0006-3495(90)82534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Derzko Z., Wu E. S., Hou Y., Poste G. Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J Supramol Struct. 1976;5(4):565(417)–576(428). doi: 10.1002/jss.400050411. [DOI] [PubMed] [Google Scholar]
- Jain R. K., Baxter L. T. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 1988 Dec 15;48(24 Pt 1):7022–7032. [PubMed] [Google Scholar]
- Jain R. K., Stock R. J., Chary S. R., Rueter M. Convection and diffusion measurements using fluorescence recovery after photobleaching and video image analysis: in vitro calibration and assessment. Microvasc Res. 1990 Jan;39(1):77–93. doi: 10.1016/0026-2862(90)90060-5. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987 Jun 15;47(12):3039–3051. [PubMed] [Google Scholar]
- Kaufman E. N., Jain R. K. Measurement of mass transport and reaction parameters in bulk solution using photobleaching. Reaction limited binding regime. Biophys J. 1991 Sep;60(3):596–610. doi: 10.1016/S0006-3495(91)82089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURENT T. C., BJOERK I., PIETRUSZKIEWICZ A., PERSSON H. ON THE INTERACTION BETWEEN POLYSACCHARIDES AND OTHER MACROMOLECULES. II. THE TRANSPORT OF GLOBULAR PARTICLES THROUGH HYALURONIC ACID SOLUTIONS. Biochim Biophys Acta. 1963 Oct 29;78:351–359. doi: 10.1016/0006-3002(63)91645-7. [DOI] [PubMed] [Google Scholar]
- Lanni F., Taylor D. L., Ware B. R. Fluorescence photobleaching recovery in solutions of labeled actin. Biophys J. 1981 Aug;35(2):351–364. doi: 10.1016/S0006-3495(81)84794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaoui M., Benlyas M., Wahl P. Diffusion of proteins in Sepharose Cl-B gels. J Chromatogr. 1992 Feb 7;591(1-2):115–120. doi: 10.1016/0021-9673(92)80228-m. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Wayland H. Macromolecular transport in the cat mesentery. Microvasc Res. 1975 Jan;9(1):1–21. doi: 10.1016/0026-2862(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Nugent L. J., Jain R. K. Extravascular diffusion in normal and neoplastic tissues. Cancer Res. 1984 Jan;44(1):238–244. [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Raj T., Flygare W. H. Diffusion studies of bovine serum albumin by quasielastic light scattering. Biochemistry. 1974 Jul 30;13(16):3336–3340. doi: 10.1021/bi00713a024. [DOI] [PubMed] [Google Scholar]
- Tanke H. J., van Oostveldt P., van Duijn P. A parameter for the distribution of fluorophores in cells derived from measurements of inner filter effect and reabsorption phenomenon. Cytometry. 1982 May;2(6):359–369. doi: 10.1002/cyto.990020602. [DOI] [PubMed] [Google Scholar]
- Tsay T. T., Jacobson K. A. Spatial Fourier analysis of video photobleaching measurements. Principles and optimization. Biophys J. 1991 Aug;60(2):360–368. doi: 10.1016/S0006-3495(91)82061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yguerabide J., Schmidt J. A., Yguerabide E. E. Lateral mobility in membranes as detected by fluorescence recovery after photobleaching. Biophys J. 1982 Oct;40(1):69–75. doi: 10.1016/S0006-3495(82)84459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]




