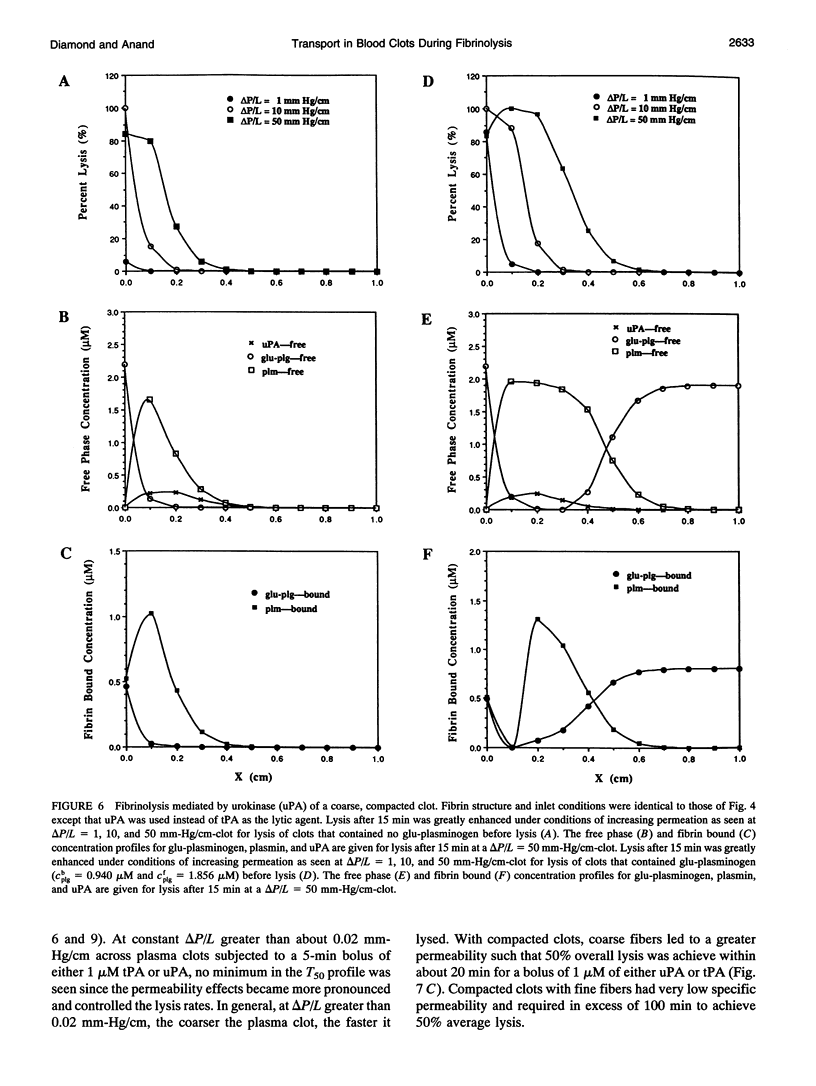
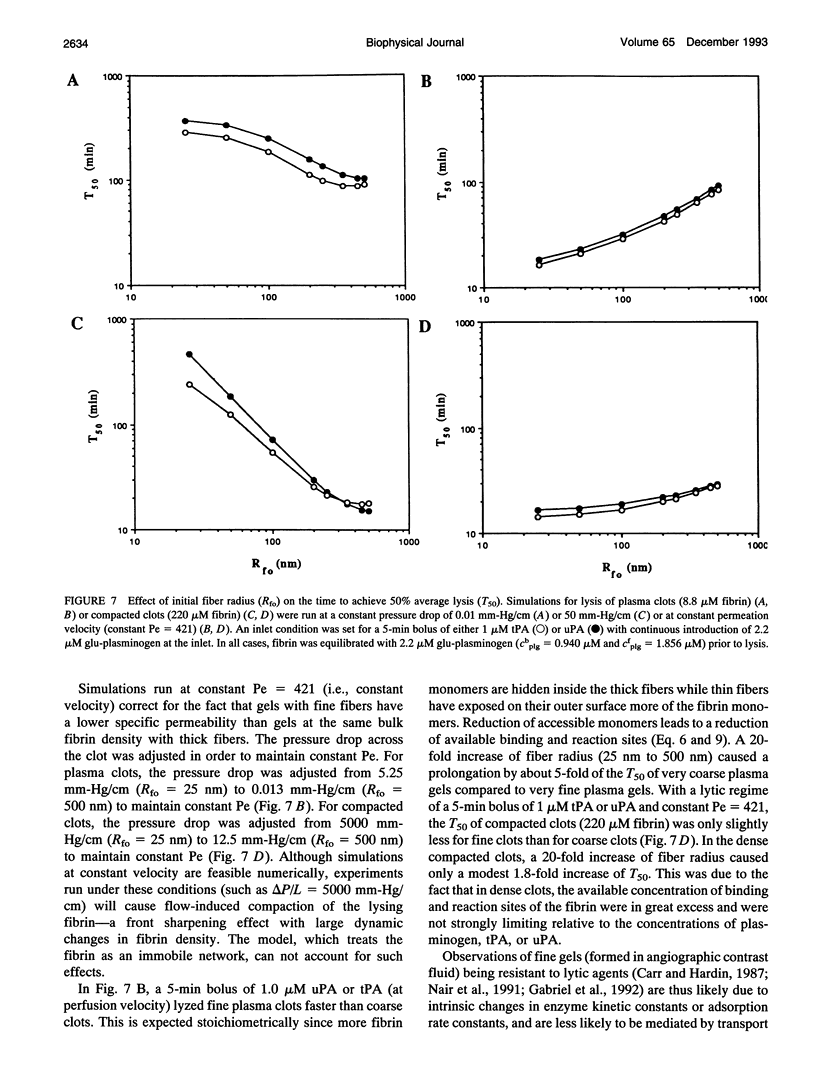
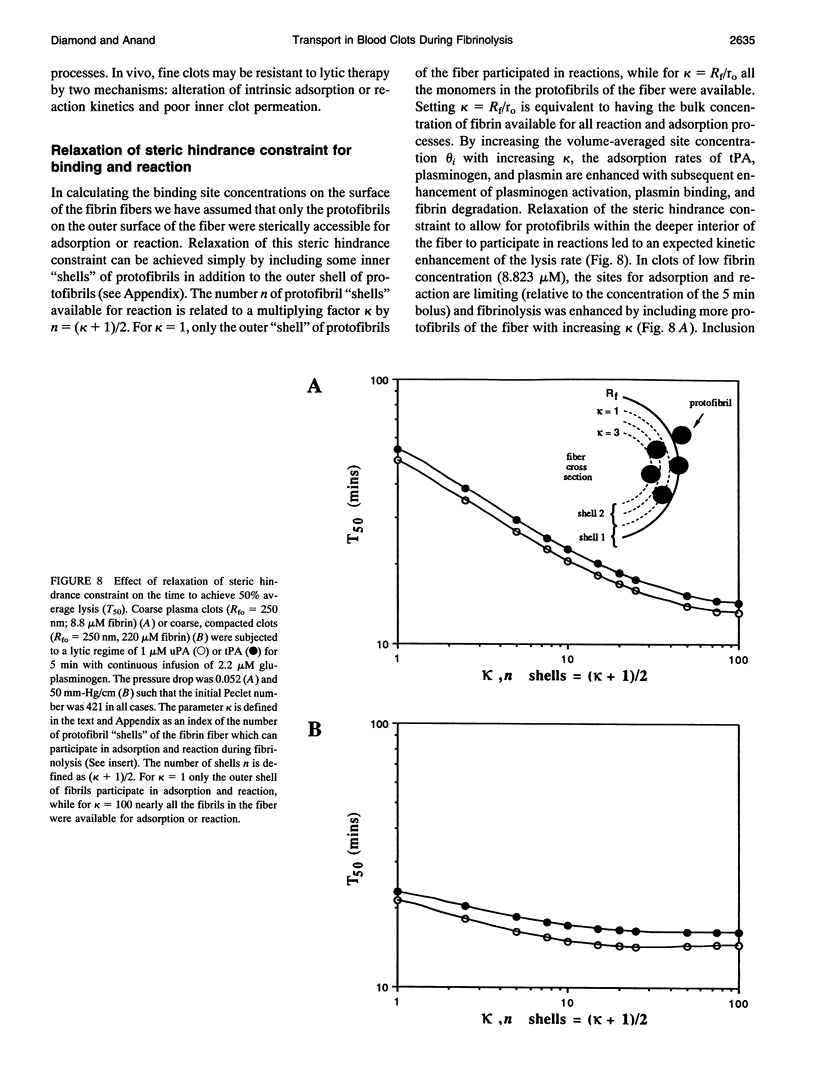
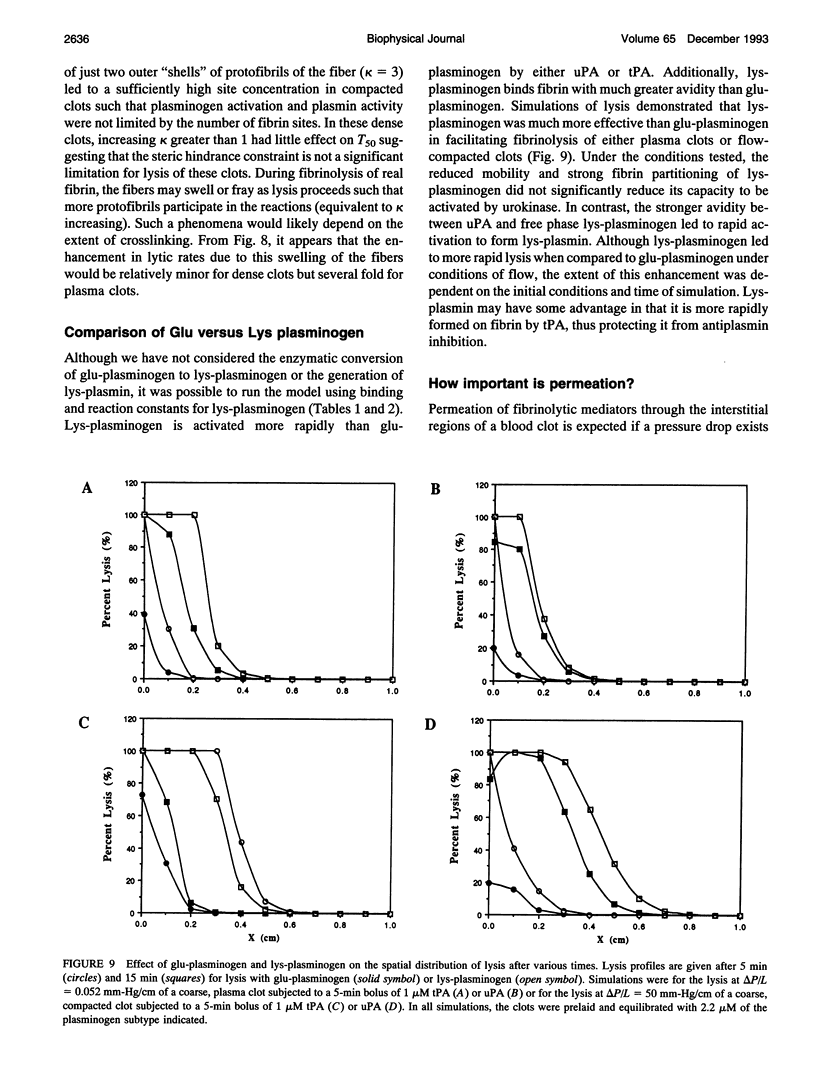
Abstract
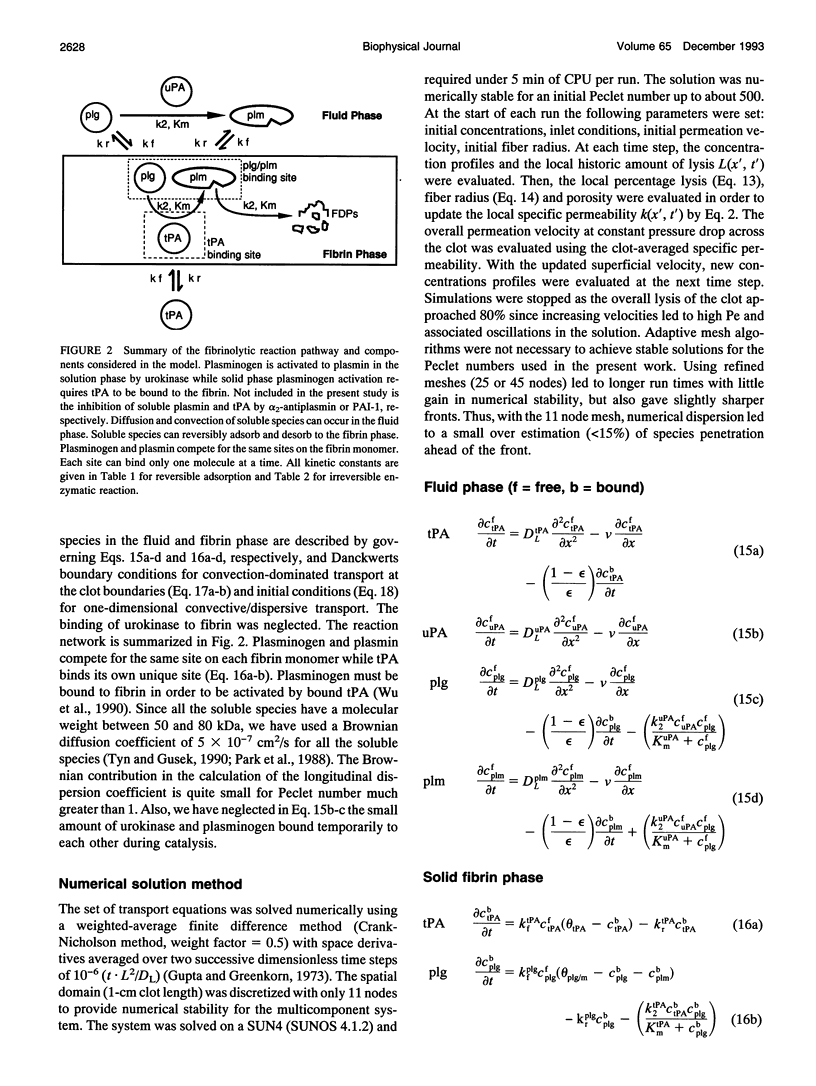
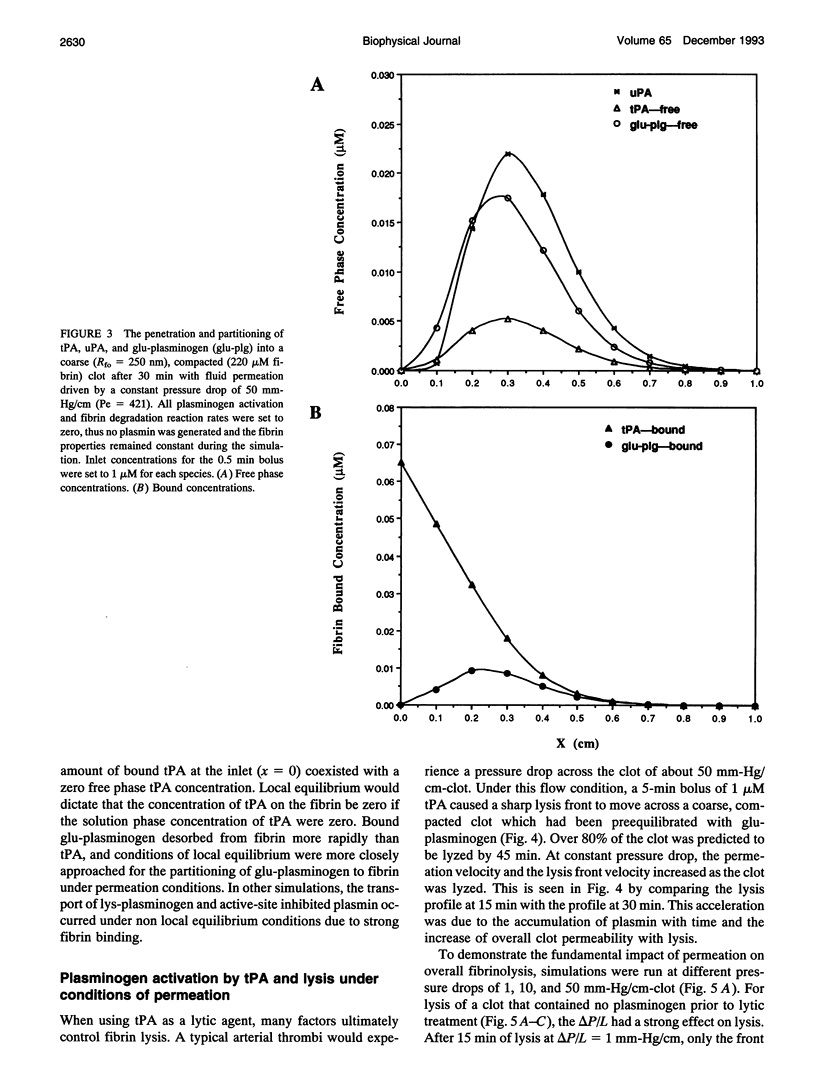
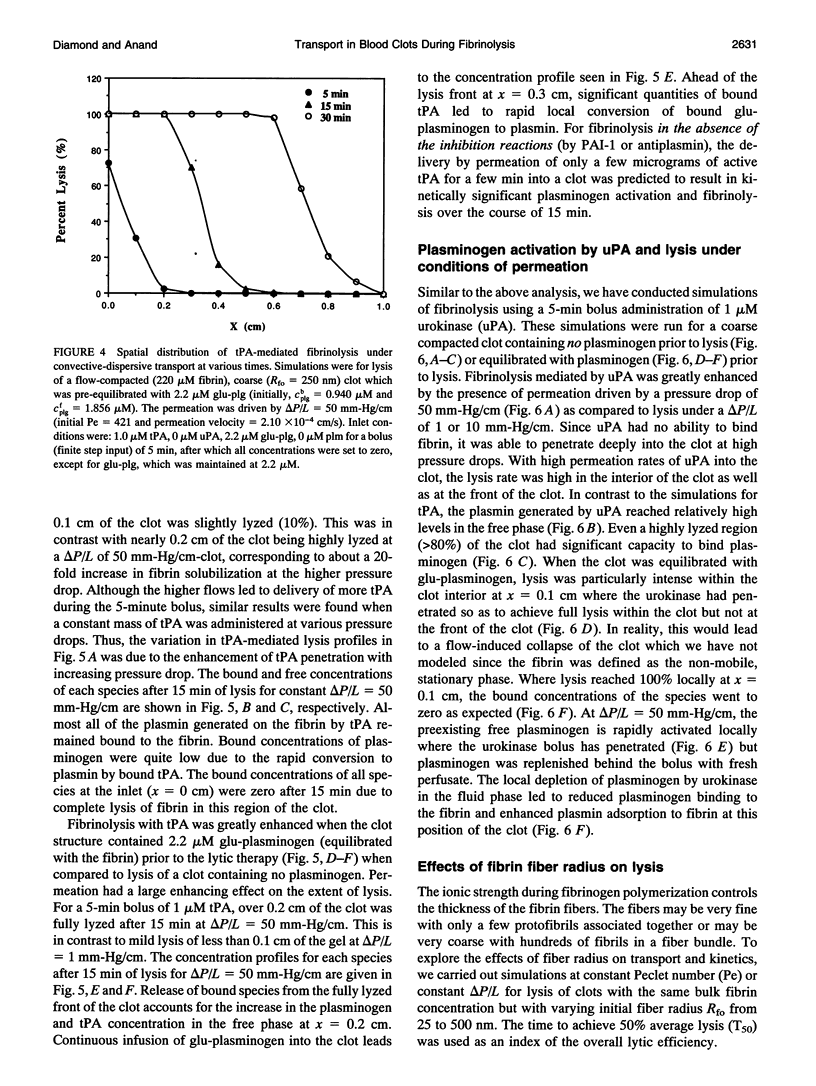
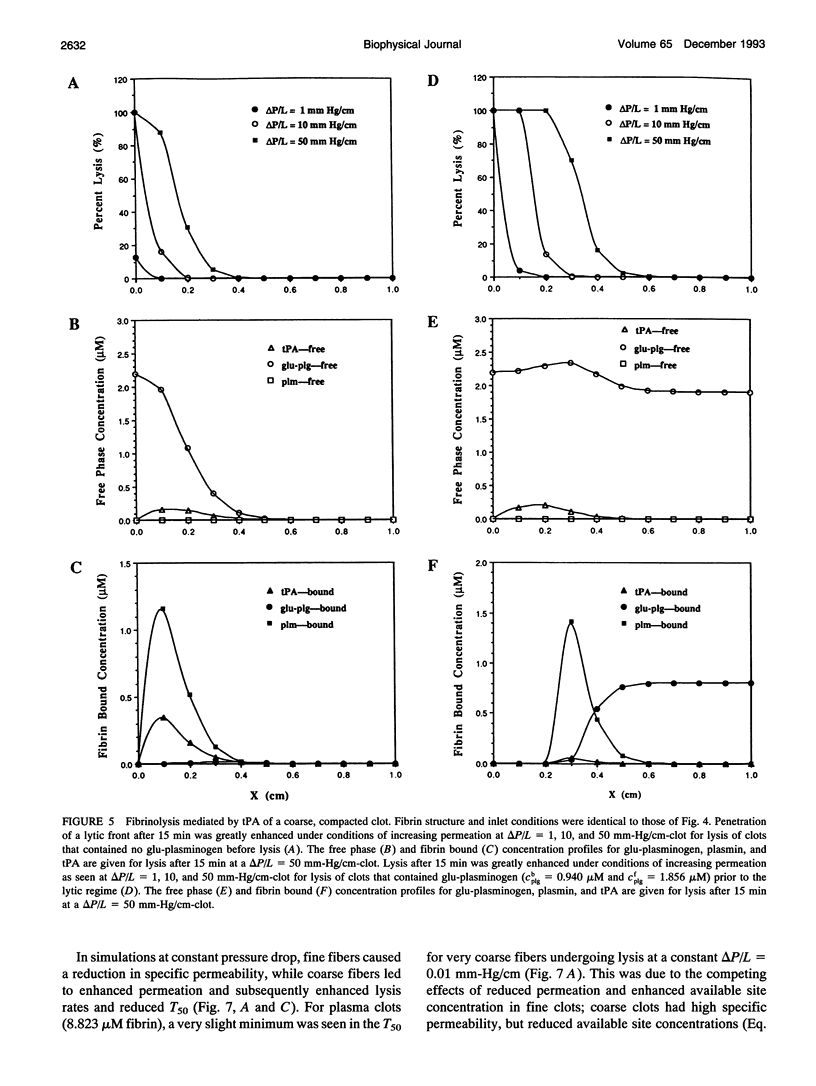
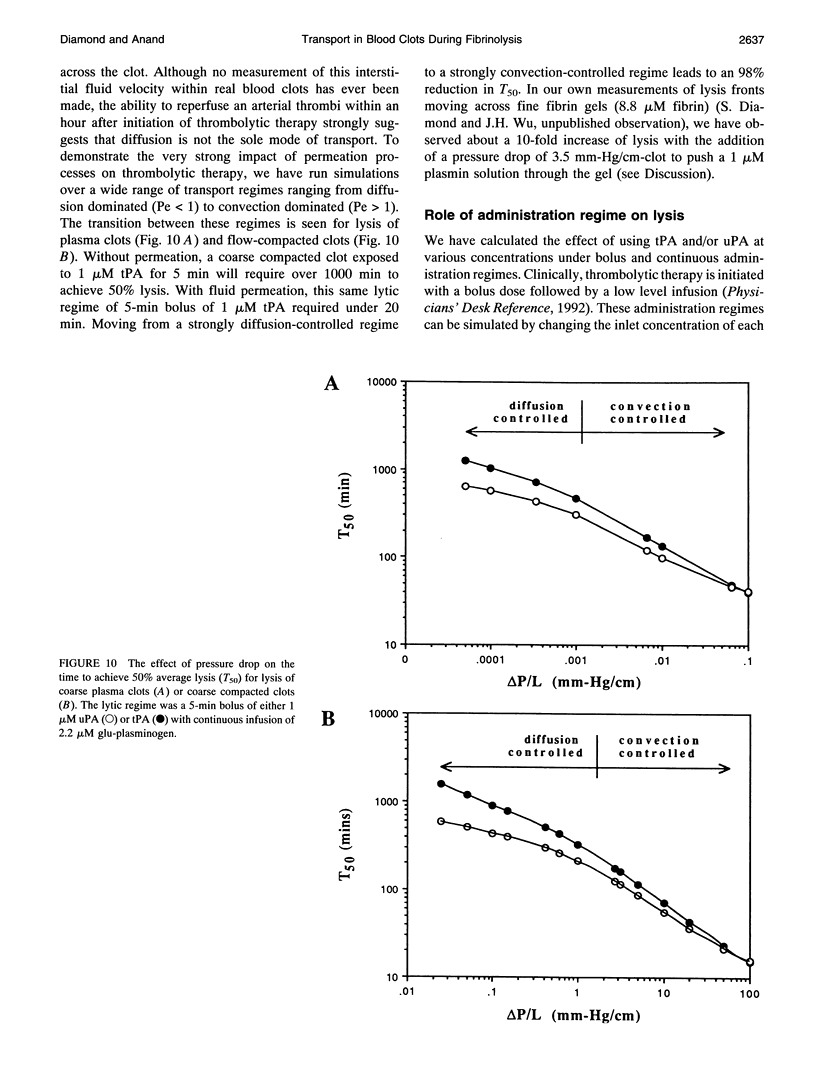
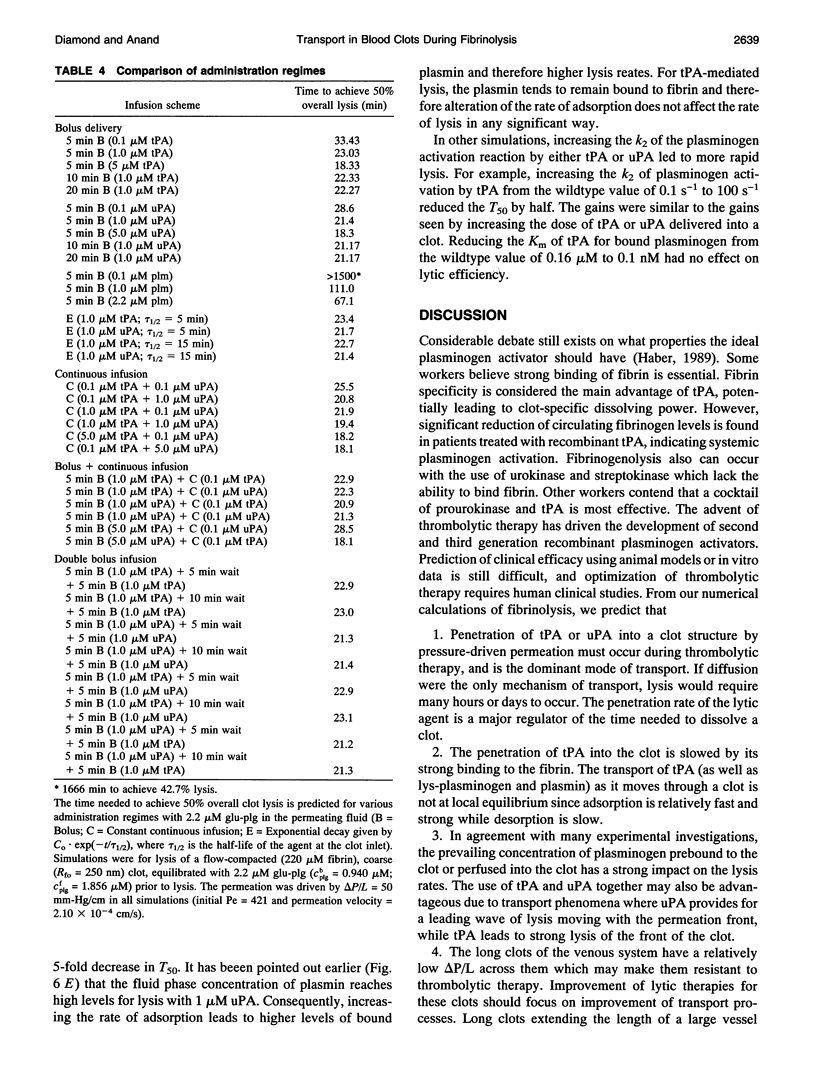
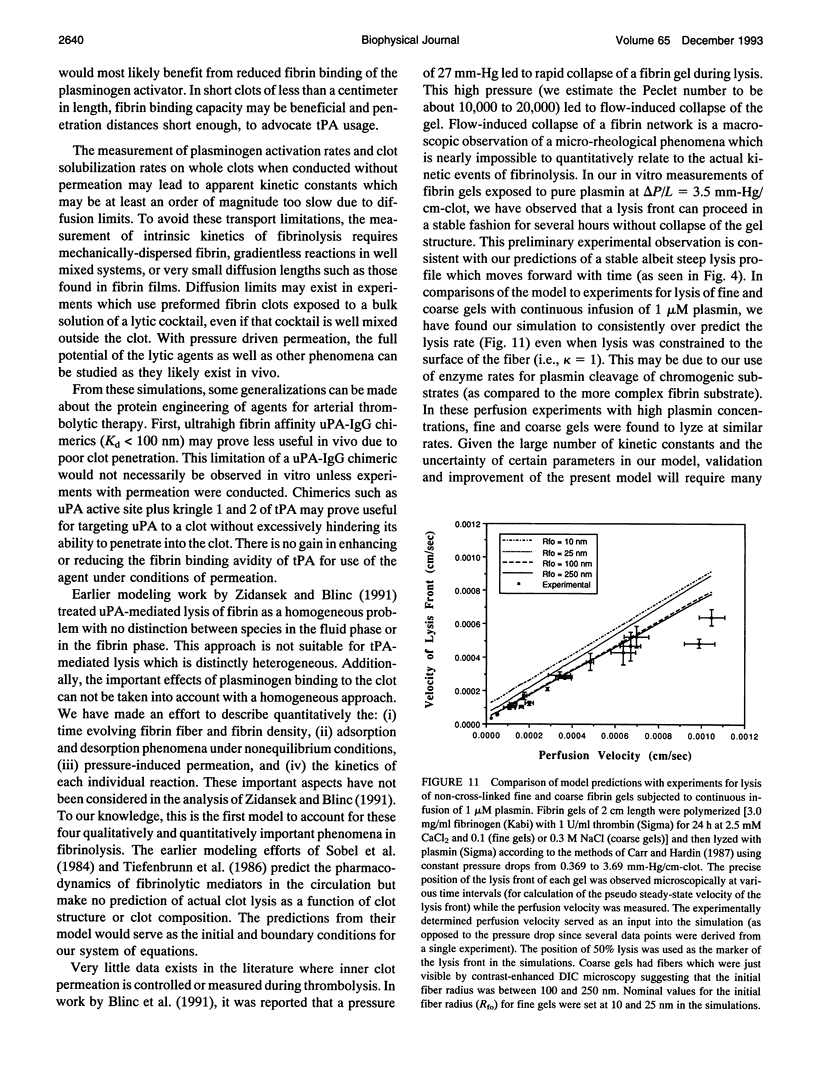
A model of fibrinolysis was developed using multicomponent convection-diffusion equations with homogeneous reaction and heterogeneous adsorption and reaction. Fibrin is the dissolving stationary phase and plasminogen, tissue plasminogen activator (tPA), urokinase (uPA), and plasmin are the soluble mobile species. The model is based on an accurate molecular description of the fibrin fiber and protofibril structure and contains no adjustable parameters and one phenomenological parameter estimated from experiment. The model can predict lysis fronts moving across fibrin clots (fine or coarse fibers) of various densities under different administration regimes using uPA and tPA. We predict that pressure-driven permeation is the major mode of transport that allows for kinetically significant thrombolysis during clinical situations. Without permeation, clot lysis would be severely diffusion limited and would require hundreds of minutes. Adsorption of tPA to fibrin under conditions of permeation was a nonequilibrium process that tended to front load clots with tPA. Protein engineering efforts to design optimal thrombolytics will likely be affected by the permeation processes that occur during thrombolysis.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blinc A., Planinsic G., Keber D., Jarh O., Lahajnar G., Zidansek A., Demsar F. Dependence of blood clot lysis on the mode of transport of urokinase into the clot--a magnetic resonance imaging study in vitro. Thromb Haemost. 1991 May 6;65(5):549–552. [PubMed] [Google Scholar]
- Blombäck B., Carlsson K., Hessel B., Liljeborg A., Procyk R., Aslund N. Native fibrin gel networks observed by 3D microscopy, permeation and turbidity. Biochim Biophys Acta. 1989 Jul 27;997(1-2):96–110. doi: 10.1016/0167-4838(89)90140-4. [DOI] [PubMed] [Google Scholar]
- Blombäck B., Okada M. Fibrin gel structure and clotting time. Thromb Res. 1982 Jan 1;25(1-2):51–70. doi: 10.1016/0049-3848(82)90214-6. [DOI] [PubMed] [Google Scholar]
- Blum J. J., Lawler G., Reed M., Shin I. Effect of cytoskeletal geometry on intracellular diffusion. Biophys J. 1989 Nov;56(5):995–1005. doi: 10.1016/S0006-3495(89)82744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok R. A., Mangel W. F. Quantitative characterization of the binding of plasminogen to intact fibrin clots, lysine-sepharose, and fibrin cleaved by plasmin. Biochemistry. 1985 Jun 18;24(13):3279–3286. doi: 10.1021/bi00334a031. [DOI] [PubMed] [Google Scholar]
- Carr M. E., Jr, Hardin C. L. Fibrin has larger pores when formed in the presence of erythrocytes. Am J Physiol. 1987 Nov;253(5 Pt 2):H1069–H1073. doi: 10.1152/ajpheart.1987.253.5.H1069. [DOI] [PubMed] [Google Scholar]
- Carr M. E., Jr, Hermans J. Size and density of fibrin fibers from turbidity. Macromolecules. 1978 Jan-Feb;11(1):46–50. doi: 10.1021/ma60061a009. [DOI] [PubMed] [Google Scholar]
- Carr M. E., Jr, Krishnamurti C., Alving B. M. Effect of plasminogen activator inhibitor-1 on tissue-type plasminogen activator-induced fibrinolysis. Thromb Haemost. 1992 Jan 23;67(1):106–110. [PubMed] [Google Scholar]
- Carr M. E., Jr, Shen L. L., Hermans J. Mass-length ratio of fibrin fibers from gel permeation and light scattering. Biopolymers. 1977 Jan;16(1):1–15. doi: 10.1002/bip.1977.360160102. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- Fears R. Binding of plasminogen activators to fibrin: characterization and pharmacological consequences. Biochem J. 1989 Jul 15;261(2):313–324. doi: 10.1042/bj2610313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel D. A., Muga K., Boothroyd E. M. The effect of fibrin structure on fibrinolysis. J Biol Chem. 1992 Dec 5;267(34):24259–24263. [PubMed] [Google Scholar]
- Haber E., Quertermous T., Matsueda G. R., Runge M. S. Innovative approaches to plasminogen activator therapy. Science. 1989 Jan 6;243(4887):51–56. doi: 10.1126/science.2492113. [DOI] [PubMed] [Google Scholar]
- Harpel P. C., Chang T. S., Verderber E. Tissue plasminogen activator and urokinase mediate the binding of Glu-plasminogen to plasma fibrin I. Evidence for new binding sites in plasmin-degraded fibrin I. J Biol Chem. 1985 Apr 10;260(7):4432–4440. [PubMed] [Google Scholar]
- Higgins D. L., Vehar G. A. Interaction of one-chain and two-chain tissue plasminogen activator with intact and plasmin-degraded fibrin. Biochemistry. 1987 Dec 1;26(24):7786–7791. doi: 10.1021/bi00398a038. [DOI] [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Hunziker E. B., Straub P. W., Haeberli A. A new concept of fibrin formation based upon the linear growth of interlacing and branching polymers and molecular alignment into interlocked single-stranded segments. J Biol Chem. 1990 May 5;265(13):7455–7463. [PubMed] [Google Scholar]
- Husain S. S., Hasan A. A., Budzynski A. Z. Differences between binding of one-chain and two-chain tissue plasminogen activators to non-cross-linked and cross-linked fibrin clots. Blood. 1989 Aug 15;74(3):999–1006. [PubMed] [Google Scholar]
- Jen C. J., McIntire L. V. Platelet microtubules in clot structure formation and contractile force generation: investigation of a controversy. Thromb Haemost. 1986 Aug 20;56(1):23–27. [PubMed] [Google Scholar]
- Kaufman E. N., Jain R. K. Quantification of transport and binding parameters using fluorescence recovery after photobleaching. Potential for in vivo applications. Biophys J. 1990 Oct;58(4):873–885. doi: 10.1016/S0006-3495(90)82432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijnen H. R., Van Hoef B., De Cock F., Collen D. The mechanism of plasminogen activation and fibrin dissolution by single chain urokinase-type plasminogen activator in a plasma milieu in vitro. Blood. 1989 May 15;73(7):1864–1872. [PubMed] [Google Scholar]
- Lottenberg R., Christensen U., Jackson C. M., Coleman P. L. Assay of coagulation proteases using peptide chromogenic and fluorogenic substrates. Methods Enzymol. 1981;80(Pt 100):341–361. doi: 10.1016/s0076-6879(81)80030-4. [DOI] [PubMed] [Google Scholar]
- Lucas M. A., Fretto L. J., McKee P. A. The binding of human plasminogen to fibrin and fibrinogen. J Biol Chem. 1983 Apr 10;258(7):4249–4256. [PubMed] [Google Scholar]
- Matveyev MYu, Domogatsky S. P. Penetration of macromolecules into contracted blood clot. Biophys J. 1992 Sep;63(3):862–863. doi: 10.1016/S0006-3495(92)81656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles L. A., Plow E. F. Binding and activation of plasminogen on the platelet surface. J Biol Chem. 1985 Apr 10;260(7):4303–4311. [PubMed] [Google Scholar]
- Nair C. H., Azhar A., Wilson J. D., Dhall D. P. Studies on fibrin network structure in human plasma. Part II--Clinical application: diabetes and antidiabetic drugs. Thromb Res. 1991 Nov 15;64(4):477–485. doi: 10.1016/0049-3848(91)90347-y. [DOI] [PubMed] [Google Scholar]
- Pizzo S. V., Schwartz M. L., Hill R. L., McKee P. A. The effect of plasmin on the subunit structure of human fibrin. J Biol Chem. 1973 Jul 10;248(13):4574–4583. [PubMed] [Google Scholar]
- ROBBINS K. C., SUMMARIA L., ELWYN D., BARLOW G. H. FURTHER STUDIES ON THE PURIFICATION AND CHARACTERIZATION OF HUMAN PLASMINOGEN AND PLASMIN. J Biol Chem. 1965 Jan;240:541–550. [PubMed] [Google Scholar]
- Sabovic M., Lijnen H. R., Keber D., Collen D. Correlation between progressive adsorption of plasminogen to blood clots and their sensitivity to lysis. Thromb Haemost. 1990 Nov 30;64(3):450–454. [PubMed] [Google Scholar]
- Sabovic M., Lijnen H. R., Keber D., Collen D. Effect of retraction on the lysis of human clots with fibrin specific and non-fibrin specific plasminogen activators. Thromb Haemost. 1989 Dec 29;62(4):1083–1087. [PubMed] [Google Scholar]
- Sobel B. E., Gross R. W., Robison A. K. Thrombolysis, clot selectivity, and kinetics. Circulation. 1984 Aug;70(2):160–164. doi: 10.1161/01.cir.70.2.160. [DOI] [PubMed] [Google Scholar]
- Suenson E., Thorsen S. Secondary-site binding of Glu-plasmin, Lys-plasmin and miniplasmin to fibrin. Biochem J. 1981 Sep 1;197(3):619–628. doi: 10.1042/bj1970619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiefenbrunn A. J., Graor R. A., Robison A. K., Lucas F. V., Hotchkiss A., Sobel B. E. Pharmacodynamics of tissue-type plasminogen activator characterized by computer-assisted simulation. Circulation. 1986 Jun;73(6):1291–1299. doi: 10.1161/01.cir.73.6.1291. [DOI] [PubMed] [Google Scholar]
- Weisel J. W., Nagaswami C. Computer modeling of fibrin polymerization kinetics correlated with electron microscope and turbidity observations: clot structure and assembly are kinetically controlled. Biophys J. 1992 Jul;63(1):111–128. doi: 10.1016/S0006-3495(92)81594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. L., Chang B. I., Wu D. H., Chang L. C., Gong C. C., Lou K. L., Shi G. Y. Interaction of plasminogen and fibrin in plasminogen activation. J Biol Chem. 1990 Nov 15;265(32):19658–19664. [PubMed] [Google Scholar]
- Zidansek A., Blinc A. The influence of transport parameters and enzyme kinetics of the fibrinolytic system on thrombolysis: mathematical modelling of two idealised cases. Thromb Haemost. 1991 May 6;65(5):553–559. [PubMed] [Google Scholar]
- de Vries C., Veerman H., Pannekoek H. Identification of the domains of tissue-type plasminogen activator involved in the augmented binding to fibrin after limited digestion with plasmin. J Biol Chem. 1989 Jul 25;264(21):12604–12610. [PubMed] [Google Scholar]


