Abstract
Here, we report the identification of Fc receptor homolog expressed in B cells (FREB), a unique B cell-specific molecule that is distantly related to FcγRI (receptor I for the Fc fragment of IgG) and is encoded on human chromosome 1q, within the FcγR gene region. FREB has an intracellular distribution and lacks a canonical transmembrane domain. In addition, FREB lacks bona fide Fc fragment binding regions and does not bind immunoglobulins. By using specific monoclonal antibodies, we show that FREB is preferentially expressed in germinal center centroblasts, which undergo affinity maturation and class-switch recombination. Together, these characteristics indicate that FREB may have a unique role in B cell differentiation. FREB is also expressed in some B cell lymphomas, most of which have centroblast origin. Remarkably, FREB is expressed in a subset of diffuse large B cell lymphomas, providing a unique marker for the characterization of this B cell malignancy.
Receptors for the Fc fragment of IgG (FcγR) are cell surface glycoproteins of the Ig-superfamily (Ig-SF; refs. 1 and 2). They include the high affinity receptor FcγRI (CD64) and the low affinity receptors FcγRII (CD32) and FcγRIII (CD16). FcγRs mediate phagocytosis of IgG-coated pathogens and promote activation of effector cells, leading to inflammatory responses and antibody-mediated cellular cytotoxicity (ADCC; refs. 1 and 2). One FcγRII isoform, called FcγRIIb, transmits inhibitory signals that promote the maintenance of peripheral tolerance, increase the threshold of activation responses, and, ultimately, terminate IgG-mediated effector stimulation (1, 2). All human FcγR genes map to chromosome 1q. Recent reports indicate that this chromosomal region harbors additional genes encoding FcγR homologs, called immunoglobulin-superfamily receptor translocation associated genes 1 and 2 (IRTA1 and –2; ref. 3) and Fc receptor homologs (FcRHs; ref. 4), which are selectively expressed in B cells and may be implicated in B cell development and lymphomagenesis (3). In this study, we searched cDNA databases for novel Ig-SF inhibitory receptors. We found a molecule that is similar to FcγRI and maps to human chromosome 1q, but is quite different in terms of primary structure, exclusive intracellular distribution, inability to bind immunoglobulins, and preferential expression in germinal center centroblasts.
Materials and Methods
Cloning of Fc Receptor Homolog Expressed in B Cells (FREB) cDNA.
GenBank human expressed sequence-tagged database (dbEST) was searched with the amino acid sequences of Ig-like transcript (ILT) 2–5 (5) and FcγRIIb (1, 2) by using the tblastn algorithm. An ORF encoding FREB was found in a contig assembled from 28 distinct cDNAs. FREB cDNA was amplified from Epstein–Barr virus (EBV)-transformed B cell RNA by reverse transcriptase–PCR. PCR primers were: 5′-gagaggtttcatgttgaaga, 3′-ctattcagcagtagcctttgtgg. An ORF encoding mouse FREB was found in a contig of seven mouse dbEST cDNAs. Human and mouse FREB cDNAs and related dbEST cDNAs are deposited in GenBank under accession nos. AF426461 and AF426462, respectively.
Production of FREB-HuIgG Fusion Protein and Anti-FREB mAb.
J558L mouse myeloma cells were engineered to secrete a chimeric protein consisting of FREB (from nucleotide 62 to nucleotide 865) and human IgG1 constant regions (FREB-HuIgG) as previously described (6). Anti-FREB mAbs N28.1 and N28.2 (mouse IgG1) were raised by immunizing BALB/c mice against FREB-HuIgG. Hybridoma supernatants were screened for their ability to bind FREB-HuIgG by immunoassay and to stain 293 cells in which FREB was targeted to the surface (see below).
Transfections.
FREB cDNA was subcloned into pCDNA3 (Invitrogen) and transiently expressed in 293 cells by using Lipofectin (Bethesda Research Laboratories). In Ig binding experiments, FREB was transiently expressed as a chimeric protein with two IgSF domains from rat CD4 (CD4d3 + 4). Fusion with rat CD4d3 + 4 has been previously shown to facilitate expression of several Ig-SF domains for ligand analysis (7). A construct encoding rat CD4 leader (CD4L) and rat CD4d3 + 4 was kindly provided by M. H. Brown (Oxford, U.K.; ref. 7). The XbaI-SalI fragment encoding CD4L was replaced with a FREB cDNA fragment encoding leader sequence, pre-Ig and Ig-SF domains (from nucleotide 62 to nucleotide 859) amplified from plasmid DNA. An XbaI site was introduced upstream of the initiation methionine of FREB. A SalI site was added at the end of the second Ig-SF domains of FREB. The junction at the SalI site (g tcg acc) with CD4d3 was SSSST (the amino acid residues containing the SalI site are underlined). To target FREB-CD4 to the cell surface, a fragment of FREB-CD4 chimeric gene encoding pre-Ig and Ig-SF FREB domains and rat CD4d3 + 4 domains was amplified by PCR and cloned into pDisplay (Invitrogen). The cDNA fragment was in-frame with the vector's N-terminal secretion signal and C-terminal transmembrane anchoring domain of platelet-derived growth factor receptor (PDGFR). FREB was also targeted to the cell surface by cloning FREB pre-Ig and Ig-SF domains into pDisplay (Invitrogen), in-frame with the vector's N-terminal secretion signal and the C-terminal transmembrane domain of PDGFR. However, FREB-CD4-PDGFR reached a higher level of cell surface expression than FREB-PDGFR alone (data not shown).
Immunoglobulin Binding Assay.
293 cells were transfected with either FREB-rat CD4-pDisplay or CD1a-pCDNA3 as control (kindly provided by P. Dellabona, Milan, Italy). Cell surface expression of FREB and CD1a was assessed by flow cytometry using anti-FREB (N28.1) and anti-CD1a (OKT6, American Type Culture Collection) mAbs. Transfected cells were incubated with human IgA, IgE, IgM, and IgG at concentrations ranging from 50 to 250 μg/ml. Human IgG and IgM derived from human serum (Sigma), human IgA from colostrum (Sigma), and human IgE from human myeloma serum (Calbiochem) were used. In some experiments, immunoglobulins were aggregated by incubation at 65°C for 30 min. After washing cells once with PBS-2% FCS, bound IgA and IgE were detected by flow cytometry using biotinylated mouse anti-human IgA and IgE mAbs (PharMingen) followed by allophycocyanin-conjugated streptavidine (Molecular Probes). Bound IgM and IgG were detected by using phycoerythrin-conjugated goat anti-human IgM and -IgG antibodies (Southern Biotechnology Associates).
Human Cells and Flow Cytometry.
Human B cells were purified from peripheral blood mononuclear cells by magnetic sorting using anti-CD19-coated magnetic beads (Miltenyi Biotec, Bergisch-Gladbach, Germany). Purified B cells were stimulated with inactivated Staphylococcus aureus (Pansorbin cells; Calbiochem) either in the absence or in the presence of IL-2, -3, -4, -6, -10, or -12 (R & D Systems). To detect intracellular expression, cells were fixed with 2% formaldehyde and permeabilized with 0.5% saponin before staining with N28.1 or N28.2 mAbs followed by a FITC- or phycoerythrin-conjugated goat-anti-mouse antibodies (Southern Biotechnology Associates). In confocal microscopy analysis, 293-transfected cells were counterstained with Texas-red-X Phalloidin (Molecular Probes) to detect actin cytoskeleton.
Tissue Specimens and Immunohistochemistry.
Immunohistochemistry was performed on cryostat sections from normal spleen, lymph nodes, tonsils, and thymus, as well as on a series of Hodgkin and non-Hodgkin's lymphomas (some provided by M. Chilosi, Verona, Italy). Sections were stained with anti-FREB N28.1 mAb, which was detected by the Labeled Streptavidin-Biotin (LSAB) procedure (Dako). Two-color immunofluorescence was performed combining anti-FREB N28.1 mAb (mouse IgG1) with anti-CD20 (IgG2a; Dako), anti-proliferating cell nuclear antigen (PCNA; IgG2a, Dako), or FITC-conjugated anti-human IgA, IgM, IgG, and IgD (Dako). FREB was identified by using Texas red-conjugated anti-mouse IgG1 (Southern Biotechnology Associates), whereas CD20 and PCNA were detected with a biotinylated anti-mouse IgG2a antibody followed by FITC-conjugated streptavidin (Southern Biotechnology Associates). Immunostained tissue sections were examined with the fluorescence microscope Olympus BX60.
Results
FREB Is a Member of the Ig-SF.
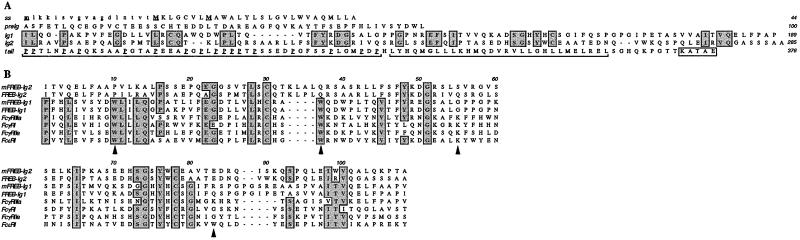
The GenBank human EST database was searched with Ig-SF inhibitory receptor polypeptides, including Ig-like transcripts (ILT; ref. 5) and FcγRIIb (1, 2). We found an ORF encoding a protein with highest similarity to FcγRI (29% identity; Fig. 1). A cDNA containing the entire ORF was amplified by reverse transcriptase–PCR from EBV-transformed B cells, but not from T lymphocytes, monocytes, neutrophils, or other cell types (data not shown). Therefore, we designated this molecule Fc receptor homolog expressed in B cells (FREB). Consistent with the selective expression in B cells, RNA blot analysis of multiple human tissues revealed a transcript of ≈2.3 kb only in the spleen (data not shown). FREB cDNA contains three potential initiating methionines (Fig. 1A). The second and/or the third methionine are most likely used, because they are directly followed by a hydrophobic peptide with the characteristics of a leader sequence. FREB continues with a hydrophilic peptide and two Ig-SF domains of the C2 type and ends with a long tail consisting of a proline-rich region and a leucine-rich region. The latter, however, does not generate a typical transmembrane region because it is frequently interrupted by charged amino acids (Fig. 1A). This leucine-rich sequence is a potentially coiled-coil region, which could mediate FREB oligomerization. FREB contains a C-terminal sequence that resembles the di-lysine (KKXX) and possibly the lysine-aspartic acid-glutamic acid-leucine (KDEL) motifs previously identified as endoplasmic reticulum retention signals (8). FREB lacks any consensus N-linked glycosylation sites. The predicted molecular mass of the protein is either 38.1 or 38.9 kDa, depending on the starting site.
Figure 1.
Predicted amino acid sequences of FREB. (A) FREB consists of a putative signal peptide (ss) followed by a hydrophilic peptide (pre-Ig) that precedes two Ig-SF domains (Ig1 and Ig2). FREB ends with a tail consisting of a proline-rich region, a leucine-rich region (both highlighted by brackets), and a putative endoplasmic reticulum retention signal (boxed). Potential starting methionines are marked in bold and underlined. The putative signal sequence after the second and third starting methionines is indicated in capital letters. Conserved residues between the two Ig-SF folds are boxed, including the cysteines potentially involved in generating the intrachain disulfide bridge of the Ig-SF fold. Proline residues in the tail are underlined. FREB lacks any N-linked glycosylation site. Human and mouse FREB sequences have been submitted to GenBank database under accession nos. AF426461 and AF426462, respectively. (B) Alignment of human and mouse FREB Ig-SF domains with the membrane proximal Ig-SF domains of human Fc receptors. Arrows indicate the four Fc receptor interface residues that are invariant among all human FcγRs. The pre-Ig domain has a limited sequence identity to a classical Ig-fold (data not shown), although the fold topology could not be adopted because of the deletion of several core elements.
Mutational and crystal structure analyses have shown that the Fc binding region of FcRs is primarily in the second, membrane proximal Ig-SF domain, and involves several residues located in both the BC and FG loops (9–12). Alignment of the Ig-SF domains of FREB and Fc receptors revealed that the FREB membrane proximal domain lacks an Fc binding interface (Fig. 1B). However, the FREB membrane distal domain contains two of the four interface residues that are invariant among all human FcγRs (Fig. 1B), suggesting a potential Fc binding capacity. The gene encoding FREB is located within the human chromosome 1q genomic sequence (accession no. NT_004668.7 Hs1_4825) centromeric to FcγRIIIa and FcγRIIb and telomeric to FcγRIIa genes. A cDNA identical to FREB was recently reported in the mammalian gene collection (accession no. NP_116127, protein designation MGC4595). By searching the GenBank mouse EST database, we found mouse FREB cDNA, which is 64% identical to its human counterpart and displays similar signal sequence, pre-Ig-SF peptide, Ig-SF domains and tail (Ig-SF domains are detailed in Fig. 1B). The C-terminal amino acid sequence is lysine-valine-alanine-aspartic acid-lysine (KVADK).
FREB Is a B Cell-Specific Intracellular Protein That Does Not Bind Immunoglobulins.
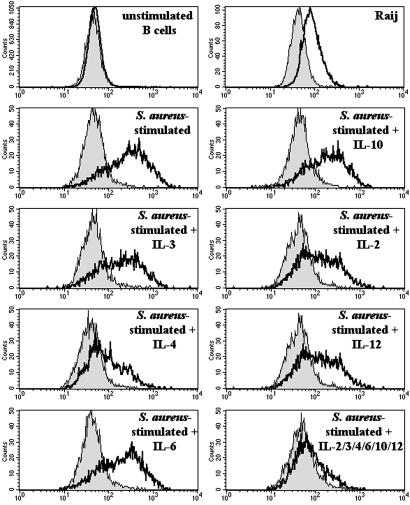
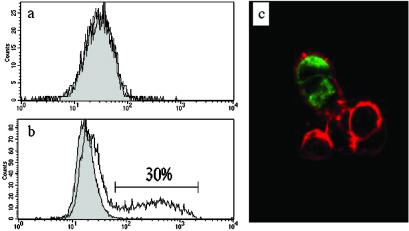
The absence of a putative transmembrane region suggested that FREB might have an intracellular distribution. To address this hypothesis, we produced two anti-FREB mAbs and stained B cells isolated from peripheral blood and EBV-transformed B cell lines. Peripheral B cells were negative for FREB expression by flow cytometry (Fig. 2). Stimulation of purified B cells with inactivated S. aureus induced intracellular expression of FREB, which was inhibited in the presence of IL-4 (Fig. 2). Exclusive intracellular expression of FREB was also observed in some EBV-transformed B cell lines, such as Raji (Fig. 2), and in 293 cells transfected with FREB cDNA (Fig. 3). We were unable to detect secretion of FREB by immunoassay of culture supernatants derived from FREB+ EBV-transformed B cells or FREB-transfected cells (data not shown). Thus, FREB has a predominant intracellular distribution.
Figure 2.
Intracellular expression of FREB in EBV-B cell lines and activated B cells. FREB is expressed in some EBV-B cell lines, such as Raji, but not in resting B cells purified from peripheral blood (Top). Activation of B cells with S. aureus results in intracellular expression, which is prevented by incubation of B cells with IL-4. Other cytokines, such as IL-2, IL-3, IL-6, IL-10, and IL-12 have no effect on the intracellular expression of FREB. FREB was detected by staining fixed and permeabilized cells with anti-FREB mAb (N28.2) followed by a phycoerythrin-labeled secondary antibody.
Figure 3.
Intracellular localization of FREB in transfected cells. 293 cells transiently transfected with full-length FREB cDNA were analyzed for cell surface (a) and intracellular (b) expression of FREB by staining with N28.1 mAb followed by a phycoerythrin-labeled secondary antibody. FREB is detectable only inside transfected cells (30% of 293 cells). Intracellular distribution of FREB is further demonstrated by confocal analysis of FREB-transfected cells (c). In this experiment, cells were stained with anti-FREB mAb (N28.1; green) and counterstained with Texas red-X Phalloidin (red), which stains actin, outlining the cytoskeleton underneath the cell surface.
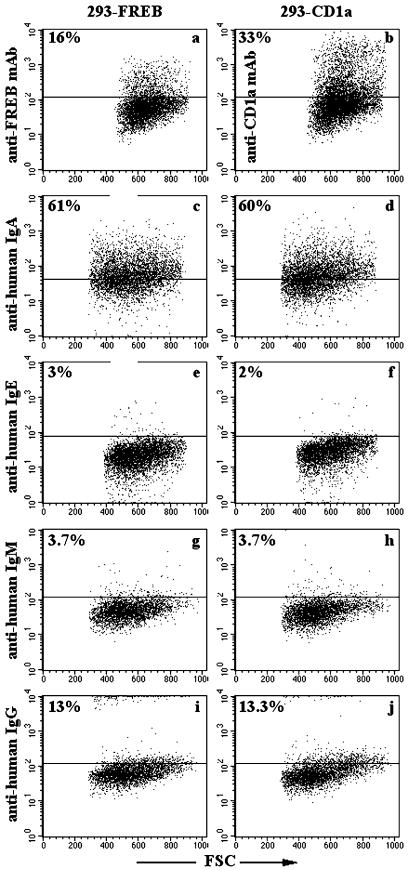
The similarity between FREB and FcγRI and the presence of potential Fc binding residues in the membrane distal domain of FREB prompted us to investigate whether FREB binds immunoglobulins. To test this, FREB was targeted to the cell surface of 293 cells by fusing FREB with two Ig-SF domains of rat CD4 and the transmembrane region of PDGFR. FREB-CD4-PDGFR-transfected 293 cells were incubated with human IgM, IgG, IgA, and IgE. In some experiments, immunoglobulins were aggregated by exposure to 65°C for 30 min before incubation with transfectants, to increase binding avidity. Under all conditions, flow cytometry of FREB-CD4-PDGFR-transfected cells revealed no specific immunoglobulins binding (Fig. 4 and data not shown). Furthermore, no binding of IgG, IgA, and IgM to soluble FREB-human IgG fusion protein was revealed by immunoassay (data not shown). The inability of FREB to bind immunoglobulins is consistent with the lack of a full set of conserved residues, which are critically involved in Fc fragment recognition by classical Fc receptors.
Figure 4.
Immunoglobulins do not bind FREB targeted to the cell surface of 293 cells. FREB was expressed on the cell surface of 293 cells as a FREB–CD4 fusion protein (Left). Control 293 cells were transfected with CD1a cDNA (Right). Cell surface expression of FREB and CD1a was confirmed by flow cytometry by using anti-FREB (a) and anti-CD1a (b) mAbs. Transfected cells were incubated with human IgA (c and d), IgE (e and f), IgM (g and h), and IgG (i and j), which had been previously aggregated by treatment at 65°C for 30 min. Immunoglobulins bound to transfected cells were detected by flow cytometry. Percentages of stained cells are indicated in the upper quadrants. Human IgA partially bound 293 cells, regardless of FREB expression (c and d). Cells stained only with the secondary antibodies fell within the lower quadrants. FSC, forward scatter.
FREB Is Preferentially Expressed in Germinal Center Centroblasts and in a Subset of Diffuse Large B Cell Lymphomas (DLBCLs).
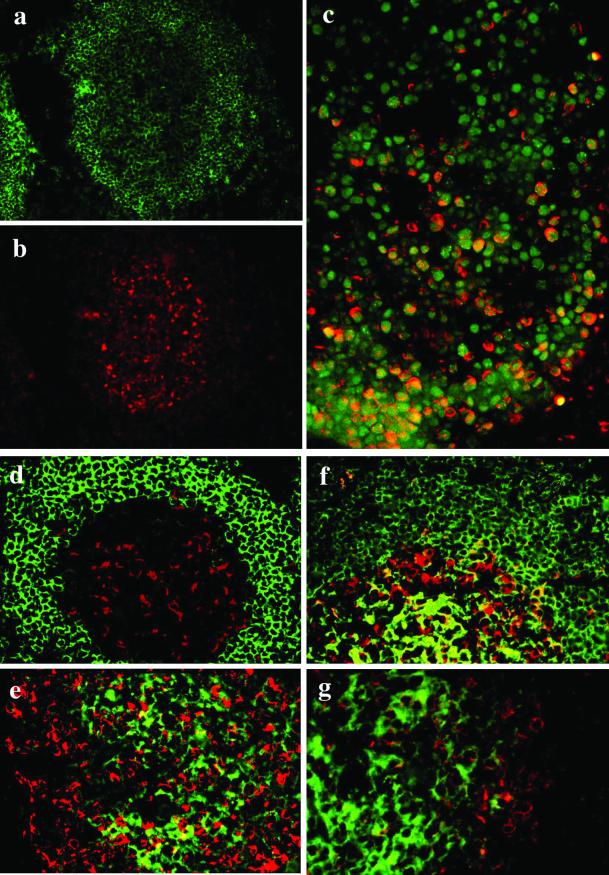
To investigate the function of FREB in B cells, we monitored its expression at different stages of B cell differentiation. Immunohistochemical analysis of lymphoid organs revealed strong and predominant expression of FREB in germinal centers, especially in large cells corresponding to centroblasts (Fig. 5). To further characterize FREB+ cells in germinal centers, we performed two-color immunofluorescence analysis with anti-FREB and either anti-IgD, -IgA, -IgM, and -IgG antibodies. Remarkably, expression of FREB and IgG, IgA, or IgD was mutually exclusive, and only 5–20% of IgM+ cells coexpressed FREB (Fig. 5). We also compared distribution of FREB with that of PCNA, a processivity factor for DNA polymerases that is expressed in actively dividing cells (13). All FREB+ cells coexpressed PCNA, indicating that FREB is expressed in proliferating centroblasts (Fig. 5). PCNA was also expressed in FREB− B cells, most of which corresponded to centrocytes (Fig. 5).
Figure 5.
Preferential expression of FREB in germinal center centroblasts. Stainings of a secondary follicle with anti-CD20 (a, green) and anti-FREB (b, red) show preferential location of FREB in large cells of the germinal center corresponding to centroblasts. (c) FREB (red) shows a perinuclear pattern of expression around the nuclear PCNA (green). Coexpression of PCNA and FREB is particularly evident at the base of the germinal center (bottom), where centroblasts are predominant. In the upper part of the germinal center, where centrocytes predominate, PCNA-positive cells are mostly FREB negative. (d–g) Expressions of FREB (red) and IgD (d), IgA (e), IgM (f), or IgG (g; green) are mutually exclusive. In f, only rare cells coexpress FREB and IgM.
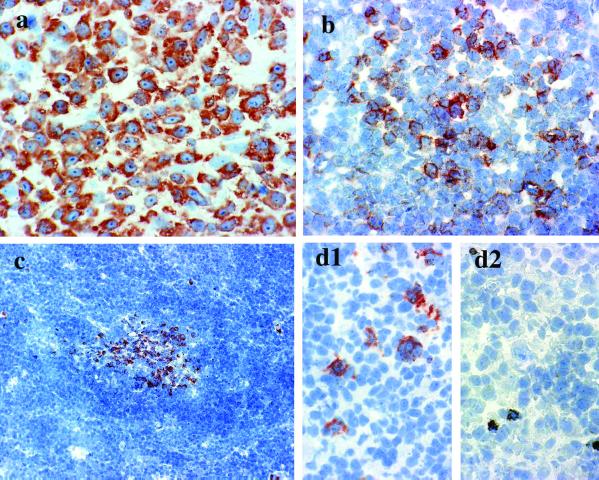
Analysis of FREB distribution in a large number of lymphomas revealed no expression in T cell lymphomas and weak or no expression in prefollicular B cell lymphomas (Table 1). In contrast, FREB was expressed in large cells of most of the examined follicular lymphomas (Fig. 6 and Table 1). Remarkably, FREB was found in cells of ≈70% of the DLBCLs examined, whereas the remaining 30% were entirely negative (Fig. 6 and Table 1). In Hodgkin's lymphomas, FREB was detectable only in cases of lymphocyte predominance (Fig. 6 and Table 1). Thus, analysis of lymphomas is consistent with the selective expression of FREB in centroblasts, and, more importantly, FREB expression divides DLBCL into two phenotypically distinct groups.
Table 1.
Expression of FREB in different types of lymphoid neoplasms
| Type | Positive/all |
|---|---|
| Hodgkin's lymphoma (HL) | |
| HL, classical* | 0/7 |
| Nodular lymphocyte-predominant | 2/2 +++ |
| Non-Hodgkin's lymphoma | |
| T cell lymphomas† | 0/7 |
| B cell lymphomas | |
| Small lymphocytic | 2/3 + |
| Mantle cell | 0/3 |
| Lymphoplasmacytic | 1/1 + |
| Follicular | |
| Grade I and II‡ | 3/5 +/++ |
| Grade III | 2/2 +++ |
| DLBCL | 25/35 |
| 13 +++ | |
| 6 ++ | |
| 6 + |
+, <10% cells positive. ++, <50% cells positive. +++, >50% cells positive.
Classical HL included nodular sclerosis (three cases), mixed cellularity (two cases), and lymphocyte-rich lymphomas (two cases).
T cell lymphomas included cutaneous epidermotropic T cell lymphoma (three cases), peripheral T-cell lymphoma (two cases), and anaplastic large cell lymphoma (two cases).
Immunoreactivity is particularly evident in large centroblasts.
Figure 6.
Expression of FREB in B cell lymphomas. FREB was found in a subset of DLBCL (a) and in the large cells of follicular lymphomas (b). Mantle cell lymphomas do not express FREB, with the exception of residual germinal center cells (c). FREB is expressed in Hodgkin's lymphoma (HL) with lymphocyte predominance (d1), whereas it is undetectable in Reed-Sternberg cells of HL with mixed cellularity (d2). Positive dots in d2 represent unblocked endogenous peroxidase in eosinophils.
Discussion
In summary, we have identified an Ig-SF molecule, FREB, which is similar to FcγRI but has intriguing features. FREB lacks a canonical transmembrane region and N-linked glycosylation sites, displays a long tail rich in prolines, and contains a C-terminal sequence that distantly resembles two endoplasmic reticulum retention motifs. These sequence features are most likely responsible for the exclusive intracellular expression of FREB, although mutational analysis of the FREB tail will be important to confirm this hypothesis. Whereas we did not detect FREB in culture supernatants of FREB+ EBV-transformed B cells, a potential secretion of FREB should not be entirely ruled out, because FREB displays a classical leader sequence. Particular stimuli may be required to induce FREB secretion by B cells. Despite amino acid sequence similarity with FcγRI, FREB membrane proximal domain lacks an Fc binding site, and the membrane distal domain contains only two of the four Fc binding residues that are conserved in all human FcγR. Indeed, we did not detect any significant binding of FREB-transfected 293 cells to human IgM, IgG, IgA, and IgE (Fig. 4 and data not shown). However, the binding affinity of FREB for immunoglobulins may be too low to be detected by flow cytometry. In addition, present results do not exclude a possible binding of FREB to IgD.
Another important feature of FREB is its preferential expression in germinal center centroblasts. On entering germinal centers, B cells down-regulate cell surface immunoglobulins, acquire a centroblastic morphology, and undergo affinity maturation by somatic hypermutation of the Ig variable region genes as well as class-switch recombination (14). After affinity maturation and class-switch recombination, B cells reexpress surface immunoglobulins, acquire a centrocitic morphology, and undergo negative selection (14). Thus, FREB identifies a stage of B cell differentiation that largely overlaps with somatic hypermutation and class-switch recombination. This unique pattern of expression, together with the intracellular localization and the apparent inability of binding immunoglobulins, suggests a unique yet unknown role of FREB in B cell differentiation. This conclusion is further supported by the observation that FREB expression is down-regulated by IL-4, a cytokine inducing class switch recombination to IgE. The identification of FREB, together with the recent discovery of IRTA1, IRTA2, and FcRH molecules (3, 4), indicates that mammals have evolved an array of Fc receptor-like molecules to fulfill functions other than Fc binding.
Many diagnostic tools are presently available for the classification, diagnosis, and prognosis of B cell lymphomas (15, 16). These tools include identification of B cell differentiation antigens and detection of DNA modifications such as gene rearrangements, translocations, and somatic hypermutations. Despite this progress, some lymphomas are poorly characterized and remain classified in heterogeneous groups (15, 16). This is the case for DLBCL, a malignancy affecting 25,000 patients per year, with highly variable clinical courses (16). The recent introduction of gene expression profiling by DNA microarrays has allowed the designation of two types of DLBCL, presumably derived from germinal center cells and activated B cells (17). Importantly, the two types correlate with different survival rates among patients. It will be important to establish whether there is a correlation between FREB+/FREB− lymphomas and the types distinguished by expression profiles. In any event, the differential expression of FREB in DLBCLs may further contribute to the diagnosis and prognosis of affected patients.
Acknowledgments
We thank Susan Gilfillan, Barry Sleckman, and Wayne M. Yokoyama for reviewing the manuscript, Chris Nelson for advice on the analysis of FREB primary structure, Marion H. Brown and Paolo Dellabona for plasmid DNAs, and Marco Chilosi for specimens of large B cell lymphomas. F.F. was partially supported by a grant from Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica (MURST).
Abbreviations
- FcγR
receptor for the Fc fragment of IgG
- Ig-SF
Ig-superfamily
- FREB
Fc receptor homolog expressed in B cells
- EBV
Epstein–Barr virus
- PDGFR
platelet-derived growth factor receptor
- EST
expressed sequence tag
- DLBCL
diffuse large B cell lymphoma
- PCNA
proliferating cell nuclear antigen
Note Added in Proof.
Mechetina et al. (18) have recently reported a molecule identical to FREB.
Footnotes
References
- 1.Ravetch J V, Bolland S. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 2.Daeron M. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 3.Hatzivassiliou G, Miller I, Takizawa J, Palanisamy N, Rao P H, Iida S, Tagawa S, Taniwaki M, Russo J, Neri A, et al. Immunity. 2001;14:277–289. doi: 10.1016/s1074-7613(01)00109-1. [DOI] [PubMed] [Google Scholar]
- 4.Davis R S, Wang Y H, Kubagawa H, Cooper M D. Proc Natl Acad Sci USA. 2001;98:9772–9777. doi: 10.1073/pnas.171308498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietrich J, Nakajima H, Colonna M. Microbes Infect. 2000;2:323–329. doi: 10.1016/s1286-4579(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 6.Traunecker A, Oliveri F, Karjalainen K. Trends Biotechnol. 1991;9:109–113. doi: 10.1016/0167-7799(91)90038-j. [DOI] [PubMed] [Google Scholar]
- 7.Brown M H, Barclay A N. Protein Eng. 1994;7:515–521. doi: 10.1093/protein/7.4.515. [DOI] [PubMed] [Google Scholar]
- 8.Teasdale R D, Jackson M R. Annu Rev Cell Dev Biol. 1996;12:27–54. doi: 10.1146/annurev.cellbio.12.1.27. [DOI] [PubMed] [Google Scholar]
- 9.Sondermann P, Kaiser J, Jacob U. J Mol Biol. 2001;309:737–749. doi: 10.1006/jmbi.2001.4670. [DOI] [PubMed] [Google Scholar]
- 10.Radaev S, Motyka S, Fridman W H, Sautes-Fridman C, Sun P D. J Biol Chem. 2001;276:16469–16477. doi: 10.1074/jbc.M100350200. [DOI] [PubMed] [Google Scholar]
- 11.Garman S C, Wurzburg B A, Tarchevskaya S S, Kinet J P, Jardetzky T S. Nature (London) 2000;406:259–266. doi: 10.1038/35018500. [DOI] [PubMed] [Google Scholar]
- 12.Maxwell K F, Powell M S, Hulett M D, Barton P A, McKenzie I F, Garrett T P, Hogarth P M. Nat Struct Biol. 1999;6:437–442. doi: 10.1038/8241. [DOI] [PubMed] [Google Scholar]
- 13.Bruck I, O'Donnell M. Genome Biol. 2001;2:3001.1–3001.3. doi: 10.1186/gb-2001-2-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLennan I C. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 15.Harris N L, Jaffe E S, Stein H, Banks P M, Chan J K, Cleary M L, Delsol G, De Wolf-Peeters C, Falini B, Gatter K C. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 16.The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 17.Alizadeh A A, Eisen M B, Davis R E, Ma C, Lossos I S, Rosenwald A, Boldrick J C, Sabet H, Tran T, Yu X, et al. Nature (London) 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 18.Mechetina L V, Najakshin A M, Volkova O Y, Guselnikov S V, Faizulin R Z, Alabyev B Y, Chikaev N A, Vinogradova M S, Taranin A V. Eur J Immunol. 2002;32:87–96. doi: 10.1002/1521-4141(200201)32:1<87::AID-IMMU87>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]