Abstract
Deep vein thrombosis (DVT) is a common and serious complication of spontaneous intracerebral hemorrhage (ICH). The timely recognition and treatment of DVT can significantly mitigate several negative consequences. To investigate the association between the neutrophil-to-high-density lipoprotein cholesterol ratio (NHR) and the occurrence of DVT in ICH patients. This multicenter, retrospective cohort study included patients diagnosed with ICH from three hospitals. The primary outcome was the occurrence of DVT. The NHR was calculated using complete blood counts. Receiver operating characteristic (ROC) analysis was performed to determine the optimal NHR threshold for predicting DVT and to compare its predictive performance with that of other biomarkers. Univariate and multivariate logistic regression analyses were conducted to identify factors associated with DVT and to explore clinical complications related to NHR. A total of 5,004 patients were ultimately included in this study, among whom 359 (7.2%) were diagnosed with DVT. The optimal NHR cutoff for predicting DVT was 6.55, based on the Youden Index from the ROC curve, and was used to dichotomize the study population. In multivariable analyses, even after adjustment, a higher NHR was associated with an increased occurrence of DVT (adjusted odds ratio 1.69, 95% confidence interval 1.33–2.16) and its complications. Additionally, ROC analysis showed that NHR had superior predictive ability for DVT compared to single markers. In patients with ICH, a high NHR (≥ 6.55) independently predicts DVT and may serve as a laboratory marker for its occurrence.
Keywords: Neutrophil-to-high-density lipoprotein cholesterol ratio, Mortality, Complication, Intracerebral hemorrhage
Introduction
Spontaneous intracerebral hemorrhage (ICH) is associated with high mortality and morbidity rates1, highlighting a significant clinical challenge despite advancements in medical treatment2. Effective therapeutic options for ICH remain limited3, emphasizing the need for ongoing research and attention to this serious health issue4. ICH patients are particularly susceptible to complications such as deep venous thrombosis (DVT), which can severely impact their recovery and quality of life5,6. If left untreated, lower limb DVT following an ICH episode can progress and lead to sudden death due to pulmonary embolism7–10. Despite the critical importance of DVT prevention in ICH patients, data on effective prophylactic measures are scarce11. Therefore, identifying reliable predictors for DVT in ICH patients is essential to improve patient outcomes and guide timely therapeutic interventions12.
Previous studies have identified several risk factors for predicting DVT, including advanced age, current smoking status, admission Glasgow Coma Scale (GCS) score, National Institutes of Health Stroke Scale (NIHSS) score13. Laboratory biomarkers have garnered significant attention due to their practicality, sensitivity, and convenience. However, the limited predictive value of single biomarkers restricts their clinical utility in the early prediction of DVT14. Therefore, there is a need for more reliable and easily accessible blood markers for the early prediction of DVT and to guide timely therapeutic interventions.
The neutrophil-to-high-density lipoprotein cholesterol ratio (NHR) is a well-established serum marker for inflammation and infection and has demonstrated strong predictive capabilities for metabolic-related diseases, as well as adverse cardiovascular outcomes15,16. However, its potential as a predictor for DVT in patients with ICH has not yet been explored. Therefore, the objective of this multicenter cohort study is to evaluate the association between NHR, which can be easily obtained through routine laboratory tests, and the occurrence of DVT in ICH patients. Our study aims to fill this gap in the literature and provide valuable insights into this topic.
Methods
Study design
Our study was a retrospective, multicenter cohort study involving 5,004 patients diagnosed with ICH. Data were collected from three hospitals: West China Hospital of Sichuan University (December 2010 to August 2019), The First People’s Hospital of Longquanyi District Chengdu (December 2016 to November 2020), and the Affiliated Hospital of Chengdu University (August 2012 to November 2020). The study received approval from the institutional review boards of the ethics committees of the three hospitals: West China Hospital (No. 2021–624), The First People’s Hospital of Longquanyi District Chengdu (No. AF-AK86 2,022,010), and the Affiliated Hospital of Chengdu University (No. PJ2021-017-03). As this study involved a clinical audit, informed consent was waived. The study adhered to the STROBE criteria and followed the ethical principles outlined in the Declaration of Helsinki 1964.
Patient selection
All patients diagnosed with spontaneous ICH were included in the study. Diagnosis of ICH was confirmed at admission through computed tomography (CT) or magnetic resonance imaging (MRI), and intraoperative diagnosis by a neurosurgeon during hospitalization. Only patients aged 18 years and older were included. Exclusion criteria were as follows: (1) ischemic stroke with hemorrhagic transformation, trauma, cerebral aneurysm, arteriovenous malformations, brain tumor bleeding, hemorrhagic disorders due to coagulation abnormalities, and other conditions distinct from primary ICH; (2) DVT before admission; (3) patients whose serum NHR levels were not obtained within 24 h of admission.
Data collection
We collected the following baseline information: demographic variables (age and sex); physiological indicators (body mass index and systolic blood pressure); lifestyle history (smoking and alcohol consumption); relevant comorbidities (hypertension, diabetes, and chronic kidney disease); treatment details at baseline (antithrombotic therapy); hematoma characteristics (including size and location); and the GCS score at admission. Additionally, baseline laboratory parameters, defined as the first available results within the first 7 days of admission, included NHR, albumin, lymphocyte count, glucose, leukocyte count, platelet count, and APTT. Admission NHR, given its early predictive value and clinical feasibility, was used, and its optimal cutoff value was determined through ROC curve analysis and the Youden index.
Outcomes
The primary outcome was the occurrence of DVT. DVT was diagnosed based on clinical evaluation and confirmed by imaging. Specifically, if a patient developed signs or symptoms suggestive of DVT (such as limb swelling, pain, or Homans’ sign), Doppler ultrasound of the lower extremities was performed to confirm the presence of thrombosis. All DVT diagnoses, including clinical presentation and imaging confirmation, were documented in the electronic medical record and served as the basis for outcome determination.
Given the high risk of rebleeding and the complexity of anticoagulation management in patients with ICH, all participating centers adopted a cautious and standardized approach to DVT prevention. Upon admission, patients without contraindications were started on mechanical prophylaxis measures, including but not limited to graduated compression stockings, intermittent pneumatic compression devices, and other standard supportive modalities. Pharmacological prophylaxis with low-molecular-weight heparin was reserved for a small proportion of patients and initiated only when imaging confirmed stability and there was no evidence of ongoing hemorrhage.
Additionally, we evaluated several secondary outcomes, which were categorized into three main groups: (1) mortality-related outcomes, scuh as 30-day, 90-day, and 180-day mortality, as well as overall survival; (2) infectious complications, including hospital-acquired infections, pneumonia, intracranial infections, urinary tract infections, and bloodstream infections; (3) other clinical complications, including acute kidney injury (AKI) and seizures. The date of death was obtained from the Chinese Hukou System. Hospital-acquired infections were defined as infections occurring after 48 h of hospital admission and included all of the aforementioned infection types. AKI was defined based on KDIGO criteria—specifically, a rise in serum creatinine ≥ 0.3 mg/dL within 48 h or ≥ 1.5 times baseline within one week, consistent with stage 1 AKI17.
Statistical analysis
Continuous variables were presented as means and standard deviations and were compared using the Student’s t-test and the Mann–Whitney U-test. Categorical data were analyzed by calculating frequencies or percentages and compared using the chi-square test or Fisher’s exact test. All available demographics, baseline variables, and laboratory tests were selected as factors in logistic regression models based on prior studies and clinical expertise. Univariate logistic regression models were conducted separately to analyze the association between each factor and the outcomes. Factors with P-values less than 0.05 in univariate analysis were included as confounders in the multivariable logistic regression analysis. ROC curves were used to compare the predictive abilities of NHR and other biomarkers (including albumin, lymphocytes, neutrophils, white blood cells, platelets, glucose, high-density lipoprotein, and activated partial thromboplastin time) for DVT. ROC curves were also used to evaluate the discriminative ability of admission NHR, maximum NHR, and median NHR in predicting the occurrence of DVT within 7 days.
Further subgroup analyses were conducted based on age (≤ 65 years and > 65 years), sex (male and female), smoking status, alcohol consumption, hypertension, diabetes, hematoma location (infratentorial and supratentorial), hematoma volume (≤ 30 ml and > 30 ml), presence of intraventricular hematoma, and GCS scores to explore the heterogeneity of outcomes. All factors were selected based on clinical advice, prior studies, and a review of risk scores. All statistical analyses were performed using R software (version 4.2.1; Foundation for Statistical Computing). A P-value of less than 0.05 was considered statistically significant, and all P-values were two-sided.
Results
A total of 5,004 patients from West China Hospital, The First People’s Hospital of Longquanyi District Chengdu, and The Affiliated Hospital of Chengdu University, who were diagnosed with ICH and were over 18 years old, were included in the study. Patients with conditions other than primary ICH and those without NHR measurements at admission were excluded.
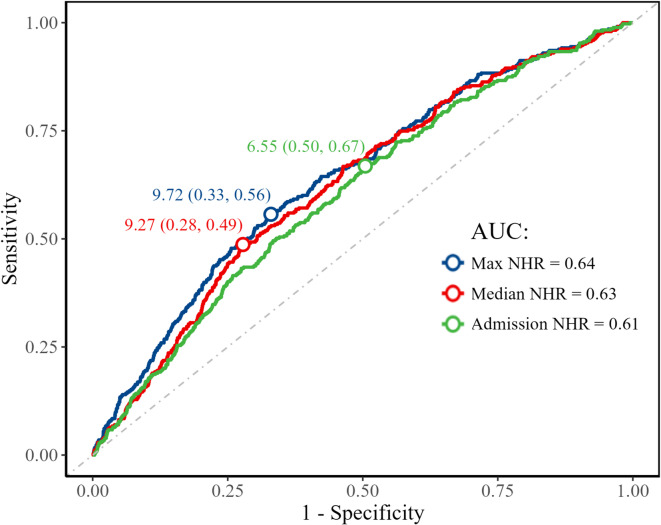
Figure 1 shows the ROC curves of admission, maximum, and median NHR within the first 7 days of hospitalization for predicting DVT. Due to its practicality and early availability, admission NHR was chosen as the primary variable for analysis. An NHR value of 6.55 at admission was identified as the optimal threshold for distinguishing patients with DVT. Based on this value, patients were divided into two groups: a lower NHR group (< 6.55) and a higher NHR group (≥ 6.55). The higher NHR group comprised 2,584 patients, while the lower NHR group comprised 2,420 patients. Table 1 shows the comparison of patient demographics, medical history, and other factors between the two groups. Specifically, a higher NHR was generally associated with younger age and male sex, and was more likely to be observed in patients with a history of smoking and hypertension, lower GCS scores, higher systolic blood pressure, and larger hematoma volumes. Additionally, NHR levels were significantly associated with hematoma location.
Fig. 1.
Receiver operating characteristic curve analysis of different NHR measures for predicting deep venous thrombosis. NHR, neutrophil-to-high-density lipoprotein cholesterol ratio.
Table 1.
Baseline characteristics of the patients by neutrophil-to-high density lipoprotein cholesterol ratio.
| Characteristics | Overall (n = 5,004) | Lower NHR (< 6.55, n = 2,420) | Higher NHR (≥ 6.55, n = 2,584) | P |
|---|---|---|---|---|
| Demographics | ||||
| Age, year, mean (SD) | 56.75 (14.58) | 58.50 (14.69) | 55.10 (14.28) | < 0.001 |
| Female, n (%) | 1,769 (35.4) | 968 (40.0) | 801 (31.0) | < 0.001 |
| Smoking, n (%) | 1,338 (26.7) | 586 (24.2) | 752 (29.1) | < 0.001 |
| Alcohol, n (%) | 1,605 (32.1) | 751 (31.0) | 854 (33.0) | 0.134 |
| Medical history, n (%) | ||||
| Hypertension | 3,615 (72.2) | 1,682 (69.5) | 1,933 (74.8) | < 0.001 |
| Diabetes | 553 (11.1) | 248 (10.2) | 305 (11.8) | 0.088 |
| Chronic kidney disease | 147 ( 2.9) | 75 (3.1) | 72 (2.8) | 0.568 |
| Hematoma characteristics, n (%) | ||||
| Size of hematoma, cm, mean (SD) | 22.40 (27.32) | 17.84 (23.59) | 26.74 (29.81) | < 0.001 |
| Infratentorial hematoma, n (%) | 994 (19.9) | 438 (18.1) | 556 (21.5) | 0.003 |
| Intraventricular hematoma, n (%) | 1,045 (20.9) | 415 (17.1) | 630 (24.4) | < 0.001 |
| Glasgow coma score, n (%) | ||||
| 3–8 | 1,689 (33.8) | 448 (18.5) | 1,241 (48.0) | < 0.001 |
| 9–15 | 3,282 (65.6) | 1,959 (81.0) | 1,323 (51.2) | |
| SBP, mean (SD) | 159.04 (31.83) | 155.26 (29.28) | 162.57 (33.68) | < 0.001 |
| Antithrombotic therapy, n (%) | 413 (8.3) | 228 (9.4) | 185 (7.2) | < 0.001 |
| Laboratory tests | ||||
| Albumin, g/L, mean (SD) | 38.20 (6.07) | 39.14 (5.16) | 37.31 (6.70) | < 0.001 |
| Lymphocyte, 109/L, mean (SD) | 1.10 (0.66) | 1.15 (0.61) | 1.06 (0.69) | < 0.001 |
| Neutrophil, 109/L, mean (SD) | 8.95 (5.01) | 6.02 (2.43) | 11.69 (5.25) | < 0.001 |
| White blood cell, 109/L, mean (SD) | 10.91 (10.46) | 7.79 (4.01) | 13.84 (13.39) | < 0.001 |
| Platelets, 109/L, mean (SD) | 162.38 (73.48) | 154.02 (64.29) | 169.21 (79.44) | < 0.001 |
| Glucose, mmol/L, mean (SD) | 7.47 (3.14) | 6.73 (2.59) | 8.16 (3.44) | < 0.001 |
| High-density lipoprotein, mmol/L, mean (SD) | 1.27 (0.45) | 1.46 (0.45) | 1.09 (0.37) | < 0.001 |
| Activated partial thromboplastin time, s, mean (SD) | 28.81 (8.49) | 28.19 (7.34) | 29.37 (9.37) | < 0.001 |
SD, standard deviation; NHR, neutrophil-to-high-density lipoprotein cholesterol ratio.
The overall DVT rate in the entire plation was 7.2% (n = 359). Patients with a higher NHR had a mortality rate of 9.3% (n = 240), compared to 4.9% (n = 119) in patients with a lower NHR (P < 0.001). Logistic regression analysis showed that age, GCS score, hematoma size and location, history of diabetes, chronic kidney disease, and antithrombotic therapy were independent risk factors for the development of DVT. The adjusted OR for the association between NHR level and DVT was 1.69 (95% CI: 1.33–2.16), as shown in Table 2. This finding was consistent with the associations observed for AKI (adjusted OR: 1.84; 95% CI: 1.52–2.22; P < 0.001), hospital-acquired infections (adjusted OR: 1.63; 95% CI: 1.43–1.86; P < 0.001), pneumonia (adjusted OR: 2.03; 95% CI: 1.78–2.31; P < 0.001), intracranial infection (adjusted OR: 2.28; 95% CI: 1.50–3.47; P < 0.001), urinary tract infection (adjusted OR: 1.44; 95% CI: 1.19–1.74; P < 0.001), and bloodstream infection (adjusted OR: 2.04; 95% CI: 1.44–2.91; P < 0.001). Moreover, NHR was also found to be significantly associated with both short- and long-term mortality in patients with ICH, such as 30-day mortality (adjusted OR: 1.50; 95% CI: 1.21–1.86) and long-term mortality (adjusted OR: 1.41; 95% CI: 1.18–1.69).
Table 2.
Comparison of unadjusted and risk-adjusted outcomes by neutrophil-to-high density lipoprotein cholesterol ratio.
| Outcomes | Events, n (%) | Unadjusted | Adjusted | ||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Primary outcome | |||||
| Deep Vein Thrombosis | 240 (9.3) | 1.98 (1.58–2.48) | < 0.001 | 1.69 (1.33–2.16) | < 0.001 |
| Complications | |||||
| Seizures | 65 (2.5) | 1.33 (0.91–1.95) | 0.141 | 1.39 (0.92–2.11) | 0.115 |
| Acute Kidney Injury | 572 (22.1) | 2.42 (2.07–2.84) | < 0.001 | 1.84 (1.52–2.22) | < 0.001 |
| Hospital-acquired infections | 1,192 (46.1) | 2.09 (1.86–2.35) | < 0.001 | 1.63 (1.43–1.86) | < 0.001 |
| Pneumonia | 1,232 (47.7) | 2.46 (2.18–2.76) | < 0.001 | 2.03 (1.78–2.31) | < 0.001 |
| Intracranial infection | 113 (4.4) | 3.41 (2.30–5.07) | < 0.001 | 2.28 (1.50–3.47) | < 0.001 |
| Urinary tract infection | 364 (14.1) | 1.61 (1.35–1.92) | < 0.001 | 1.44 (1.19–1.74) | < 0.001 |
| Bloodstream infection | 141 (5.5%) | 2.85 (2.05–3.98) | < 0.001 | 2.04 (1.44–2.91) | < 0.001 |
| Mortality | |||||
| Mortality at 30 days | 429 (27.6) | 2.27 (1.87–2.74) | < 0.001 | 1.50 (1.21–1.86) | < 0.001 |
| Mortality at 90 days | 493 (31.7) | 2.28 (1.90–2.73) | < 0.001 | 1.50 (1.22–1.85) | < 0.001 |
| Mortality at 180 days | 541 (34.7) | 2.31 (1.94–2.75) | < 0.001 | 1.58 (1.29–1.93) | < 0.001 |
| Mortality at 1 year | 591 (38.0) | 2.21 (1.87–2.61) | < 0.001 | 1.56 (1.28–1.90) | < 0.001 |
| Mortality at 2 years | 658 (42.3) | 2.06 (1.76–2.42) | < 0.001 | 1.46 (1.21–1.76) | < 0.001 |
| Mortality at 3 years | 698 (44.8) | 2.02 (1.72–2.36) | < 0.001 | 1.46 (1.22–1.76) | < 0.001 |
| Mortality at 4 years | 718 (46.1) | 1.90 (1.63–2.22) | < 0.001 | 1.40 (1.16–1.68) | < 0.001 |
| Mortality at 5 years | 744 (47.8) | 1.88 (1.61–2.18) | < 0.001 | 1.40 (1.17–1.68) | < 0.001 |
| In-hospital mortality | 294 (12.5) | 2.77 (2.21–3.47) | < 0.001 | 1.57 (1.22–2.02) | 0.001 |
| Long-term mortality | 782 (50.2) | 1.83 (1.57–2.13) | < 0.001 | 1.41 (1.18–1.69) | < 0.001 |
Hospital-acquired infections (≥ 48 h after admission), including pneumonia, intracranial, urinary tract, and bloodstream infections. OR, odds ratio; CI: confidence interval.
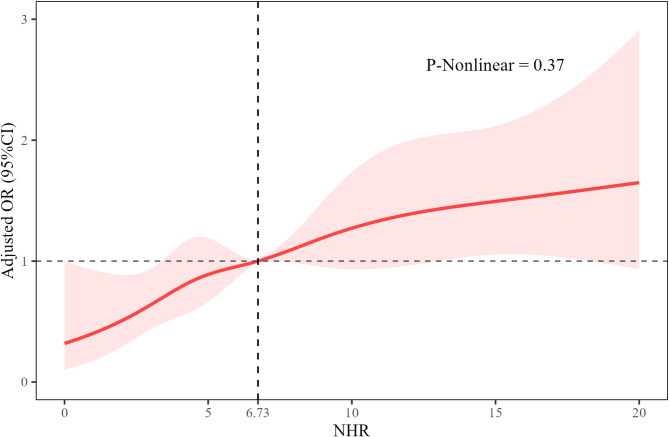
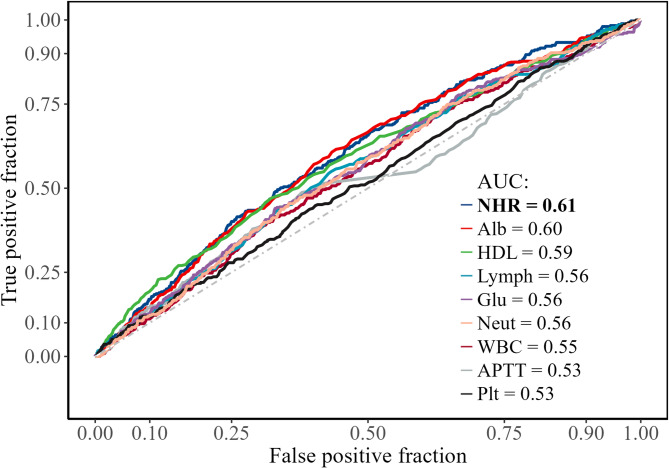
The restricted cubic spline (RCS) demonstrated the association between NHR and the risk of DVT (Fig. 2). As NHR increased, the OR showed a slight upward trend, suggesting a potential positive dose–response relationship between NHR and DVT risk. This relationship appeared to be non-linear (P = 0.37). ROC curve analysis was conducted to assess the predictive value of NHR and other laboratory indicators for DVT (Fig. 3). Among all variables, NHR had the highest area under the curve (AUC = 0.61), followed by albumin (Alb, AUC = 0.60), high-density lipoprotein (HDL, AUC = 0.59), lymphocytes (Lymph, AUC = 0.56), glucose (Glu, AUC = 0.56), neutrophils (Neut, AUC = 0.56), white blood cells (WBC, AUC = 0.55), activated partial thromboplastin time (APTT, AUC = 0.53), and platelets (Plt, AUC = 0.53). These results indicate that NHR has relatively superior discriminatory power compared to the other parameters.
Fig. 2.
Restricted cubic spline analysis of the association between neutrophil-to-high-density lipoprotein cholesterol ratio and deep venous thrombosis. NHR, neutrophil-to-high-density lipoprotein cholesterol ratio; OR, odds ratio; CI: confidence interval.
Fig. 3.
Receiver operating characteristic curve analysis of different biomarkers for predicting deep venous thrombosis. NHR, neutrophil-to-high-density lipoprotein cholesterol ratio; Alb, albumin; HDL, high-density lipoprotein cholesterol; Lymph, lymphocyte; Glu, glucose; Neut, neutrophil; WBC, white blood cell count; APTT, activated partial thromboplastin time; Plt, platelet count.
The subgroup analysis further demonstrates the association between NHR distribution across different clinical factors and event occurrence (Fig. 4). The results show that in most subgroups, no significant interaction was observed between NHR levels and event occurrence (P > 0.05). However, in the subgroups of age, alcohol use, and chronic kidney disease, the effect of NHR levels on the occurrence of DVT showed significant differences (P < 0.05), suggesting that these factors may exacerbate the risk of DVT in the high NHR group, particularly among individuals who are younger, have alcohol consumption habits, and have chronic kidney disease.
Fig. 4.
Subgroup analysis of the association between neutrophil-to-high-density lipoprotein cholesterol ratio and deep venous thrombosis.
Discussion
In this study, we found a strong association between elevated NHR levels and the risk of developing DVT in patients with ICH. Patients with admission NHR levels above 6.55 had a significantly higher likelihood of DVT. This association followed a clear linear dose–response pattern, as confirmed by RCS analysis. Moreover, elevated NHR levels were also significantly linked to other complications, such as AKI and various infections, including pneumonia, urinary tract infections, and bloodstream infections, as well as increased short- and long-term mortality. Subgroup analyses further revealed that in certain subgroups—specifically younger patients, individuals with a history of alcohol use, and those with chronic kidney disease—the association between elevated NHR levels and DVT was significantly stronger.
DVT prevention is explicitly recommended for patients with ICH in clinical guidelines from multiple countries3,18,19. Studies have shown that the median time from ICH onset to DVT diagnosis is 7 days, suggesting that earlier prevention may be critical20. Therefore, this study aimed to evaluate the predictive value of the NHR at admission for the development of DVT, in order to facilitate early identification of high-risk patients and guide timely clinical interventions. Previous studies have demonstrated that NHR can predict early hematoma expansion and poor outcomes in patients with ICH21,22. For example, Yu et al.23 reported that a higher NHR is associated with an increased risk of acute ischemic stroke. Similarly, Liu et al.24 found that elevated NHR levels are predictive of adverse cardiovascular outcomes in individuals with pre-diabetes. Our study is the first to investigate the association between NHR levels and the occurrence of DVT in patients with ICH. The findings are consistent with these studies, demonstrating that elevated NHR levels are significantly associated with DVT and other adverse outcomes in patients with ICH.
Several potential mechanisms may explain the association between NHR levels and DVT in ICH patients. First, the inflammatory response following ICH can activate the coagulation cascade, increasing the risk of thrombosis, and may also exacerbate endothelial injury, further promoting a pro-thrombotic state25,26. Second, neutrophils release extracellular traps that serve as both a scaffold and a prothrombotic stimulus by capturing platelets, red blood cells, and plasma proteins, while histones within NETs directly trigger platelet activation and aggregation, thereby promoting blood clot formation27,28. HDL-C not only reduces platelet sensitivity to aggregation stimuli but also inhibits the formation of neutrophil extracellular traps, thereby reducing thrombosis formation29,30. Moreover, they are all closely related to the coagulation cascade31,32. These mechanisms underscore the potential value of the NHR in predicting DVT in this patient population.
Our study has several strengths. Firstly, the large sample size of 5,004 patients from multiple centers enhances the generalizability of our findings. Secondly, we utilized both univariate and multivariate analyses to adjust for potential confounders, providing robust evidence for the independent predictive value of NHR. Thirdly, the practical application of NHR, derived from routine blood tests, makes it an accessible and cost-effective tool for clinicians. There are several limitations to this study that should be acknowledged. First, its retrospective design may have introduced selection bias and unmeasured confounding variables. Second, the use of electronic medical records for data collection may be associated with reporting bias. Third, the study population consisted exclusively of patients from China, which may limit the generalizability of the findings to other populations. Despite these limitations, our results suggest that measuring the NHDL ratio at baseline may have clinical relevance in identifying ICH patients at higher risk of DVT and in guiding more personalized prophylactic strategies. Nevertheless, further prospective, multicenter studies with larger and more diverse populations are needed to validate the findings of this study.
Conclusions
An NHR exceeding 6.55 was established as an independent predictor of DVT in patients with ICH. Furthermore, NHR emerged as an independent predictor of various clinical complications, particularly infectious ones. Due to its ease of measurement and cost-effectiveness, NHR may prove to be a valuable tool for prognostication in ICH patients.
Abbreviations
- ICH
Spontaneous intracerebral hemorrhage
- NHR
Lactate dehydrogenase
- GCS
Glasgow Coma Scale
- ROC
Receiver operating characteristic
- AUROC
The area under the receiver operating characteristic curve
Author contributions
Study concept and design: YZ, FF Acquisition, analysis, or interpretation of data: WX, JW, PW, XC, LP, JH, YF, and FF Statistical analysis: WX, YX Drafting of the manuscript: WX, PW Critical revision of the manuscript for important intellectual content: All authors.
Funding
This work is supported by National Natural Science Foundation of China (82271364), the innovation team project of Affiliated Hospital of Clinical Medicine College of Chengdu University (CDFYCX202203), and the project of Sichuan Science and Technology Bureau (2024NSFSC0657), the 1·3·5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (21HXFH046), the project of health commission of Sichuan province (2019HR50), Experimental Teaching Research and Reform Project of Chengdu University (cdsyjg2022022), Chengdu Science and Technology Project (2024-YF05-00871-SN), and Science and Technology Research Program of Chongging Municipal Education Commission (KJQN202200452).
Data availability
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The ethics committee of West China Hospital (No. 2021-624). The ethics committee of Affiliated Hospital of Chengdu University (No. PJ2021-017-03). The ethics committee of The First People’s Hospital of Longquanyi District Chengdu (No.AF-AK-2022010). The committee waived the requirement for informed consent because the data were analyzed anonymously.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenhao Xu, Peng Wang and Jun Wan contributed equally to this work.
Contributor Information
Fang Fang, Email: fangfang01@scu.edu.cn.
Yu Zhang, Email: zhangyu1057@cdu.edu.cn.
References
- 1.Cordonnier, C., Demchuk, A., Ziai, W. & Anderson, C. S. Intracerebral haemorrhage: Current approaches to acute management. Lancet392, 1257–1268. 10.1016/s0140-6736(18)31878-6 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Dong, C., Li, Y. & Ma, M. Venous thromboembolism prophylaxis after spontaneous intracerebral hemorrhage: A review. Neurologist29, 54–58. 10.1097/nrl.0000000000000509 (2024). [DOI] [PubMed] [Google Scholar]
- 3.Hemphill, J. C. 3rd. et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A Guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke46, 2032–2060. 10.1161/str.0000000000000069 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Zhao, Y., Xie, Y., Li, S. & Hu, M. The predictive value of neutrophil to lymphocyte ratio on 30-day outcomes in spontaneous intracerebral hemorrhage patients after surgical treatment: A retrospective analysis of 128 patients. Front. Neurol.13, 963397. 10.3389/fneur.2022.963397 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheth, K. N. Spontaneous intracerebral hemorrhage. N. Engl. J. Med.387, 1589–1596. 10.1056/NEJMra2201449 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Greenberg, S. M. et al. 2022 guideline for the management of patients with spontaneous intracerebral hemorrhage: A guideline from the American Heart Association/American Stroke Association. Stroke53, e282–e361. 10.1161/str.0000000000000407 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Sacco, S., Marini, C., Toni, D., Olivieri, L. & Carolei, A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke40, 394–399. 10.1161/strokeaha.108.523209 (2009). [DOI] [PubMed] [Google Scholar]
- 8.An, S. J., Kim, T. J. & Yoon, B. W. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J. Stroke19, 3–10. 10.5853/jos.2016.00864 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregory, P. C. & Kuhlemeier, K. V. Prevalence of venous thromboembolism in acute hemorrhagic and thromboembolic stroke. Am. J. Phys. Med. Rehabil.82, 364–369. 10.1097/01.Phm.0000064725.62897.A5 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Naccarato, M., Chiodo Grandi, F., Dennis, M. & Sandercock, P. A. Physical methods for preventing deep vein thrombosis in stroke. Cochrane Database Syst. Rev.2010, Cd001922 (2010). 10.1002/14651858.CD001922.pub3. [DOI] [PMC free article] [PubMed]
- 11.Otite, F. O. et al. Ten-year temporal trends in medical complications after acute intracerebral hemorrhage in the United States. Stroke48, 596–603. 10.1161/strokeaha.116.015746 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Prabhakaran, S. et al. Is prophylactic anticoagulation for deep venous thrombosis common practice after intracerebral hemorrhage?. Stroke46, 369–375. 10.1161/strokeaha.114.008006 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Qian, X., Ge, Y. & Luo, J. Risk factors for deep vein thrombosis after traumatic lower extremity fracture: A systematic review and meta-analysis. Medicine (Baltimore)103, e38439 (2024). 10.1097/md.0000000000038439. [DOI] [PMC free article] [PubMed]
- 14.Lattanzi, S., Cagnetti, C., Provinciali, L. & Silvestrini, M. Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke47, 1654–1657. 10.1161/strokeaha.116.013627 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Chuang, S.-M. et al. Neutrophil-to-high-density lipoprotein ratio (NHR) and neutrophil-to-lymphocyte ratio (NLR) as prognostic biomarkers for incident cardiovascular disease and all-cause mortality: A comparison study. Am. J. Prev. Cardiol.20, 100869. 10.1016/j.ajpc.2024.100869 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia, Z., Li, Z. & Chen, S. The association between neutrophil/high-density lipoprotein cholesterol ratio and non-alcoholic fatty liver disease in a healthy population. Diabetes Metab. Syndr. Obes.17, 2597–2605. 10.2147/dmso.S464406 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract.120, c179–c184 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Steiner, T. et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int. J. Stroke9, 840–855. 10.1111/ijs.12309 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Ageno, W. et al. Prevention of venous thromboembolism in immobilized neurological patients: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb. Res.124, e26-31. 10.1016/j.thromres.2009.06.032 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Ji, R. et al. Higher risk of deep vein thrombosis after hemorrhagic stroke than after acute ischemic stroke. J. Vasc. Nurs.37, 18–27. 10.1016/j.jvn.2018.10.006 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Cai, Q., Zhang, X. & Chen, H. Patients with venous thromboembolism after spontaneous intracerebral hemorrhage: A review. Thromb. J.19, 93. 10.1186/s12959-021-00345-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu, F., Ji, Y., Song, J. H., Huang, G. X. & Zhang, Y. F. Correlations between NLR, NHR, and clinicopathological characteristics, and prognosis of acute ischemic stroke. Medicine (Baltimore)102, e33957 (2023). 10.1097/md.0000000000033957. [DOI] [PMC free article] [PubMed]
- 23.Yu, L., Ma, K., Hao, J. & Zhang, B. Neutrophil to high-density lipoprotein cholesterol ratio, a novel risk factor associated with acute ischemic stroke. Medicine (Baltimore)102, e34173 (2023). 10.1097/md.0000000000034173. [DOI] [PMC free article] [PubMed]
- 24.Liu, S. L. et al. Neutrophil to high-density lipoprotein cholesterol ratio predicts adverse cardiovascular outcomes in subjects with pre-diabetes: A large cohort study from China. Lipids Health Dis.21, 86. 10.1186/s12944-022-01695-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, J. et al. Proteomics reveals plasma biomarkers for ischemic stroke related to the coagulation cascade. J. Mol. Neurosci.70, 1321–1331. 10.1007/s12031-020-01545-4 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Cui, J. et al. Thrombo-inflammation and immunological response in ischemic stroke: Focusing on platelet-tregs interaction. Front. Cell Neurosci.16, 955385. 10.3389/fncel.2022.955385 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, C. et al. Neutrophil extracellular traps exacerbate ischemic brain damage. Mol. Neurobiol.59, 643–656. 10.1007/s12035-021-02635-z (2022). [DOI] [PubMed] [Google Scholar]
- 28.Fuchs, T. A. et al. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA107, 15880–15885. 10.1073/pnas.1005743107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang, Y., Ge, H., Wang, X. & Zhang, X. Association between blood lipid levels and lower extremity deep venous thrombosis: A population-based cohort study. Clin. Appl. Thromb. Hemost.28, 10760296221121282. 10.1177/10760296221121282 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obama, T. & Itabe, H. Neutrophils as a novel target of modified low-density lipoproteins and an accelerator of cardiovascular diseases. Int. J. Mol. Sci.21, 8312 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi, Y. et al. Neutrophils can promote clotting via FXI and impact clot structure via neutrophil extracellular traps in a distinctive manner in vitro. Sci. Rep.11, 1718. 10.1038/s41598-021-81268-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Stoep, M., Korporaal, S. J. & Van Eck, M. High-density lipoprotein as a modulator of platelet and coagulation responses. Cardiovasc. Res.103, 362–371. 10.1093/cvr/cvu137 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.