Abstract
Stress and traumatic experiences have significant and lasting effects on sensory systems. We recently identified unique expression of proteins associated with epidermal skin cells (keratinocytes) and mechanosensory Merkel cells (MC) in circulating extracellular vesicles from adult women who had experienced sexual trauma specifically during adolescence, biologically linking trauma exposure with a specific neuron-like skin cell. Here, we aimed to develop and validate a preclinical mouse model utilizing chemogenetic (DREADD Gq) activation of a population of MC. Using a reporter line, we confirmed the expected pattern of the Krt14 Cre in specific MC skin areas and that these tissues expressed relevant MC marker genes similarly between male and female mice. Chemogenetic stimulation of MC produced robust neuronal activation of the insular cortex (IC), a brain region relevant to somatosensory and valence integration. To determine if the mice could detect MC activation, home cage behaviors following CNO treatment significantly increased nest grooming time. Conditioned place preference further revealed an avoidance response following MC stimulation; an effect that was stronger in female mice. Finally, to connect back to our trauma question, we examined MC activation in fear conditioning and identified deficits in fear extinction. Overall, these studies validate utilization of this preclinical model in further investigating the mechanosensory system and its potential involvement in PTSD symptoms and therapeutic interventions. Ongoing studies will focus on critical developmental periods relevant to both MC development and sex differences associated with trauma vulnerability and potential sensory based therapeutic options for PTSD-related symptoms.
Subject terms: Stress and resilience, Cellular neuroscience
Introduction
The impact of severe trauma on the central nervous system (CNS) has been the focus of post-traumatic stress disorder (PTSD) research and treatments [1]. Changes to the structure and function of brain regions that process threat learning, memory, and symptomatology are effective targets for identifying potential treatment options. However, recent evidence suggests that sensory systems are also susceptible to alterations following severe trauma and may underlie vulnerabilities important for identifying individuals at risk for developing PTSD [2–10]. Similarly, fMRI and fear conditioning data suggest deficits in fear extinction in women who experienced violent trauma [11–16]. Survivors of sexual trauma also show decreased activation of the somatosensory and insular cortices [5, 17, 18]. These studies highlight the importance of identifying the mechanisms by which somatosensory systems are involved in trauma exposure and PTSD risk.
We recently demonstrated that trauma experienced during key developmental periods increased the expression of proteins associated with epidermal skin cells (keratinocytes) and mechanosensory Merkel cells (MC) in circulating extracellular vesicles (EVs) [14]. MC are the only keratinocyte-derived neuron-like mechanosensory cells in the epidermis of skin and are responsible for the detection of light touch in touch domes of hairy skin and rete ridges of glabrous skin [19–21]. MC synapse with Aβ-afferents and express the mechanosensitive ion channel, Piezo2 [22–26]. Piezo2-containing mechanoreceptors and afferents are associated with social and reproductive functions, and disruptions in their function have been implicated in neurodevelopmental disorders including attention-deficit/hyperactivity disorder and autism spectrum disorders [27–31]. Further, genes uniquely expressed in keratinocytes and MC have been implicated in preclinical models of PTSD and genome-wide association studies of individuals with PTSD [32–36]. However, there are insufficient tools with which the mechanisms linking MC to lasting effects of stress or trauma can be investigated. Therefore, based on our previous work, we developed a transgenic mouse model that allows for chemogenetic activation of a population of MC-enriched keratinocytes [14].
Overall, our goal was to validate a novel, preclinical model of MC activation in stress- and PTSD-relevant behaviors providing for future studies to utilize this mouse to ask mechanistic questions about the developmental timing of stress experience on sensory systems in the brain. Therefore, utilizing a chemogenetic (Gq-DREADD) approach to acutely activate MC with clozapine N-oxide (CNO), we first examined in vivo imaging and ex vivo gene expression to confirm the correct and limited expression of our transgenic MC markers in male and female mice [37–39]. Next, we examined c-Fos expression in the insular cortex following CNO activation of MC [40–42]. Then, based on similar somatosensory models, we examined the influence of MC stimulation on tactile sensitivity and naturalistic behaviors to determine if mice could sense MC activation [24, 43–47]. Finally, we tested the valence of, and fear responding to, MC stimulation in a translationally relevant PTSD model [29, 30, 48, 49]. These studies validate the use of this novel preclinical model and establish a foundation for continued investigation of MC in the mechanistic underpinnings of type, timing, and sex differences in PTSD.
Methods
Animals
Adult (8-12 weeks; n = 114) male and female mice were used for all studies. In vivo imaging studies were conducted with heterozygous KRT14Cre mice (Jax stock #018964; Bar Harbor, ME, USA) bred to homozygous floxed Ai14 mice (Ai14+; Jax stock #007914). DREADD studies were conducted with heterozygous KRT14Cre mice (MC; Jax stock #018964) maintained on a C57BL/6 J background bred to heterozygous hM3Dq Designer Receptor Exclusively Activated by Designer Drugs (DREADD) mice (DRD+; Jax stock #026220) on a mixed C57BL/6J x 129S1/SvlmJ (Jax stock #002448) background to target a MC-enriched population of keratinocytes with CNO administration. Separate cohorts of mice were used for each behavioral task, except conditioned place preference and home cage behavior were conducted on the same cohort, with home cage observations occurring immediately prior to conditioned place preference (CPP) training and 7 days after CPP testing. Mice were administered chow (Teklad 2920X global soy protein-free extruded; Madison, WI, USA) and water ad libitum. Lights were maintained on a 10:14 schedule with lights on at 0600 hr and lights off at 2000 h. All animal experiments were approved by the University of Colorado Anschutz Medical Campus Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Clozapine N-oxide administration
CNO (Hello Bio #HB6149; Bristol, UK) was prepared in cookie dough at 0.1 mg CNO/g dough (bacon (Transgenic Dough Diet, Bio-Serv #S3472; Flemington, NJ, USA), Nutella (Ferrero, Alba, IT), sugar cookie (Pillsbury; Minneapolis, MN, USA), or Reese’s Peanut Butter (Pillsbury)) [42]. For all CNO dosing experiments, mice were primed with CNO-free dough for 3 days, followed by 2 days of no dough. Acute CNO was administered at 5 mg/kg unless otherwise noted (Table S1). Previous development of DREADD-Gq models in our lab found that varied flavors and dosages of CNO were necessary to maintain consistent consumption of CNO cookie dough, especially if the cell type being activated by CNO is associated with a negative valence [42].
In Vivo imaging system (IVIS)
Keratinocyte and MC bioluminescence were measured using an in vivo imaging system (IVIS Spectrum, Perkin Elmer; Springfield, IL, USA) in the Anschutz Medical Campus Small Animal Imaging Shared Resource. Ai14+ mice were anesthetized with isoflurane and placed in the IVIS. Ai14 imaging was performed with an excitation/emission wavelength pair of 570/620 nm, maximized for TdTomato signal-to-noise ratio.
TaqMan qPCR gene expression analysis
Mice were anesthetized with isoflurane, treated with Nair for hair removal, and cervically dislocated. Lips and paws were harvested, immediately snap-frozen in liquid nitrogen, and homogenized as previously described [50]. Total RNA was isolated using Qiagen RNeasy Fibrous Tissue Kit according to manufacturer’s protocol (Qiagen #74704; Germantown, MD, USA). cDNA was transcribed using the Applied Biosystems High-Capacity cDNA reverse transcriptase kit (Thermo Fisher #4368814; Waltham, MA, USA). Quantitative real-time PCR (qRT-PCR) was performed using TaqMan Gene Expression Assays, TaqMan Fast Advanced Master Mix (Thermo Fisher #44445556), and primers for KRT7 (Mm00466676_m1), KRT14 (Mm00516876_m1), Piezo2 (Mm01265861_m1), and Actb (Mm02619580_g1). Samples were run in triplicate with a no-template control for each gene in the same qRT-PCR experiment. Relative quantification of gene expression was calculated with the comparative Ct (∆CT) method using Actb as a reference gene.
FFPE preparation of mouse skin
Following isoflurane anesthesia, mouse lips and paws were carefully dissected. Samples were fixed in 10% neutral buffered formalin (Sigma Aldrich, #HT501128) overnight. Samples were then dehydrated in an ethanol gradient (50%, 50%, 80%, 95%, 95%,100%, 100%, xylene x3, and paraffin wax x4) in a Tissue-Tek VIP (Sakura, Torrance, CA, USA) and embedded with Paraplast Plust (Millipore Sigma, #P3683). 4 µm sections were cut on a Microm microtome (HM310) and mounted on TOMO hydrophilic slides (Matsunami Glass Ind., LTD., #TOM-12). Slides were then processed on an Autostainer Link 48 (Agilent Dako, Santa Clara, CA, USA). Slides were incubated (1 hr at 90̊ °C) with Citrate Buffer (pH 6; Bio SB, #BSB 0021, Santa Barbara, CA, USA), washed with TBS-T, blocked in 10% BSA in PBS (1 hr at RT), and rinsed with TBS-T. Slides were then incubated (1 hr at RT) with primary antibodies for TROMA-I anti-cytokeratin 8 (KRT8; DSHB, #AB_531826; 1:300; Iowa City, IA, USA) and HA (Proteintech, 51064-2-AP; 1:100; Rosemont, IL, USA). Sections were then rinsed with TBS-T and incubated with secondary antibodies (1 hr at RT; Alexa Fluor 488 chicken anti-rat IgG; Thermo Fisher #A-21470 and Alexa Fluor 568 goat anti-rabbit IgG; Thermo Fisher #A-11036; 1:2000), washed in TBS-T, and cover-slipped EverBrite Hardset mounting medium containing DAPI (Biotium #23004; Fremont, CA, USA). Images were acquired at 40x magnification on an Olympus APX100 microscope system.
Immunohistochemistry
Two hours after CNO treatment, mice were anesthetized with isoflurane and transcardially perfused with PBS and fixed with 4% Paraformaldehyde. Brains were post-fixed for 24 hr, placed in cryoprotection (30% sucrose), and frozen at −80 °C. Frozen brains were mounted in OCT and sectioned on a Leica cryostat at 45 µm. Sections were selected at 0.85 to −0.59 mm from Bregma for S1 and IC and at −4.95 to −5.63 from Bregma for PBN based on the Mouse Brain Atlas [51]. Sections were washed in PBS, pretreated in 0.1 M Glycine (30 min at RT), washed in PBS, and incubated in SDS (10 min at RT). Sections were then washed in PBS-T, blocked in 4% NGS in PBS-T (1 hr at RT), and then incubated overnight in c-Fos antibody (Synaptic Systems, #226308; 1:2500; Goettingen, DE) in 4% NGS. Sections were then washed in PBS-T at RT, incubated with secondary antibody (2 hr at RT; Alexa Fluor 488 goat anti-guinea pig IgG; Thermo Fisher #A-11073; 1:2000), washed in PBS, and mounted on slides (VWR Superfrost Plus, 48311-703; Radnor, PA, USA) with antifade mounting medium containing DAPI (Enzo #53003; Farmingdale, NY, USA). Images were acquired at 20x magnification on an Olympus APX100 microscope system. Six images per mouse were used to quantify c-Fos positive cells within a collection of S1 and IC regions of interest and two images per mouse within the PBN regions of interest [52, 53]. The total number of positive cells per image was quantified using “GT_annotations tool” ImageJ plugin [54].
von Frey filament test
Mice were placed on a suspended wire mesh to habituate for 1 hr the day before testing. Mice received CNO cookie dough 15 min prior to testing. The von Frey filament test consists of monofilaments of increasing diameter with forces from 0.008 to 11.0 g (North Coast Medical #NC12775-99; Morgan Hill, CA, USA). Each filament was pressed against the hind paw skin, and withdrawal/no withdrawal responses were recorded until the paw was withdrawn on 5 consecutive trials [42, 55]. The proportion of paw withdrawals during ascending trials was used to calculate the frequency of the withdrawal curve. The von Frey threshold (vF50) corresponds to the 50% frequency of withdrawal for each mouse [42].
Home cage behavior
Mice received CNO cookie dough and were observed for 72 min with the frequency of behaviors recorded every 3 min. Recorded behaviors included eating, drinking, grooming, nest building, and the location of the behavior (in/out of nest). Home cage observations occurred within 1 hr of lights on. Chronic CNO was administered daily for 14 days with varying flavors and dosages (0.25–5 mg/kg; Table S1) [42].
Open-field test (OFT)
Prior to testing, mice were habituated to the testing room for 30 min. The OFT consists of an open box (40 cm×40 cm x 30.5 cm), with a defined perimeter (6 inches from any wall) and a 20 ×20 cm center square. Mice were placed in the center square to start each 10 min session. Overhead digital cameras recorded center times and were calculated with EthoVision XT (Noldus, v17.5; Leesburg, VA, USA). Experiments were performed in the morning (from 0800 h to 1200 h). The OFT was cleaned with 70% EtOH between trials.
Conditioned place preference (CPP)
The CPP test was conducted like previous DREADD-based designs [29, 30, 48]. Prior to each testing or training procedure, mice were habituated to the testing room for 30 min. On day 1, mice were allowed to explore the CPP arena (compartments: 20 cm × 20 cm x 28 cm; transition area: 19 cm × 9 cm x 28 cm with a 2.5 cm door) for 20 min. One arena contained a smooth metal floor with circular patterns on the walls and the other arena contained an angled polyurethane foam floor with stripe patterns on the walls. From days 2-6, mice were conditioned to associate a saline cookie dough treat to the preferred zone (defined during the first day) and a CNO cookie dough treat (varied flavors and doses; Table S1) to the other arena. During conditioning, mice received cookie dough ~15 min prior to conditioning in the designated compartment for 20 min. To avoid CNO remaining active between the two conditioning sessions, saline conditioning was conducted in the morning, and CNO conditioning was conducted in the afternoon. On day seven, mice were free to explore the entire arena for 20 min. The position of the animal was detected by overhead digital camera,s and arena times were calculated with EthoVision XT (Noldus, v17.5; Leesburg, VA, USA). Experiments were performed in the morning (from 0800 h to 1200 h) and afternoon (from 1300 hr to 1700 hr).
Contextual fear conditioning and extinction
Mice were habituated to the fear conditioning chambers for 10 min (Med Associates Inc., Fairfax, VT, USA) in context A (wire grid floor cleaned with 70% EtOH), similar to previous studies [56, 57]. The next day, in context A, acquisition of the conditioned stimulus response was elicited by pairing a tone (75 dB) with a foot shock (0.4 mA) four times with a 60 sec delay between pairings. For experiment 1, extinction day 1 testing (15 tone presentations) was conducted in the same context A with CNO treats administered ~15 min prior to testing. Extinction day 2 was conducted as on day 1, but without CNO administration. For experiment 2, extinction was conducted as before, but in context B (solid metal floor cleaned with 70% EtOH plus 10% apple cider vinegar). All fear conditioning occurred in the morning (0800 h–1200 h). Freezing behavior was analyzed with ezTrack Freeze Analysis [58].
Statistical analysis
Data are represented as mean ± SEM. All statistical analyses were performed in GraphPad Prism, (V10.4.1; Boston, MA, USA). Schematics and figures were prepared with BioRender and GraphPad Prism. Outlier analysis utilized the ROUT method (Q = 1% cutoff threshold). For all statistical tests, a value of p < 0.05 was considered significant. All testing was completed by experimenters blinded to treatment and genotype groups. All statistical comparisons and results are detailed in Table S2.
Results
All statistical results are reported within the figure legends and in Table S2.
Validation of Merkel cell-enriched keratinocyte populations
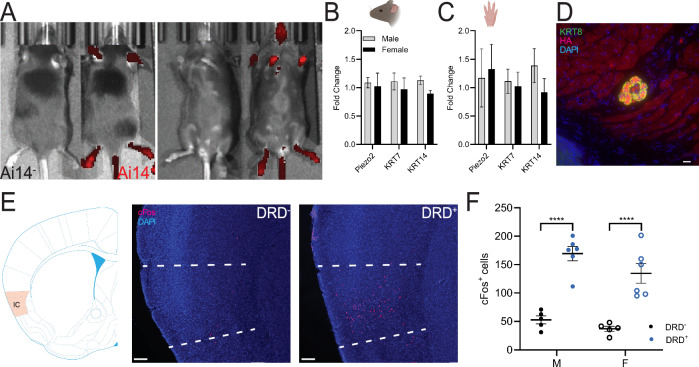
Based on our previous findings, we aimed to validate a mouse chemogenetic model of MC-enriched keratinocyte activation in altering behaviors in adult male and female mice [14]. First, we identified KRT14+ cells (MC) in all visible dorsal and ventral skin using IVIS imaging of Ai14+ cells in KRT14Cre x Ai14 mice compared to wildtype littermates (Fig. 1A). No sex differences were detected in MC-related gene expression in lips (Fig. 1B) or paws (Fig. 1C) of wildtype mice [26, 59]. Individual channel images of DAPI (Fig. S1A), α-KRT8 (Fig. S1B), and HA (Fig. S1C) reveal the robust expression of hM3dq DREADD and the α-KRT8 MC marker. Co-labeling of α-KRT8 (MCs) and HA (DREADD hM3Dq) in DRD+ mice shows DREADD Gq expression in KRT8 + MC (Fig. 1D and Fig. S1D).
Fig. 1. Validation of Merkel cell-enriched keratinocyte populations.
A Dorsal (left) and ventral (right) visible skin showed robust expression of keratinocytes and Merkel cells (MC) in Ai14+ mice compared to Ai14- littermates. B, C qPCR analysis confirmed expression of keratinocyte (KRT7, KRT14) and MC (KRT14, Piezo2) genes in lip and paw tissue. There is no significant sex differences for Piezo2 in lip (n = 4; unpaired t test: t(6) = 0.3654, p = 0.9101) or paw (n = 4; unpaired t test: t(6) = 0.5186, p = 0.7599), KRT7 in lip (n = 4; unpaired t test: t(6) = 0.0439, p = 0.9102) or paw (n = 4; unpaired t test: t(6) = 0.7002, p = 0.7599), or KRT14 in lip (n = 4; unpaired t test: t(6) = 0.3654, p = 0.9102) or paw (n = 4; unpaired t test: t(6) = 0.5186, p = 0.7599). D Co-labeling of KRT8 (green) and HA (red) associated with the hM3Dq transgene in DRD+ mice shows Gq expression in MC. Scale bar = 20 μm. E Representative images of c-Fos immunostaining in the Insular Cortex (IC) from DRD- (left) and DRD+ (right) mice following stimulation of MC-enriched keratinocyte populations. Scale bar = 100μm. F Quantification of c-Fos positive cells in IC. DRD+ mice had significantly higher c-Fos positive cells compared to DRD- controls with no significant difference between males and females (n = 5; two-way ANOVA: Fsex*genotype(1,18) = 0.5821, p = 0.4554; Fsex(1,18) = 4.046, p = 0.0595; Fgenotype(1,18) = 73.72, p < 0.0001). Ai14+ mice are KRT14Cre+ x Ai14+, Ai14- mice are wildtype littermates. DRD+ mice are KRT14Cre+ x hM3Dq+, DRD- mice are wildtype littermates. (****p < 0.0001).
We next identified cortical activation of neurons in KRT14Cre+ x hM3Dq+ (DRD+) mice compared to wildtype littermates (DRD-). Following CNO administration, the number of c-Fos positive cells was significantly higher in the insular cortex (IC) of male and female DRD+ mice (Fig. 1E, F). Additionally, c-Fos positive cell counts were increased in the somatosensory cortex (S1) and parabrachial nucleus (PBN) of male and female DRD+ mice (Fig. S1E, F). We did not detect sex differences in c-Fos positive cell counts in the IC or S1 following CNO administration. DRD+ male mice had more c-Fos positive cells in the PBN compared to DRD+ females (Fig. S1G, H).
Merkel cell stimulation does not alter tactile sensitivity, but does increase grooming behavior and codes for negative valence
To identify the influence of MC stimulation on tactile sensitivity, we utilized the von Frey filament test. There was no effect of genotype on paw withdrawal curves following CNO treatment for male (Fig. 2A) or female (Fig. 2B) mice. Similarly, von Frey thresholds (vF50) were not influenced by MC stimulation in either male or female mice of either genotype (Fig. 2C). No sex differences were detected in paw withdrawal curves or vF50.
Fig. 2. Tactile sensitivity, home-cage behavior, and valence following MC stimulation.
The von Frey filament test was used to measure tactile sensitivity. The paw withdrawal curve was not different in male (A; Nonlinear fit: n = 5; F(1,72) = 0.08362, p = 0.9689) or female (B; Nonlinear fit: n = 5; F(1,72) = 0.4887, p = 0.6907) DRD+ mice, compared to DRD- littermates following MC stimulation. C There was also no difference in vF50 for tactile sensitivity following MC stimulation (n = 5; two-way ANOVA: Fsex*genotype(1,16) = 0.0578, p = 0.8131; Fsex(1,16) = 0.1361, p = 0.7171; Fgenotype(1,16) = 2.215, p = 0.1561). Home-cage observations were conducted following the first dose of CNO. D, E Grooming frequency was increased in DRD+ mice compared to DRD- littermates following the initial MC stimulation independent of sex (n = 10; two-way ANOVA: Fsex*genotype(1,36) = 3.527, p = 0.0685; Fsex(1,36) = 1.2, p = 0.2806; Fgenotype(1,36) = 543.7, p < 0.0001). F The total nest frequency was also increased in DRD+ mice compared to DRD- littermates after MC stimulation (n = 10; two-way ANOVA: Fsex*genotype(1,36) = 0.2489, p = 0.6209; Fsex(1,36) = 2.476, p = 0.1244; Fgenotype(1,36) = 45.1, p < 0.0001). G, H Following 14 d chronic CNO, grooming frequency was still increased in DRD+ mice compared to DRD- littermates (n = 10; two-way ANOVA: Fsex*genotype(1,36) = 0.003, p = 0.9566; Fsex(1,36) = 0.0271, p = 0.8702; Fgenotype(1,36) = 72.29, p < 0.0001) following MC stimulation. I Total nest frequency was also increased in DRD+ mice following 14 d chronic CNO, compared to DRD- littermates (n = 10; two-way ANOVA: Fsex*genotype(1,36) = 5.881, p = 0.0205; Fsex(1,36) = 7.443, p = 0.0098; a=Fgenotype(1,36) = 18.01, p = 0.0001). Female DRD- mice spent less time in the nest than male DRD- mice (b = Tukey post-hoc p = 0.0045) and female DRD+ mice (c = Tukey post-hoc p = 0.0002). J Conditioned place preference revealed a negative valence of MC stimulation. Male DRD- mice did not show a preference for either control or CNO-paired arenas (n = 10; paired t test: t(9) = 0.2522, p = 0.8065). Male DRD+ mice preferred the control-paired arena compared to the CNO-paired arena (n = 10; paired t test: t(9) = 2.410, p = 0.0393). K Female DRD- mice did not show a preference for either control or CNO-paired arenas (n = 10; paired t test: t(9) = 0.0968, p = 0.9520). Female DRD+ mice preferred the control-paired arena compared to the CNO-paired arena (n = 10; paired t test: t(9) = 5.632, p = 0.0003). L Overall, the preference for the CNO-paired arena (%) was decreased in male and female DRD+ mice (n = 10; two-way ANOVA: Fsex*genotype(1,36) = 0.8244, p = 0.3699; Fsex(1,36) = 1.138, p = 0.2932; a=Fgenotype(1,36) = 7.797, p = 0.0083) compared to DRD- mice; especially in female DRD+ compared to female DRD- mice (b=Tukey post-hoc p = 0.0129). DRD+ mice are KRT14Cre+ x hM3Dq+, DRD- mice are wildtype littermates. (*p < 0.05, ****p < 0.0001).
Home cage observations were conducted following the first CNO dose and after two weeks of daily CNO dosing to examine the effect of MC stimulation on naturalistic behaviors. After the first CNO dose, male and female DRD+ mice spent significantly more time grooming (Fig. 2D, E and supplemental video 1) and more time in their nest (Fig. 2F) than DRD- littermates (supplemental video 2). DRD+ mice also spent more time grooming in their nest (Fig. S2A), had a higher ratio of in nest grooming: in nest time (Fig. S2B), and lower frequency of all other observed behaviors (Fig. S2C). To examine if these effects were due to novelty of MC stimulation, we examined these behaviors following two weeks of daily CNO. DRD+ mice maintained a similar response to MC stimulation, with both males and females spending more time grooming than DRD- littermates (Fig. 2G, H). Total nest frequency revealed an interaction, driven by decreased nest time in DRD- female mice compared to male DRD- mice and female DRD+ mice (Fig. 2I). Compared to DRD- littermates, DRD+ male and female mice also spent significantly more time grooming in their nest (Fig. S2D), had an increased ratio of in nest grooming: in nest time (Fig. S2E), and had a lower frequency of all other observed behaviors, including feeding, drinking, and nest building, (Fig. S2F). These responses do not appear to be driven exclusively by an anxiety-like response, as there was no difference in OFT center time following acute (~10 min) CNO stimulation of MC (Fig. S2G).
To determine the valence of MC stimulation, we conducted conditioned place preference (CPP). Following training, both male (Fig. 2J) and female (Fig. 2K) DRD+ mice spent significantly more time in the saline-paired arena compared to the CNO-paired arena. DRD- mice did not display an arena preference following training. The percentage of time and difference in time spent in the CNO-paired arena was decreased in DRD+ male and female mice (Fig. 2L, S2H). While patterns in the data suggest potential for sex differences in magnitude of effects, no interaction between sexes was identified. Future studies should dissect unique aspects of sex specific central integration of this sensory stimulation.
Merkel cell stimulation attenuates fear extinction
Given the potential for MC stimulation to alter tactile perception, we conducted fear conditioning with extinction paradigms occurring in different tactile and odor settings. On extinction day 1, with CNO in context A, both male (Fig. 3A) and female (Fig. 3B) DRD+ mice displayed increased freezing compared to DRD- littermates. However, on extinction day 2, without CNO, freezing time was not significantly different between DRD+ male or female mice and their DRD- littermates (Fig. 3C, D). To identify the impact of context and tactile perception on fear extinction, we also conducted extinction trials in context B. On extinction day 1 with CNO, while a similar pattern of increased freezing appears for DRD+ male (Fig. 3E) and female (Fig. 3F) mice, neither group reached statistical significance compared to DRD- littermates. On extinction day 2 without CNO, the increased freezing in male DRD+ mice compared to DRD- littermates was statistically significant (Fig. 3G), but a similar pattern of differences did not reach statistical significance in female mice (Fig. 3H). AUC analyses for extinction curves revealed similar patterns; increased freezing in both male and female DRD+ mice on extinction day 1, with CNO in context A and increased freezing only in male DRD+ mice on extinction day 2, without CNO in context B (Fig. S2I, J).
Fig. 3. Fear extinction is dependent on MC stimulation.
A Male DRD+ mice spent more time freezing following CNO, on extinction day 1 (n = 15; two-way RM ANOVA: Ftime*genotype(14, 392) = 0.6724, p = 0.8016; Ftime(5.856, 164) = 10.03, p < 0.0001; Fgenotype(1, 28) = 6.677, p = 0.0153). B Female DRD+ mice also spent more time freezing with CNO, on extinction day 1 (n = 14; two-way RM ANOVA: Ftime*genotype(14, 364) = 0.9085, p = 0.5498; Ftime(4.86, 126.4) = 6.12, p < 0.0001; Fgenotype(1, 26) = 10.81, p = 0.0029). C On extinction day 2, without CNO, there was no difference in freezing behavior in male mice (n = 15; two-way RM ANOVA: Ftime*genotype(14, 252) = 0.9439, p = 0.5122; Ftime(7.535, 135.6) = 2.077, p = 0.0456; Fgenotype(1, 18) = 1.142, p = 0.2994). D On extinction day 2, without CNO, DRD+ female mice had different freezing patterns than DRD- mice (n = 14; two-way RM ANOVA: Ftime*genotype(14, 196) = 1.911, p = 0.0272; Ftime(14, 196) = 2.258, p = 0.0072; Fgenotype(1, 14) = 0.0066, p = 0.9664). E In context B, on day 1 with CNO, male DRD+ and WT spent similar amounts of time freezing (two-way RM ANOVA: n = 8; Ftime*genotype(14, 210) = 1.091, p = 0.367; Ftime(4.313, 64.69) = 7.786, p < 0.0001; Fgenotype(1, 15) = 4.012, p = 0.0636). F Female mice did not show significantly different freezing behavior in context B on day 1, with CNO (two-way RM ANOVA: n = 7; Ftime*genotype(14, 154) = 0.8588, p = 0.6046; Ftime(4.131, 45.44) = 2.106, p = 0.0935; Fgenotype(1, 11) = 2.999, p = 0.1112). G On extinction day 2, without CNO, male DRD+ mice spent more time freezing compared to WT littermates (two-way RM ANOVA: n = 8; Ftime*genotype(14, 210) = 0.6007, p = 0.8629; Ftime(6.537, 98.05) = 2.189, p = 0.0454; Fgenotype(1, 15) = 6.413, p = 0.023). H Female mice did not spend more time freezing on extinction day 2, without CNO (two-way RM ANOVA: n = 7; Ftime*genotype(14, 168) = 1.108, p = 0.3538; Ftime(14, 168) = 2.177, p = 0.0105; Fgenotype(1, 12) = 1.965, p = 0.1863). DRD+ mice are KRT14Cre+ x hM3Dq+, DRD- mice are wildtype littermates. (*p < 0.05).
Discussion
Recent studies have identified a significant impact of stress and trauma on sensory pathways [4, 5, 60–63]. We previously identified keratinocyte and MC-related protein expression in an unbiased proteomics screen from circulating extracellular vesicles in adult women who had experienced sexual trauma [14]. Sexual trauma alters insular and somatosensory cortices, as well as heightened fear conditioning, however, a preclinical model to assess the underlying mechanisms of sensory involvement in fear memory processing has not been developed [5, 17, 18, 64]. Therefore, we utilized chemogenetic activation of MC in mice as a model to assess potential MC involvement in specific fear and stress behavioral assays.
First, to validate this model, we examined expression patterns of the KRT14cre utilizing in vivo imaging system (IVIS) and an Ai14 fluorescent reporter to visualize MC-enriched populations of keratinocytes [24, 43, 45, 46, 65, 66]. IVIS imaging revealed fluorescence in KRT14+ cells, including lips, ears, paws, genitals, and tail, consistent with expected MC expression [20, 21, 24, 37, 39, 43, 45, 46, 67]. Additionally, we compared expression levels of known genes important to MC differentiation and function in lips and paws from male and female mice using real-time PCR. We did not detect sex differences in the levels of pre- and post-differentiation keratinocyte (KRT7 and KRT14) and MC (KRT14 and Piezo2) related genes [26, 59]. Additionally, co-labeling of the hM3Dq DREADD receptor HA with the TROMA-I α-KRT8 confirmed the presence of hM3Dq DREADD expression in MC. Combined, these results validate that our KRT14Cre mouse line expresses in specific MC skin areas and that these tissues express relevant MC marker genes at similar levels between male and female mice.
Next, we focused on the CNS to identify the impact of MC activation on neural activity in DREADD positive (DRD+) mice as evidence that CNO treatment could produce neuronal activation within the insular cortex (IC) based on its role in somatosensory and valence perception [30, 40, 41, 68, 69]. As expected, male and female DRD+ mice showed significantly increased c-Fos positive cells in IC compared to DRD- controls following CNO treatment. Additionally, MC stimulation increased c-Fos-positive cells in the S1 and PBN [53, 68, 70–72]. MCs form a synapse at the Merkel disc in touch domes projecting to the dorsal root ganglion via Aβ-afferents [22, 26, 73–75]. C-LTMR (C-fiber low threshold mechanoreceptor) afferents are largely responsible for ‘emotional touch’, however, significant overlap exists in the distribution of Aβ- and C-LTMRs in hairy skin, their innervation of hair follicles (C-LTMRs are not present in glabrous skin), and the brain regions that they activate [30, 40, 41, 53, 76–82]. While activation of Aβ-afferents is well defined, our study demonstrates the novel identification of IC neuronal activation following MC stimulation [22–26, 43–46]. Interestingly, beyond its role in affiliative touch processing, the IC is highly involved in valence detection and the sensory integration of fear signals [52, 69, 72, 83–88].
Based on the role of MC in the detection of light touch, we next validated the role of acute MC stimulation on tactile sensitivity [19–21]. To identify the influence of MC activation on local tactile sensitivity, we tested mice in the von Frey monofilament test and found no genotypic differences in paw withdrawal following CNO treatment. Previous studies did not identify tactile differences with chemogenetic or optogenetic stimulation of MC-enriched populations of keratinocytes, but describe increased frequencies of grooming or itching [43–46, 65, 66]. Interestingly, decreased tactile sensitivity and reduced Aβ-afferent responses in mice with MC-specific Piezo2 knockout or optogenetic inhibition have been reported [23–25, 89].
As acute CNO stimulation did not alter tactile sensitivity, we examined mice for a behavioral confirmation that they were detecting CNO stimulation of MC. In a home cage behavioral assessment following acute CNO administration, male and female DRD+ mice performed significantly more grooming behavior in their nest compared to DRD- littermates. Based on the significant increase in time spent grooming, not surprisingly DRD+ mice spent less time engaging in other naturalistic behaviors. The grooming phenotype aligns with previous reports of chemogenetic and optogenetic stimulation of MC and a potential role of MC involvement in converting the perception of touch to itch [43–46, 65].
To determine if these effects of CNO on grooming were due to the novelty of acute MC stimulation, we also examined mice following daily CNO treatment. Following 14 days of CNO administration, male and female DRD+ mice still performed grooming behavior at a significantly higher rate than DRD- mice, while also performing fewer other naturalistic behaviors, including feeding, drinking, and nest building. However, the effect was less robust following the chronic CNO administration and suggests that mice do partially habituate to MC stimulation. The persistence of the grooming phenotype (~60 min) in DRD+ mice indicates a potentially aversive or stressful perception of MC stimulation associated with IC activation, despite the lack of an effect of MC stimulation in the OFT [90–93].
We next wanted to identify the valence of MC stimulation. To quantify positive or negative valence, we conducted a conditioned place preference test. DRD+ mice displayed a significant avoidance response to the CNO-paired arena compared to the saline-paired arena; an effect not found in the DRD- mice. Additionally, this effect was greater in DRD+ female mice compared to males, suggesting potential sex differences in the intensity of CNO stimulation of MC or central integration of this sensory perception. Previous studies have identified sex differences in preference or valence responses to emotional stimuli that are associated with IC activity [85, 86, 94–96]. Interestingly, while we found that MC stimulation produced a CNO-paired aversion, previous studies found that C-LTMR stimulation was associated with CNO-paired preference [29, 30, 48]. These opposing behavioral outcomes may reflect the heterogeneity of cell types and subregions within the IC that are activated by ascending Aβ and C-LTMR pathways [30, 40, 41, 52, 68, 77–82, 85, 86]. Overall, this data aligns with previous reports of changes in IC activity during conditioned aversion, with negative valence, and following stress or trauma [97–102].
Changes in sensory systems have been described in PTSD, and survivors of sexual trauma display decreased activation of the somatosensory and insular cortices [2–10, 17, 18]. Additionally, violent or sexual trauma is associated with heightened fear conditioning and deficits in fear extinction [11–16, 64]. These effects are often stronger in women [13, 14, 16, 103]. While sex differences have not been directly examined in mechanosensory systems, RNA sequencing of Aβ-afferent containing dorsal root ganglia neurons has identified sex-specific gene expression differences [104–106]. To this end, we next validated our MC stimulation model in a PTSD-relevant fear conditioning task. We found that CNO administration prior to extinction day 1 produced increased freezing behavior, indicating an augmented fear response in both male and female DRD+ mice. However, in the absence of further CNO treatment on day 2, extinction in both male and female mice was indistinguishable from DRD- littermates, suggesting that this effect might be reflective of a heightened sensory sensitivity following acute MC activation that impacts extinction in this same context.
Next, to examine the potential role of context in extinction learning following MC activation, we examined mice following acute CNO treatment. Again, similar to context A, when CNO was administered prior to fear extinction day 1 but in a novel context (context B), we saw a similar pattern as previously with increased freezing responses in DRD+ male and female mice. However, with the increased variance in both genotypes in this novel context, these differences did not reach statistical significance, likely due to the stress of novelty for all mice or a potential floor effect of freezing in mice [107]. Interestingly, on the second extinction day, in context B, male DRD+ mice reached significance for increased freezing, potentially due to pairing context B with CNO administration on extinction day one, leading to reduced variance on the second extinction day. Female mice showed similar increased freezing on day 2, but again, this did not reach significance. We note that the lack of sex differences in fear extinction is surprising, despite females showing a larger negative valence effect in the conditioned place preference. This could be explained by an increased stress sensitivity to the novel context for both DRD+ and DRD- female mice [108–113]. Combined, these findings validate a role for MC sensory activation in the expression of fear memory and identify exciting targets of future studies, especially known circuits between the somatosensory cortex, insular cortex, prefrontal cortex, and amygdala [114–121].
Increased fear-related responses in female rodents and risk for developing PTSD in women have been previously reported [94–96, 110, 113, 122–126]. Meta-analyses report significant sex differences not only in PTSD vulnerability, but also for differences in the experience of the traumatizing event and the underlying neural activation in response to stimuli with positive and negative emotional valence [94–96, 117, 122, 124]. Further, biological differences in fear responding may be influenced by sex hormones and an evolutionary ability to differentially adapt to stress or trauma [12, 96, 124, 127, 128]. Ongoing studies should focus on mechanistic questions to resolve the neural and biological underpinnings of MC function as they pertain to sex differences in valence perception and conditioned fear. Finally, as MCs reach full developmental differentiation during critical pubertal windows, a period that also represents increased vulnerability to trauma, future studies can utilize preclinical models to focus on the impact of stress and MC activation during these developmental periods [14, 78, 103, 129–134]. These studies support an opportunity to utilize a preclinical model to explore potential sensory-based therapeutic options for anxiety and PTSD-related symptoms [135–143].
Supplementary information
Acknowledgements
We thank the CU Anschutz Animal Behavior Core, Drs. Michael Mesches and Nicolas Busquet, for their guidance, training, and feedback on all mouse behavior tasks. Mouse imaging support was supported in part by the National Institutes of Health P30CA046934 funded University of Colorado Anschutz Medical Campus Cancer Center Animal Imaging Shared Resource Core Facility [RRID: SCR_021980] and the NIH SIFAR shared instrumentation grant S10OD27023. We thank Jenna Steiner, AAS, CVT, and Toni Mufford, LVT, RLATG, for assistance with IVIS imaging and analysis. We thank the CU Anschutz Gates Institute Histology Core and Laura Hoaglin, HT(ASCP,) for processing FFPE skin samples. The TROMA-I anti-cytokeratin 8 monoclonal antibody was developed by Dr. Kannan Athilakshmi at the University of Illinois at Urbana-Champaign and obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at the University of Iowa, Department of Biology, Iowa City, IA 52242. We thank Dr. Brian Dias for his excellent suggestions and feedback on the contextual fear conditioning protocol.
Author contributions
ACK, TJ, and TLB conceived the studies and designed the experiments. ACK, RO, IJS, and KEP performed the experiments. ACK, RO, and IJS analyzed the data and prepared the figures. ACK and TLB wrote and edited the manuscript with input from all authors. TJ and TLB supervised the research.
Funding
These studies were supported by National Institutes of Health funding to TJ and TLB (MH129495).
Data availability
All relevant data are available upon response request to the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-025-02144-w.
References
- 1.Fenster RJ, Lebois LAM, Ressler KJ, Suh J. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat Rev Neurosci. 2018;19:535–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fani N, Carter SE, Harnett NG, Ressler KJ, Bradley B. Association of racial discrimination with neural response to threat in Black women in the US exposed to Trauma. JAMA Psychiatry. 2021;78:1005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearney BE, Lanius RA. The brain-body disconnect: A somatic sensory basis for trauma-related disorders. Front Neurosci. 2022;16:1015749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming LL, Harnett NG, Ressler KJ. Sensory alterations in post-traumatic stress disorder. Curr Opin Neurobiol. 2023;84:102821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowland GE, Roeckner A, Ely TD, Lebois LAM, van Rooij SJH, Bruce SE, et al. Prior sexual trauma exposure impacts posttraumatic dysfunction and neural circuitry following a recent traumatic event in the AURORA Study. Biol Psychiatry Glob Open Sci. 2023;3:705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoki S, Nozawa E. Pilot study on classification of sensory symptoms in PTSD. J Child Adolesc Trauma. 2024;17:283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harnett NG, Fleming LL, Clancy KJ, Ressler KJ, Rosso IM. Affective visual circuit dysfunction in trauma and stress-related disorders. Biol Psychiatry. 2024;97:405–16. [DOI] [PMC free article] [PubMed]
- 8.Korgan AC, Prendergast K, Rosenhauer AM, Morrison KE, Jovanovic T, Bale TL. Trauma and sensory systems: biological mechanisms involving the skin and the 17q21 gene cluster. Biol Psychiatry. 2024;97:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matson R, Barnes-Brown V, Stonall R. The impact of childhood trauma on sensory processing and connected motor planning and skills: a scoping review. J Child Adolesc Trauma 2024;17:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens L, Bregulla M, Scheele D. Out of touch? How trauma shapes the experience of social touch - Neural and endocrine pathways. Neurosci Biobehav Rev. 2024;159:105595. [DOI] [PubMed] [Google Scholar]
- 11.Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;69:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry. 2012;72:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michopoulos V, Norrholm SD, Jovanovic T. Diagnostic biomarkers for posttraumatic stress disorder: promising horizons from translational neuroscience research. Biol Psychiatry. 2015;78:344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison KE, Stenson AF, Marx-Rattner R, Carter S, Michopoulos V, Gillespie CF, et al. Developmental timing of trauma in women predicts unique extracellular vesicle proteome signatures. Biol Psychiatry. 2022;91:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machlin L, Sheridan MA, Lurie LA, Kasparek SW, Kim SG, Peverill M, et al. Alterations in fear learning as a mechanism linking childhood exposure to violence with PTSD symptoms: a longitudinal study. Psychol Med. 2024;54:3389–97. [DOI] [PMC free article] [PubMed]
- 16.Moallem BI, Wen Z, Hammoud MZ, Su W, Pace-Schott EF, Milad MR. Impact of trauma type on neural mechanisms of threat conditioning and its extinction. J Psychiatr Res. 2024;178:50–58. [DOI] [PubMed] [Google Scholar]
- 17.Heim CM, Mayberg HS, Mletzko T, Nemeroff CB, Pruessner JC. Decreased cortical representation of genital somatosensory field after childhood sexual abuse. Am J Psychiatry. 2013;170:616–23. [DOI] [PubMed] [Google Scholar]
- 18.Harnett NG, Ference EW 3rd, Wood KH, Wheelock MD, Knight AJ, Knight DC. Trauma exposure acutely alters neural function during Pavlovian fear conditioning. Cortex 2018;109:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature 2007;445:858–65. [DOI] [PubMed] [Google Scholar]
- 20.Woo SH, Lumpkin EA, Patapoutian A. Merkel cells and neurons keep in touch. Trends Cell Biol. 2015;25:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handler A, Ginty DD. The mechanosensory neurons of touch and their mechanisms of activation. Nat Rev Neurosci. 2021;22:521–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haeberle H, Fujiwara M, Chuang J, Medina MM, Panditrao MV, Bechstedt S, et al. Molecular profiling reveals synaptic release machinery in Merkel cells. Proc Natl Acad Sci USA. 2004;101:14503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell 2014;157:664–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 2014;509:617–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature 2014;509:622–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman BU, Baba Y, Griffith TN, Mosharov EV, Woo SH, Roybal DD, et al. Merkel cells activate sensory neural pathways through adrenergic synapses. Neuron 2018;100:1401–13.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orefice LL, Zimmerman AL, Chirila AM, Sleboda SJ, Head JP, Ginty DD. Peripheral mechanosensory neuron dysfunction underlies tactile and behavioral deficits in mouse models of ASDs. Cell 2016;166:299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orefice LL, Mosko JR, Morency DT, Wells MF, Tasnim A, Mozeika SM, et al. Targeting peripheral somatosensory neurons to improve tactile-related phenotypes in ASD Models. Cell 2019;178:867–86.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huzard D, Martin M, Maingret F, Chemin J, Jeanneteau F, Mery PF, et al. The impact of C-tactile low-threshold mechanoreceptors on affective touch and social interactions in mice. Sci Adv. 2022;8:eabo7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elias LJ, Succi IK, Schaffler MD, Foster W, Gradwell MA, Bohic M, et al. Touch neurons underlying dopaminergic pleasurable touch and sexual receptivity. Cell 2023;186:577–90.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam RM, von Buchholtz LJ, Falgairolle M, Osborne J, Frangos E, Servin-Vences MR, et al. PIEZO2 and perineal mechanosensation are essential for sexual function. Science 2023;381:906–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glatt SJ, Tylee DS, Chandler SD, Pazol J, Nievergelt CM, Woelk CH, et al. Blood-based gene-expression predictors of PTSD risk and resilience among deployed marines: a pilot study. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:313–26. [DOI] [PubMed] [Google Scholar]
- 33.van der Merwe C, Jahanshad N, Cheung JW, Mufford M, Groenewold NA, Koen N, et al. Concordance of genetic variation that increases risk for anxiety disorders and posttraumatic stress disorders and that influences their underlying neurocircuitry. J Affect Disord. 2019;245:885–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bountress KE, Vladimirov V, McMichael G, Taylor ZN, Hardiman G, Chung D, et al. Gene expression differences between young adults based on trauma history and post-traumatic stress disorder. Front Psychiatry. 2021;12:581093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuehner JN, Walia NR, Seong R, Li Y, Martinez-Feduchi P, Yao B. Social defeat stress induces genome-wide 5mC and 5hmC alterations in the mouse brain. G3. 2023;13:jkad114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma A, Huffman JE, Rodriguez A, Conery M, Liu M, Ho YL, et al. Diversity and scale: Genetic architecture of 2068 traits in the VA Million Veteran Program. Science 2024;385:eadj1182. [DOI] [PubMed] [Google Scholar]
- 37.Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch Genome targeting in epidermal stem cellsinduced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA. 1999;96:8551–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104:5163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Keymeulen A, Mascre G, Youseff KK, Harel I, Michaux C, De Geest N, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009;187:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;5:900–4. [DOI] [PubMed] [Google Scholar]
- 41.Bjornsdotter M, Loken L, Olausson H, Vallbo A, Wessberg J. Somatotopic organization of gentle touch processing in the posterior insular cortex. J Neurosci. 2009;29:9314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montgomery KR, Bridi MS, Folts LM, Marx-Rattner R, Zierden HC, Wulff AB, et al. Chemogenetic activation of CRF neurons as a model of chronic stress produces sex-specific physiological and behavioral effects. Neuropsychopharmacology. 2023;49:443–54. [DOI] [PMC free article] [PubMed]
- 43.Baumbauer KM, DeBerry JJ, Adelman PC, Miller RH, Hachisuka J, Lee KH, et al. Keratinocytes can modulate and directly initiate nociceptive responses. Elife 2015;4:e09674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moehring F, Cowie AM, Menzel AD, Weyer AD, Grzybowski M, Arzua T, et al. Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling. Elife 2018;7:e31684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikesell AR, Isaeva O, Moehring F, Sadler KE, Menzel AD, Stucky CL. Keratinocyte PIEZO1 modulates cutaneous mechanosensation. Elife 2022;11:e65987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikesell AR, Isaeva E, Schulte ML, Menzel AD, Sriram A, Prahl MM, et al. Increased keratinocyte activity and PIEZO1 signaling contribute to paclitaxel-induced mechanical hypersensitivity. Sci Transl Med. 2024;16:eadn5629. [DOI] [PubMed] [Google Scholar]
- 47.Pando MM, Debner EK, Jacobs BA, Jamshidi RJ, Jennings EM, Clarke WP, et al. Activation of G protein gated inwardly rectifying potassium (GIRK) channels in keratinocytes mediates peripheral kappa opioid receptor-mediated antinociception. Neuropharmacology 2025;268:110326. [DOI] [PubMed] [Google Scholar]
- 48.Vrontou S, Wong AM, Rau KK, Koerber HR, Anderson DJ. Genetic identification of C fibres that detect massage-like stroking of hairy skin in vivo. Nature 2013;493:669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowers ME, Ressler KJ. An Overview of translationally informed treatments for posttraumatic stress disorder: animal models of pavlovian fear conditioning to human clinical trials. Biol Psychiatry. 2015;78:E15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LoCoco PM, Boyd JT, Espitia Olaya CM, Furr AR, Garcia DK, Weldon KS, et al. Reliable approaches to extract high-integrity RNA from skin and other pertinent tissues used in pain research. Pain Rep. 2020;5:e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paxinos G, Franklin KBJ. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates. 4th ed. Academic Press; 2012.
- 52.Gehrlach DA, Dolensek N, Klein AS, Roy Chowdhury R, Matthys A, Junghanel M, et al. Aversive state processing in the posterior insular cortex. Nat Neurosci. 2019;22:1424–37. [DOI] [PubMed] [Google Scholar]
- 53.Zhang D, Turecek J, Choi S, Delisle M, Pamplona CL, Meltzer S, et al. C-LTMRs evoke wet dog shakes via the spinoparabrachial pathway. Science 2024;386:686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beretta CA, Liu S, Stegemann A, Gan Z, Wang L, Tan LL, et al. Quanty-cFOS, a Novel ImageJ/Fiji Algorithm for automated counting of immunoreactive cells in tissue sections. Cells. 2023;12:704–23. [DOI] [PMC free article] [PubMed]
- 55.Deuis JR, Dvorakova LS, Vetter I. Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci. 2017;10:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aoued HS, Sannigrahi S, Doshi N, Morrison FG, Linsenbaum H, Hunter SC, et al. Reversing behavioral, neuroanatomical, and germline influences of intergenerational stress. Biol Psychiatry. 2019;85:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Venkataraman A, Hunter SC, Dhinojwala M, Ghebrezadik D, Guo J, Inoue K, et al. Incerto-thalamic modulation of fear via GABA and dopamine. Neuropsychopharmacology 2021;46:1658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pennington ZT, Dong Z, Feng Y, Vetere LM, Page-Harley L, Shuman T, et al. ezTrack: An open-source video analysis pipeline for the investigation of animal behavior. Sci Rep. 2019;9:19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith FJ, Porter RM, Corden LD, Lunny DP, Lane EB, McLean WH. Cloning of human, murine, and marsupial keratin 7 and a survey of K7 expression in the mouse. Biochem Biophys Res Commun. 2002;297:818–27. [DOI] [PubMed] [Google Scholar]
- 60.Harbour K, Cappel Z, Baccei ML. Effects of Corticosterone on the excitability of Glutamatergic and GABAergic neurons of the adolescent mouse superficial dorsal horn. Neuroscience 2023;526:290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harbour K, Baccei ML. Influence of early-life stress on the excitability of Dynorphin neurons in the adult mouse dorsal horn. J Pain. 2024;25:104609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harbour K, Eid F, Serafin E, Hayes M, Baccei ML. Early life stress modulates neonatal somatosensation and the transcriptional profile of immature sensory neurons. Pain. 2024;166:888–901. [DOI] [PMC free article] [PubMed]
- 63.Li M, Liu K, Xu M, Chen Z, Yu L, Zhang J, et al. Anterior cingulate cortex-anterior insular cortex circuit mediates hyperalgesia in adolescent mice experiencing early life stress. ACS Chem Neurosci. 2025;16:920–31. [DOI] [PubMed]
- 64.Rowland GE, Mekawi Y, Michopoulos V, Powers A, Fani N, Bradley B, et al. Distinctive impacts of sexual trauma versus non-sexual trauma on PTSD profiles in highly trauma-exposed, Black women. J Affect Disord. 2022;317:329–38. [DOI] [PubMed] [Google Scholar]
- 65.Feng J, Luo J, Yang P, Du J, Kim BS, Hu H. Piezo2 channel-Merkel cell signaling modulates the conversion of touch to itch. Science 2018;360:530–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng J, Zhao Y, Xie Z, Zang K, Sviben S, Hu X, et al. Miswiring of Merkel cell and pruriceptive C fiber drives the itch-scratch cycle. Sci Transl Med. 2022;14:eabn4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ames HM, Bichakjian CK, Liu GY, Oravecz-Wilson KI, Fullen DR, Verhaegen ME, et al. Huntingtin-interacting protein 1: a Merkel cell carcinoma marker that interacts with c-Kit. J Invest Dermatol. 2011;131:2113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abraira VE, Ginty DD. The sensory neurons of touch. Neuron 2013;79:618–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klein AS, Dolensek N, Weiand C, Gogolla N. Fear balance is maintained by bodily feedback to the insular cortex in mice. Science 2021;374:1010–15. [DOI] [PubMed] [Google Scholar]
- 70.Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD. Elucidating an affective pain circuit that creates a threat memory. Cell 2015;162:363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang L, Wang J, Niu C, Zhang Y, Zhu T, Huang D, et al. Activation of parabrachial nucleus - ventral tegmental area pathway underlies the comorbid depression in chronic neuropathic pain in mice. Cell Rep. 2021;37:109936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han J, Suh B, Han JH. A top-down insular cortex circuit crucial for non-nociceptive fear learning. Sci Adv. 2025;11:eadt6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doucet YS, Woo SH, Ruiz ME, Owens DM. The touch dome defines an epidermal niche specialized for mechanosensory signaling. Cell Rep. 2013;3:1759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang W, Kanda H, Ikeda R, Ling J, DeBerry JJ, Gu JG. Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals. Proc Natl Acad Sci USA. 2016;113:E5491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Severson KS, Xu D, Van de Loo M, Bai L, Ginty DD, O’Connor DH. Active touch and self-motion encoding by merkel cell-associated afferents. Neuron 2017;94:666–76.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reinisch CM, Tschachler E. The touch dome in human skin is supplied by different types of nerve fibers. Ann Neurol. 2005;58:88–95. [DOI] [PubMed] [Google Scholar]
- 77.Morrison I, Bjornsdotter M, Olausson H. Vicarious responses to social touch in posterior insular cortex are tuned to pleasant caressing speeds. J Neurosci. 2011;31:9554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bjornsdotter M, Gordon I, Pelphrey KA, Olausson H, Kaiser MD. Development of brain mechanisms for processing affective touch. Front Behav Neurosci. 2014;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hachisuka J, Baumbauer KM, Omori Y, Snyder LM, Koerber HR, Ross SE. Semi-intact ex vivo approach to investigate spinal somatosensory circuits. Elife 2016;5:e22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Case LK, Liljencrantz J, McCall MV, Bradson M, Necaise A, Tubbs J, et al. Pleasant deep pressure: expanding the social touch hypothesis. Neuroscience 2021;464:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schirmer A, Lai O, McGlone F, Cham C, Lau D. Gentle stroking elicits somatosensory ERP that differentiates between hairy and glabrous skin. Soc Cogn Affect Neurosci. 2022;17:864–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Case LK, Madian N, McCall MV, Bradson ML, Liljencrantz J, Goldstein B, et al. Aβ-CT Affective Touch: Touch pleasantness ratings for gentle stroking and deep pressure exhibit dependence on A-Fibers. eNeuro. 2023;10:1–8. [DOI] [PMC free article] [PubMed]
- 83.Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron 2015;86:646–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liberzon I, Abelson JL. Context processing and the neurobiology of post-traumatic stress disorder. Neuron 2016;92:14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Centanni SW, Janes AC, Haggerty DL, Atwood B, Hopf FW. Better living through understanding the insula: Why subregions can make all the difference. Neuropharmacology 2021;198:108765. [DOI] [PubMed] [Google Scholar]
- 86.Ng AJ, Vincelette LK, Li J, Brady BH, Christianson JP. Serotonin modulates social responses to stressed conspecifics via insular 5-HT(2 C) receptors in rat. Neuropharmacology 2023;236:109598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nicolas C, Ju A, Wu Y, Eldirdiri H, Delcasso S, Couderc Y, et al. Linking emotional valence and anxiety in a mouse insula-amygdala circuit. Nat Commun. 2023;14:5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bayer H, Hassell JE Jr, Oleksiak CR, Garcia GM, Vaughan HL, Juliano VAL, et al. Pharmacological stimulation of infralimbic cortex after fear conditioning facilitates subsequent fear extinction. Neuropsychopharmacology 2024;49:1951–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maricich SM, Wellnitz SA, Nelson AM, Lesniak DR, Gerling GJ, Lumpkin EA, et al. Merkel cells are essential for light-touch responses. Science 2009;324:1580–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hong W, Kim DW, Anderson DJ. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 2014;158:1348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci. 2016;17:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, McGoey MT, Ritchey M, et al. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci. 2018;21:404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mu MD, Geng HY, Rong KL, Peng RC, Wang ST, Geng LT, et al. A limbic circuitry involved in emotional stress-induced grooming. Nat Commun. 2020;11:2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull. 2006;132:959–92. [DOI] [PubMed] [Google Scholar]
- 95.Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia 2012;50:1578–93. [DOI] [PubMed] [Google Scholar]
- 96.Ramikie TS, Ressler KJ. Mechanisms of sex differences in fear and posttraumatic stress disorder. Biol Psychiatry. 2018;83:876–85. [DOI] [PubMed] [Google Scholar]
- 97.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–14. [DOI] [PubMed] [Google Scholar]
- 98.Miranda MI, McGaugh JL. Enhancement of inhibitory avoidance and conditioned taste aversion memory with insular cortex infusions of 8-Br-cAMP: involvement of the basolateral amygdala. Learn Mem. 2004;11:312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miranda MI, Quirarte GL, Rodriguez-Garcia G, McGaugh JL, Roozendaal B. Glucocorticoids enhance taste aversion memory via actions in the insular cortex and basolateral amygdala. Learn Mem. 2008;15:468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cisler JM, James GA, Tripathi S, Mletzko T, Heim C, Hu XP, et al. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychol Med. 2013;43:507–18. [DOI] [PubMed] [Google Scholar]
- 101.Aust S, Hartwig EA, Koelsch S, Heekeren HR, Heuser I, Bajbouj M. How emotional abilities modulate the influence of early life stress on hippocampal functioning. Soc Cogn Affect Neurosci. 2014;9:1038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Strigo IA, Craig ADB, Simmons AN. Expectation of pain and relief: A dynamical model of the neural basis for pain-trauma co-morbidity. Neurosci Biobehav Rev. 2024;163:105750. [DOI] [PubMed] [Google Scholar]
- 103.Stenson AF, Nugent NR, van Rooij SJH, Minton ST, Compton AB, Hinrichs R, et al. Puberty drives fear learning during adolescence. Dev Sci. 2021;24:e13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang C, Hu MW, Wang XW, Cui X, Liu J, Huang Q, et al. scRNA-sequencing reveals subtype-specific transcriptomic perturbations in DRG neurons of Pirt(EGFPf) mice in neuropathic pain condition. Elife 2022;11:e76063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ray PR, Shiers S, Caruso JP, Tavares-Ferreira D, Sankaranarayanan I, Uhelski ML, et al. RNA profiling of human dorsal root ganglia reveals sex differences in mechanisms promoting neuropathic pain. Brain 2023;146:749–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xie K, Cheng X, Zhu T, Zhang D. Single-cell transcriptomic profiling of dorsal root ganglion: an overview. Front Neuroanat. 2023;17:1162049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Urien L, Bauer EP. Sex differences in BNST and Amygdala activation by contextual, cued, and unpredictable threats. eNeuro 2022;9:ENEURO.0233–21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Becker JB, Monteggia LM, Perrot-Sinal TS, Romeo RD, Taylor JR, Yehuda R, et al. Stress and disease: is being female a predisposing factor? J Neurosci. 2007;27:11851–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shepard R, Page CE, Coutellier L. Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: Relevance for sex differences in stress-related disorders. Neuroscience 2016;332:1–12. [DOI] [PubMed] [Google Scholar]
- 110.Colom-Lapetina J, Li AJ, Pelegrina-Perez TC, Shansky RM. Behavioral diversity across classic rodent models is sex-dependent. Front Behav Neurosci. 2019;13:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ravi M, Stevens JS, Michopoulos V. Neuroendocrine pathways underlying risk and resilience to PTSD in women. Front Neuroendocrinol. 2019;55:100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lopez J, Bagot RC. Defining valid chronic stress models for depression with female rodents. Biol Psychiatry. 2021;90:226–35. [DOI] [PubMed] [Google Scholar]
- 113.Mitchell JR, Vincelette L, Tuberman S, Sheppard V, Bergeron E, Calitri R, et al. Behavioral and neural correlates of diverse conditioned fear responses in male and female rats. Neurobiol Stress. 2024;33:100675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi CJ, Cassell MD. Cascade projections from somatosensory cortex to the rat basolateral amygdala via the parietal insular cortex. J Comp Neurol. 1999;399:469–91. [DOI] [PubMed] [Google Scholar]
- 115.Boatman JA, Kim JJ. A thalamo-cortico-amygdala pathway mediates auditory fear conditioning in the intact brain. Eur J Neurosci. 2006;24:894–900. [DOI] [PubMed] [Google Scholar]
- 116.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43. [DOI] [PubMed] [Google Scholar]
- 117.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 2008;33:56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry. 2011;70:920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cadiz-Moretti B, Otero-Garcia M, Martinez-Garcia F, Lanuza E. Afferent projections to the different medial amygdala subdivisions: a retrograde tracing study in the mouse. Brain Struct Funct. 2016;221:1033–65. [DOI] [PubMed] [Google Scholar]
- 120.Cadiz-Moretti B, Abellan-Alvaro M, Pardo-Bellver C, Martinez-Garcia F, Lanuza E. Afferent and efferent projections of the anterior cortical amygdaloid nucleus in the mouse. J Comp Neurol. 2017;525:2929–54. [DOI] [PubMed] [Google Scholar]
- 121.Meng X, Yue L, Liu A, Tao W, Shi L, Zhao W, et al. Distinct basolateral amygdala excitatory inputs mediate the somatosensory and aversive-affective components of pain. J Biol Chem. 2022;298:102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol Mood Anxiety Disord. 2012;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen CY, Choi KW, et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. 2019;10:4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Velasco ER, Florido A, Milad MR, Andero R. Sex differences in fear extinction. Neurosci Biobehav Rev. 2019;103:81–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morena M, Nastase AS, Santori A, Cravatt BF, Shansky RM, Hill MN. Sex-dependent effects of endocannabinoid modulation of conditioned fear extinction in rats. Br J Pharm. 2021;178:983–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huckleberry KA, Calitri R, Li AJ, Mejdell M, Singh A, Bhutani V, et al. CB1R blockade unmasks TRPV1-mediated contextual fear generalization in female, but not male rats. Neuropsychopharmacology 2023;48:1500–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N. Y Acad Sci. 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 128.Ellis BJ, Del Giudice M. Beyond allostatic load: rethinking the role of stress in regulating human development. Dev Psychopathol. 2014;26:1–20. [DOI] [PubMed] [Google Scholar]
- 129.Kim DK, Holbrook KA. The appearance, density, and distribution of Merkel cells in human embryonic and fetal skin: their relation to sweat gland and hair follicle development. J Invest Dermatol. 1995;104:411–6. [DOI] [PubMed] [Google Scholar]
- 130.Jenkins BA, Lumpkin EA. Developing a sense of touch. Development 2017;144:4078–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jenkins BA, Fontecilla NM, Lu CP, Fuchs E, Lumpkin EA. The cellular basis of mechanosensory Merkel-cell innervation during development. Elife 2019;8:e42633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Michel N, Narayanan P, Shomroni O, Schmidt M. Maturational changes in mouse cutaneous touch and Piezo2-mediated Mechanotransduction. Cell Rep. 2020;32:107912. [DOI] [PubMed] [Google Scholar]
- 133.Keding TJ, Heyn SA, Russell JD, Zhu X, Cisler J, McLaughlin KA, et al. Differential patterns of delayed emotion circuit maturation in abused girls with and without internalizing psychopathology. Am J Psychiatry. 2021;178:1026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Querdasi FR, Enders C, Karnani N, Broekman B, Yap Seng C, Gluckman PD, et al. Multigenerational adversity impacts on human gut microbiome composition and socioemotional functioning in early childhood. Proc Natl Acad Sci USA. 2023;120:e2213768120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wan Yunus F, Liu KP, Bissett M, Penkala S. Sensory-based intervention for children with behavioral problems: a systematic review. J Autism Dev Disord. 2015;45:3565–79. [DOI] [PubMed] [Google Scholar]
- 136.Orefice LL. Peripheral Somatosensory neuron dysfunction: emerging roles in autism spectrum disorders. Neuroscience 2020;445:120–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hoffman BU, Baba Y, Lee SA, Tong CK, Konofagou EE, Lumpkin EA. Focused ultrasound excites action potentials in mammalian peripheral neurons in part through the mechanically gated ion channel PIEZO2. Proc Natl Acad Sci USA. 2022;119:e2115821119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cai M, Park HR, Yang EJ. Electroacupuncture modulates glutamate neurotransmission to alleviate PTSD-like behaviors in a PTSD animal model. Transl Psychiatry. 2023;13:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lv T, Wang M, Zheng HS, Mao JD, Yang F, Yang L, et al. Electroacupuncture alleviates PTSD-like behaviors by modulating hippocampal synaptic plasticity via Wnt/beta-catenin signaling pathway. Brain Res Bull. 2023;202:110734. [DOI] [PubMed] [Google Scholar]
- 140.Wojcik M, Bordoni B, Siatkowski I, Zekanowska E. The effect of Craniosacral therapy on blood levels of stress hormones in male firefighter cadets: a randomized clinical trial. Behav Sci. 2023;13:914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fricker F, Barbotte MV, Pallot G, Radoua N, Sorci G, Heitz M, et al. Positive psychological effects of seated acupressure massage are associated with a rise in plasma oxytocin without affecting CGRP levels or circulating IL-6. Compr Psychoneuroendocrinol. 2024;17:100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hollifield M, Hsiao AF, Smith T, Calloway T, Jovanovic T, Smith B, et al. Acupuncture for Combat-related posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2024;81:545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tan L, Deady M, Mead O, Foright RM, Brenneman EM, Bryant RA, et al. Yoga resilience training to prevent the development of posttraumatic stress disorder in active-duty first responders: A cluster randomized controlled trial. Psychol Trauma. 2024; Advance online publication. 10.1037/tra0001667. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are available upon response request to the authors.