Abstract
In this study, we investigated the role played by cytoplasmic catalase (Ctt1) in resistance against water loss using the yeast Saccharomyces cerevisiae as eukaryotic cell model. Comparing a mutant possessing a specific lesion in CTT1 with its parental strain, it was observed that both control and ctt1 strains exhibited increased levels of lipid peroxidation after dehydration, suggesting that catalase does not protect membranes during drying. Although the ctt1 strain has only 1 catalase isoform (peroxisomal catalase), the mutant showed the same levels of total catalase activity as the control strain. Furthermore, in cells deficient in Ctt1, the reduced glutathione:oxidized glutathione ratio (GSH: GSSG) of dry cells was higher than that of the control strain, indicating a compensatory mechanism of defense in response to dehydration. Even so, desiccation tolerance of the ctt1 strain was significantly lower than in the control strain. Using a fluorescent probe sensitive to oxidation, we observed that cells of the ctt1 strain showed levels of intracellular oxidation 70% higher than those of control strain, suggesting that Ctt1 plays a role in the maintenance of the intracellular redox balance during dehydration and, therefore, in tolerance against a water stress.
INTRODUCTION
Our increasing understanding of anhydrobiosis (life without water) holds promises because it may find technological applications. For example, knowledge of the mechanism of desiccation tolerance should lead to improved technologies in seed storage, gene banks, tissue engineering, cell transplantation, and the preservation of dry foods and pharmaceutical products.
Desiccation induces several changes in the cellular environment, such as (1) reduced hydration of macromolecules and consequent conformational changes, (2) reduced cytoplasmic and intracellular transport, (3) shifts in cytoplasmic pH and ion concentrations, and (4) accumulation of organic and inorganic ions (Senaratna and McKersie 1986). All or any of these changes might be expected to cause transient dysfunctions in enzymes or electron transport chains (or both), which may lead to the production of free radicals or promote chemical reactions that would not normally occur in a fully hydrated system.
Because susceptibility to oxidative damage may increase with drying, one may infer that free radical scavenging systems are an important component among the mechanisms of desiccation tolerance. Aerobic organisms are well endowed with an array of specific antioxidant molecules and scavenging systems to protect them against oxidative damage (Willcox et al 2004). Defense mechanisms include enzymes, such as peroxidases, catalases, and superoxide dismutases; and antioxidants, such as glutathione and vitamins C and E (Jamieson 1998). The regulation of antioxidant defense is complex, and its role in tolerance to dehydration is not yet firmly established.
Anhydrobiotes seem to apply mainly 2 strategies to cope with the danger of O2 toxicity: increased efficiency of antioxidant defenses and metabolic control of both energy-producing and energy-consuming processes (Oliver et al 2001). Resistance to drought in desiccation-tolerant plants seems to be associated with an upregulation of antioxidant genes (McKersie et al 1999; Hsieh et al 2002). Baker's yeast, when overexpressing superoxide dismutase, exhibited increased tolerance to dehydration (Pereira et al 2003). In sunflower and bean seeds, as well as in maize leaves, catalase activity increases during dehydration (Bailly et al 2001, 2004; Jiang and Zhang 2002), suggesting that this enzyme prevents dehydration-related oxidative damage. However, although these results show a correlation between catalase activation and increase in tolerance to dehydration, this correlation only suggests but does not prove that this antioxidant enzyme is necessary for cell protection under anhydrous conditions.
In this study, using a mutant strain of Saccharomyces cerevisiae that harbors a specific deficiency in cytoplasmic catalase (Ctt1), we investigated the role of this antioxidant enzyme in the maintenance of survival during dehydration. The use of S. cerevisiae as experimental model is particularly attractive because of the structural and functional similarity of genes in yeasts and mammals. In contrast to higher eukaryotes, S. cerevisiae genes can be easily engineered by molecular biology techniques and thoroughly studied very quickly, providing a considerable amount of information useful for understanding the molecular basis of desiccation tolerance.
RESULTS AND DISCUSSION
Catalase catalyzes the breakdown of H2O2 to O2 and H2O. S. cerevisiae possess 2 isoforms, catalase A (peroxisomal) and catalase T (cytoplasmic), encoded by the CTA1 and CTT1 genes, respectively. The main physiological role of catalase A seems to be the removal of H2O2 produced by fatty acid β-oxidation. The role of catalase T is less clear. CTT1 gene expression is regulated by oxidative and osmotic stresses (Ruis and Hamilton 1992).
In sunflower seeds, catalase expression is finely regulated by the cell water content (Bailly et al 2004). Catalase activity increases during maize leaf water stress (Jiang and Zhang 2002) or during bean seed desiccation (Bailly et al 2001). Hsieh et al (2002) also demonstrated that transgenic tomato plants resistant to water deficiency overexpress the catalase gene.
To investigate the importance of the cytoplasmic isoform of this antioxidant enzyme and its role in the mechanism of tolerance against dehydration, we used a mutant strain of S. cerevisiae that does not express CTT1. BY4741 (MATa; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0) and its isogenic mutant ctt1, harboring the gene CTT1 interrupted by the gene KanMX4, were acquired from Euroscarf (Frankfurt, Germany). Cells were grown up to stationary phase (4.0 mg dry weight/mL) in liquid yeast-peptone-dextrose (YPD) medium (1% yeast extract, 2% glucose, and 2% peptone), as described previously (Pereira et al 2003). Dehydration was performed at 37°C until samples reached constant weight (final water content was 4–9%). All results were expressed as mean ± SD of at least 3 independent experiments. Statistical differences were tested using analysis of variance followed by Tukey-Kramer multiple comparisons test. The latter shows homogeneity between experimental groups at P < 0.05. In all tables, different letters indicate statistically different results.
As shown in Table 1, the mutant lacking Ctt1 showed a higher sensitivity to water loss than the control strain, indicating that tolerance to dehydration of yeast cells is dependent on catalase T. To understand how catalase protects cells during dehydration, we measured the oxidative damage caused by dehydration to cell membranes. One of the molecular mechanisms of damage leading to death in desiccation-sensitive cells on drying is free radical attack to phospholipids leading to lipid peroxidation and phospholipids de-esterification (Leprince et al 1994; Hoekstra et al 2001). As previously observed (Espindola et al 2003), dehydration produced a high increase in the levels of malondialdehyde, a product of lipid peroxidation (Table 1). However, the increase in the levels of lipid peroxidation caused by dehydration in ctt1 cells was similar to that of the control strain.
Table 1.
Effect of dehydration on survival and lipid peroxidation
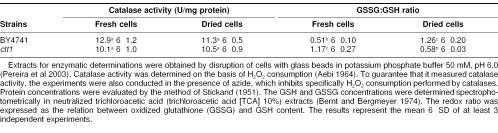
In plants, the increase in catalase activity induced by seed drying was associated with a decrease in hydrogen peroxide levels and in lipid peroxidation, suggesting that catalase plays a role during seed desiccation by preventing dehydration-related oxidative damage of membranes (Bailly et al 2004). In contrast, under the conditions of this study, catalase activity did not change in response to dehydration (Table 2), which could explain the increase in lipid peroxidation observed. Interestingly, the mutant deficient in Ctt1 showed similar levels of activity to those of control strain, indicating that a deficiency in Ctt1 is overcome by an increase in the remaining peroxisomal catalase activity. Similar to what has been shown for other antioxidant enzymes, such as glutathione peroxidases and superoxide dismutases (AM Avery and SV Avery 2001; Pereira et al 2003), there seems to be compensation in the regulation of catalase activity under a severe oxidative stress, as is the case during dehydration. In the mutant strain, as the CTT1 gene is not functional, the activity expressed is only because of peroxisomal isoform (Table 2). Then, because catalase activity in the ctt1 strain was almost the same as its parental strain, we can conclude that in the mutant, the activity of peroxisomal catalase is induced when compared with the activity of this isoform in the control strain, in such a manner that the activity of 1 isoform in the mutant strain is on the level with the activities of both catalases in the control strain. This result suggests that catalase A, which is the only isoform present in the mutant strain, is not enough to protect cells against dehydration.
Table 2.
Catalase activity and GSSG:GSH ratio
By analyzing the levels of oxidized (GSSG) and reduced glutathione (GSH), we verified that cells lacking Ctt1 showed a 2-fold reduction in the GSSG:GSH ratio after dehydration, in contrast to the control strain, which demonstrated a significant enhancement of the intracellular GSSG content (Table 2). Considering the importance of glutathione to relieve the oxidative damage of phospholipids produced by dehydration (Espindola et al 2003), the high levels of GSH in dry ctt1 cells, in addition to the high levels of peroxisomal catalase activity (Table 2), might be contributing to prevent a higher increase in lipid peroxidation, despite the deficiency of Ctt1.
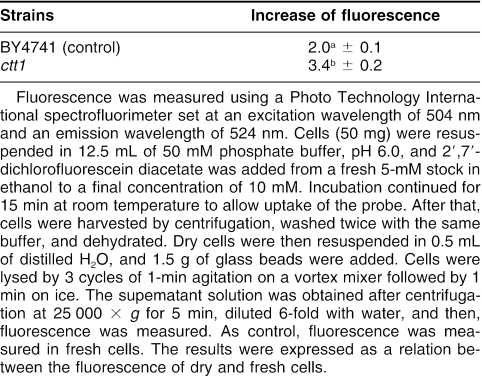
To further investigate the role of catalase T in protecting yeast during a water deficit, we analyzed the cell redox status, using the fluorescent probe, 2′, 7′-dichlorofluorescein. This probe reaches the intracellular environment by passive diffusion and, once inside the cell, becomes susceptible to attack by free radical species, producing a more fluorescent compound (Tsuchiya and Suematsu 1994). By measuring the level of fluorescence of cell extracts before and after dehydration, we can evaluate the increase in oxidation produced by this stress. According to Table 3, the ratio of fluorescence between dried and fresh cells, which is indicative of the state of cell oxidation, increased in both strains, confirming that dehydration causes an oxidative stress. However, the level of oxidation caused by the water stress in ctt1 cells was 70% higher than that in the control strain, indicating that the cytoplasmic isoform favors resistance to dehydration by controlling reactive oxygen species (ROS) production, probably through removal of H2O2. In sunflower, the increase in catalase activity during seed development after artificial drying seems to be involved in the reduction of H2O2 content and in the increase of tolerance (Bailly et al 2004). It is indeed admitted that water stress induces ROS generation, which can, in turn, produce cellular and metabolic damage (Smirnoff 1993).
Table 3.
Enhancement of intracellular oxidation measured as fold increase in fluorescence
Taken together, our investigation addresses the question of the functional role of Ctt1 with respect to dehydration-related damage, and our data confirm that the ability of cells to withstand water loss is related to the maintenance of redox homeostasis.
Acknowledgments
This work was supported by grants from FAPERJ and CNPq.
REFERENCES
- Aebi H. Catalase in vitro. Methods Enzymol. 1964;105:114–118. doi: 10.1016/s0076-6879(84)05016-3.0076-6879(1964)105[0114:CIV]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Avery AM, Avery SV. Saccharomyces cerevisiae expresses three phospholipids hydroperoxide glutathione peroxidases. J Biol Chem. 2001;276:33730–33735. doi: 10.1074/jbc.M105672200.0021-9258(2001)276[33730:SCETPH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bailly C, Audigier C, Ladonne F, Wagner MH, Coste F, Corbineau F, Come D. Changes in oligosaccharide content and antioxidant enzyme activities in developing bean seeds as related to acquisition of drying tolerance and seed quality. J Exp Bot. 2001;357:701–708. doi: 10.1093/jexbot/52.357.701.0022-0957(2001)357[0701:CIOCAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bailly C, Leymarie J, Lehner A, Rousseau S, Come D, Corbineau F. Catalase activity and expression in developing sunflower seeds as related to drying. J Exp Bot. 2004;55:475–483. doi: 10.1093/jxb/erh050.0022-0957(2004)055[0475:CAAEID]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bernt E, Bergmeyer HU 1974 Glutathione. In: Methods of Enzymatic Analysis, vol 4, ed Bergmeyer HU. Academic Press, New York. 1643–1647. [Google Scholar]
- Espindola Ade S, Gomes DS, Panek AD, Eleutherio EC. The role of glutathione in yeast dehydration tolerance. Cryobiology. 2003;47:236–241. doi: 10.1016/j.cryobiol.2003.10.003.0011-2240(2003)047[0236:TROGIY]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001;6:431–438. doi: 10.1016/s1360-1385(01)02052-0.1360-1385(2001)006[0431:MOPDT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hsieh TH, Lee JT, Charng YY, Chan MT. Tomato plants ectopically expressing Arabidopsis cbf1 show enhanced resistance to water deficit stress. Plant Physiol. 2002;130:618–626. doi: 10.1104/pp.006783.0032-0889(2002)130[0618:TPEEAC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson DJ. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast. 1998;14:1511–1527. doi: 10.1002/(SICI)1097-0061(199812)14:16<1511::AID-YEA356>3.0.CO;2-S.0749-503X(1998)014[1511:OSROTY]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot. 2002;53:2401–2410. doi: 10.1093/jxb/erf090.0022-0957(2002)053[2401:WSAAAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Leprince O, Atherton NM, Deltour R, Hendry G. The involvement of respiration in free radical processes during loss of desiccation tolerance in germinating Zea Mays l. (An electron paramagnetic resonance study) Plant Physiol. 1994;104:1333–1339. doi: 10.1104/pp.104.4.1333.0032-0889(1994)104[1333:TIORIF]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie BD, Bowley SR, Jones KS. Winter survival of transgenic alfalfa overexpressing superoxide dismutase. Plant Physiol. 1999;119:839–848. doi: 10.1104/pp.119.3.839.0032-0889(1999)119[0839:WSOTAO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver AE, Leprince O, Wolkers WF, Hincha DK, Heyer AG, Crowe JH. Non-disaccharide-based mechanisms of protection during drying. Cryobiology. 2001;43:151–167. doi: 10.1006/cryo.2001.2359.0011-2240(2001)043[0151:NMOPDD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pereira Ede J, Panek AD, Eleutherio EC. Protection against oxidation during dehydration of yeast. Cell Stress Chaperones. 2003;8:120–124. doi: 10.1379/1466-1268(2003)008<0120:paoddo>2.0.co;2.1466-1268(2003)008[0120:PAODDO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruis H, Hamilton B 1992 Regulation of yeast catalase genes. In: Molecular Biology of Free Radical Scavenging Systems. CSH Press, Cold Spring Harbor, New York. 153–172. [Google Scholar]
- Senaratna T, McKersie BD 1986 Loss of desiccation tolerance during seed germination: a free radical mechanism of injury. In: Membranes, Metabolism, and Dry Organisms, ed Leopold AC. Cornell University Press, NY, 85–101. [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x.1469-8137(1993)125[0027:TROAOI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Steels EL, Learmonth RP, Watson K. Stress tolerance and membrane lipid unsaturation in Saccharomyces cerevisiae grown aerobically or anaerobically. Microbiology. 1994;140:569–576. doi: 10.1099/00221287-140-3-569.0026-2617(1994)140[0569:STAMLU]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stickland LH. The determination of small quantities of bacteria by means of the biuret reaction. J Gen Microbiol. 1951;5:698–703. doi: 10.1099/00221287-5-4-698.0022-1287(1951)005[0698:TDOSQO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tsuchiya M, Suematsu M. In vivo visualization of oxygen radical-dependent photoemission. Methods Enzymol. 1994;233:128–140. doi: 10.1016/s0076-6879(94)33015-8.0076-6879(1994)233[0128:IVVOOR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. 2004;44:275–295. doi: 10.1080/10408690490468489.1040-8398(2004)044[0275:AAPOCD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]