Abstract
The heat shock proteins (Hsps) have an important role in the cytoprotection and repair of cells and tissues. One potential mechanism of protection is the ability of Hsp to inhibit genetic expression of proinflammatory cytokines, the transcription of which is dependent on nuclear factor–kappa B (NF-κB) activation. In this study, we evaluated the ability of ectoine, a novel natural biomolecule produced by halophilic microorganisms, to activate the hsp70 and hsp70B′. By reverse transcriptase–polymerase chain reaction and Western blot analysis, we demonstrated increased hsp70B′ gene expression in human keratinocytes treated with ectoine and heat stressed. In contrast, in the absence of heat shock, ectoine was unable to induce hsp70B′ but had the ability to induce another member of the Hsp family, the hsp70. The latter is not only elevated in response to stress but is also present at basal level in unstressed cells. In addition, ectoine had no effect on proinflammatory cytokines interleukin (IL)-1alpha, IL-6, IL-8, and tumor necrosis factor–alpha and on NF-κB and IκB-α pathway, whereas it downregulated the expression of cited proinflammatory cytokines, in lipopolysaccharides-treated keratinocytes. These results highlighted the ability of ectoine to protect cells from stress conditions and to prevent cell damage by maintaining an elevated level of the Hsp70. Overall, these data might suggest the use of this compatible solute in cosmetic and even pharmaceutical preparations aiming to activate a cytoprotective heat shock response in human cells.
INTRODUCTION
Cells have developed a number of different strategies to deal with adverse changes in their environment. The environmental stressor may produce disease conditions associated mainly to protein damage and misfolded protein structures. To restore cellular homeostasis, some mechanisms of cellular defense might be activated by inducing the expression of various genes, including the acute phase genes and the heat shock proteins (Hsps) (Giannessi et al 2003). Increased expression of Hsps occurs when cells are exposed to a number of metabolic insults other than heating, including amino acid analogues, various heavy metals, agents that modify protein sulfhydryls, various ionophores, and finally a number of other metabolic poisons (Welch 1992). In addition, Hsp synthesis was proved to be increased to protect prokaryotic or eukaryotic cells from various insults during periods of stress caused by infection, inflammation, or similar events (Zügel and Kaufmann 1999).
The Hsp70 represents the most highly induced member of the stress protein family (Welch 1992). However, in humans, Hsp70 is not only elevated in response to stress but is also present at basal level in unstressed cells (Leung et al 1990). This protein is constitutively expressed in specific skin cells, such as epidermal keratinocytes, that provide a natural barrier against potential environmental stressful attacks. The expression of hsp70 may be further induced in epidermal keratinocytes by heat treatment, providing a state of resistance against the deleterious effects of solar ultraviolet (UV)–B radiation (Souil et al 2001). The Hsp70B′, another member of Hsp70 family, is strictly stress inducible and absent in unstressed cells (Leung et al 1990; Tavoria et al 1996). The sequences of the hsp70 (also known as hsp70A or hsp72) and hsp70B′ genes are similar, but they differ in terms of their pattern and extent of inducibility in response to conditions such as heat shock and CdCl2 treatment (Leung et al 1990).
Recently, it has been demonstrated that laser photoirradiation markedly induced a long-lasting epidermal expression of hsp70 in normal rat skin (Souil et al 2001). It has been suggested that the laser-induced expression of hsp70 might contribute to improve tissue regeneration and wound healing. In addition, Hsp70 has also been associated with reduced inflammation and cell proliferation, suggesting an alternative role for this protein in cutaneous scarring (Laplante et al 1998).
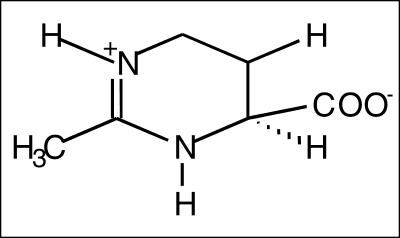
This research aims to evaluate novel natural biomolecules regarding their ability to activate the hsp70 and hsp70B′. In particular, there is growing scientific interest in the study of compatible solutes isolated by halophilic eubacteria. These peculiar microorganisms are able to live in harsh environments, specifically they developed “biochemical strategies” to cope with high salinity (up to 20% NaCl); in fact, they synthesize small organic molecules that can accumulate in the cytosol to balance the osmotic pressure of the external medium, without affecting the metabolism. Among these molecules, ectoine, a cyclic tetrahydropyrimidine (Fig 1), produced by Halomonas ssp. and Marinococcus ssp., has been extensively characterized as a stabilizer for cells and biomolecules (Louis et al 1994). In addition to the biological function as an osmoprotectant, it was shown to preserve enzymes and whole cells in vitro against harmful conditions such as freezing, drying, or heating (Lippert and Galinski 1992).
Fig 1.
Ectoine structure
However, the potential biotechnological applications of ectoine have still to be explored; in this context it is very interesting to study the effect of this biomolecule on primary keratinocytes in relation to stressful conditions.
Here, we demonstrate that ectoine has a potential to increase the expression of hsp70 and also hsp70B′ in heated cells; in addition, a possible pharmacological use of ectoine in wound healing and skin protection is discussed.
MATERIALS AND METHODS
Cell culture and treatments
Human keratinocytes were obtained from surgical specimens of normal adult skin (following informed consent). The skin was split overnight in dispase (Sigma, Milan, Italy) at 4°C. Epidermal sheets were removed from the dermis, and single-cell suspensions were obtained by placing epidermal sheets in 0.05% Trypsin-0.53 mM EDTA for 15 minutes at 37°C. After incubation, trypsin activity was stopped by adding 10 mL of Soybean Trypsin Inhibitor (Life Technologies, Milan, Italy), and the keratinocyte cell suspensions were centrifuged. The resulting cell pellet was cultured in defined keratinocyte-serum free medium (SFM) medium supplemented with growth factors (serum-free keratinocyte medium and growth factors, Life Technologies) at a density of approximately 3 × 106 cells per flask. The concentration of total calcium in the medium was maintained at 0.1 mM. Keratinocyte cultures were refreshed with complete medium every 2–3 days (Boyce and Ham 1983). For the treatments, 3 × 105 cells were plated in 6-well plates (35 mm diameter) with 2 mL complete medium supplemented with Ca++ to reach a concentration of 0.3 mM and preincubated for 30 minutes, 2, 4, and 24 hours, at 37°C, with ectoine (derived from Halomonas elongata, kindly donated by Prof Erwin Galinski, University of Bonn, Germany) at different concentrations. At the end of the incubation period, the cells were heat stressed for 40 minutes at 42°C. As control for the Hsp, heat-stressed untreated keratinocytes were used (positive control). In addition, to test the ability of ectoine to induce Hsp, a different group of cells which received the treatments, but that were not heat stressed, were used.
Another set of experiments aiming to evaluate the ability of ectoine to influence the proinflammatory response was carried out treating keratinocytes with lipopolysaccharides (LPS) from Pseudomonas aeruginosa. In particular, cells preincubated with ectoine were exposed to 10 μg/ mL of LPS. In addition, the cells were preincubated for 15 minutes with 30 μM calpain inhibitor I (nuclear factor– kappa B [NF-κB] inhibitor) (Sigma), then treated or untreated with LPS for 24 hours.
Reverse transcriptase–polymerase chain reaction analysis
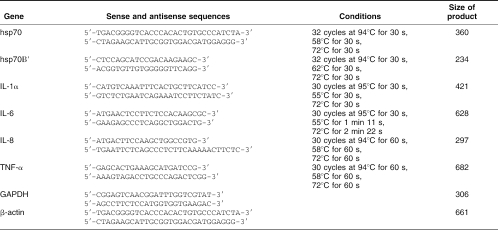
Total ribonucleic acid (RNA), isolated by High Pure RNA Isolation Kit (Roche Diagnostics, Milan, Italy), from keratinocytes before and after the treatment with ectoine, heat stressed or LPS treated, in presence or absence of calpain inhibitor I, was transcribed by reverse transcriptase (Expand Reverse Transcriptase, Roche Diagnostics) at 42°C for 45 minutes according to the manufacturer's instructions. Two microliters of complementary deoxyribonucleic acid was amplified in a reaction mixture containing 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, 200 μM deoxynucleoside triphosphates, and 2.5 U of Taq deoxyribonucleic acid (DNA) polymerase (Roche Diagnostics) in a final volume of 50 μL. For the coamplification of hsp70 and β-actin, polymerase chain reaction (PCR) was carried out in the presence of 0.5 μM sense and antisense hsp70 primers (Stressgen Biotechnologies) and of 0.5 μM sense and antisense β-actin primers. For the coamplification of hsp70B′ and IL-8 with β-actin, PCR was carried out in the presence of 0.5 μM sense and antisense hsp70B′ and IL-8 primers (Medical and Biological Laboratories [MBL] Co., Ltd, Japan) and of 0.05 μM sense and antisense β-actin primers. For the coamplification of IL-1α, IL-6, and tumor necrosis factor (TNF)–α with glyceraldehyde-3-phosphate dehydrogenase (GAPDH), PCR was carried out in the presence of 0.5 μM sense and antisense IL-1α, IL-6, and TNF-α primers (MBL) and of 0.05 μM sense and antisense GAPDH primers. The reaction was carried out in a DNA thermal cycler (Gene AMP PCR system 2400, Applied Biosystem, Milan, Italy). The PCR was performed in the exponential phase of amplification and started with a 3-minute denaturation step at 95°C. The PCR products were analyzed by electrophoresis on 1.8% agarose gel in Tris Boric Acid EDTA (TBE). For primers sequences, PCR conditions, and size of products, see Table 1. Densitometric analysis of ethidium bromide– stained agarose gel (software NIH image V 1.6) was performed to quantitate the amount of specific messenger RNAs (mRNAs) present in the total RNA prepared from the different cell samples.
Table 1.
Human primers sense and antisense and expected PCR products (bp)
Protein extraction and Western blotting analysis
The cell pellets obtained from keratinocytes before and after the treatment with 100 μg/mL ectoine, heat stressed or LPS treated, were homogenized separately with 300 μL ice-cold buffer (50 mM N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid, pH 7.5, 150 mM NaCl, 1% glycerol, 1% Triton, 1.5 mM MgCl2, 5 mM ethylene glycol-bis(aminoethylether)-tetraacetic acid) supplemented with 20 mM sodium pyrophosphate, 40 μg/mL aprotinin, 4 mM phenylmethanesulfonyl fluoride, 10 mM sodium orthovanadate, and 25 mM NaF. Total extracts were cleared by centrifugation for 30 minutes at 4°C at 10 000 × g and assayed for the protein content by Bradford's method (Bradford 1976). Fifty micrograms of proteins was electrophoresed through a 12.5% or 7.5% polyacrylamide gel and transferred to nitrocellulose membranes, and the filters were stained with 10% Ponceau S solution for 2 minutes to verify equal loading and transfer efficiency. In addition, protein normalization was verified by densitometric analysis of bands (data not shown). Blots were blocked overnight with 5% nonfat dry milk and then incubated with anti-mouse anti-hsp70 monoclonal antibody (catalog number SPA-810, Stressgen, Milan, Italy) and anti-hsp70B′ polyclonal antibody (catalog number SPA-756, Stressgen), 1 μg/mL in Tris-buffered saline (150 mM NaCl, 20 mM Tris-HCl pH 8) for 2 hours at room temperature or anti–IκB-α polyclonal antibody (Santa Cruz, CA, USA) 1 μg/mL in 5% nonfat dry milk and 0.1% Tween-20 in PBS overnight at 4°C. After washing with 0.1% Tween-20 phosphate-buffered saline (PBS), the filters were incubated with 1:2000 peroxidase-conjugated anti-mouse immunoglobulins for 1 hour at 22°C. They were extensively washed and finally analyzed using the chemiluminescence system (ECL system, Amersham Biosciences Biotech, Milan, Italy).
Analytical protocol (high-performance liquid chromatography)
Ectoine concentration in solutions used for cell treatment before stress was measured both before and after incubation. Analyses were accomplished using a high-performance chromatographic system (Dionex, HPLC) equipped with a UV detector (λ = 230 nm). Elution was based on isocratic separation (H2O/CH3CN 20/80 vol/vol) using a 250 × 4 mm Grom-Sil Amino 1PR column as described previously (Wohlfarth et al 1990).
Statistical analysis
Each experiment was performed at least 5 times. The results are expressed as means ± SD. The ANOVA test (Analysis of variance among groups) was performed for each kind of experiment (eg, dose-response curve, cytokines induction). The P value was generally evaluated between 0.01 and 0.03, confirming the statistical significance of results (P < 0.05).
RESULTS
Evaluation of ectoine concentration in the medium
To study the ectoine protection mechanism, solutions containing increasing amounts (50, 100, 250, and 500 μg/mL) of the molecule were used to incubate keratinocytes at 37°C for 24 hours before applying heat stress. Ectoine treatment, or heat-stress condition, did not produce effects on cell viability as assessed by trypan blue analysis (data not shown). Ectoine concentrations in the supernatant were measured before and after incubation to evaluate the uptake of the molecule. The results showed that the concentration in the medium decreased in all cases after 24 hours incubation and prolonging the contact up to 48 hours did not lead to a further decrement. Ectoine decrease in the medium may be because of molecule accumulation inside the cell, adhesion on cell membrane, or even metabolization. However, the ectoine decrease, evaluated about 5–15 μg/mL, was not increasing proportionally to the initial concentration of the molecule.
Ectoine induces hsp70 and hsp70B′ gene expression
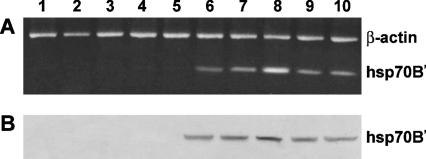
Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis was performed on mRNA derived from keratinocytes, which are preincubated for 24 hours in the presence or absence of increasing concentrations of ectoine (50, 100, 250, and 500 μg/mL) and heat stressed. A 2.5-fold increase of hsp70B′ gene expression was observed when incubating with 100 μg/mL ectoine, whereas only 15–20% increase of expression for the same gene was detected at the concentrations of 50, 250, and 500 μg/ mL, with respect to the positive control (Fig 2A). In contrast, in absence of heat shock, there was no gene induction in the control and in the ectoine-treated keratinocytes (Fig 2A). From these results, we can assume that ectoine does not induce hsp70B′ gene in the absence of a heat stress but amplifies the response in vitro of the heat-stressed keratinocytes. These data were also confirmed by Western blot analysis (Fig 2B).
Fig 2.
Effect of ectoine treatment on heat shock protein (Hsp)70B′ gene expression in keratinocyte cells. (A) Reverse transcriptase– polymerase chain reaction analysis using specific primers for hsp70B′ messenger RNA (mRNA) expression. Lane 1: untreated-cell mRNA; lanes 2–5: mRNA from keratinocytes pretreated for 24 hours with 50, 100, 250, 500 μg/mL ectoine, respectively, not heat stressed; lane 6: mRNA from heat-stressed keratinocytes; lanes 7–10: mRNA from keratinocytes pretreated for 24 hours with 50, 100, 250, 500 μg/mL ectoine, respectively, and, successively, heat stressed. The hsp70B′:β-actin fluorescence intensity ratios were as follows: lanes 6, 7, 8, 9, and 10, 0.35 ± 0.12, 0.45 ± 0.18, 0.87 ± 0.11, 0.43 ± 0.10, and 0.39 ± 0.21, respectively. The values are the mean of at least 5 independent experiments ± SD (P < 0.05). (B) Western blot analysis for the hsp70B′ content. Fifty micrograms of cell lysates from each sample was separated by a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions and subjected to Western blot analysis using 1 μg/mL anti-hsp70B′ polyclonal antibody as a probe
Experiments in which keratinocytes were placed in presence of 100 μg/mL ectoine for shorter incubation time (30 minutes, 2 and 4 hours) did not evidence hsp induction (data not shown).
To better understand the ability of ectoine to protect cells from stress conditions, we analyzed another member of the Hsp family, the hsp70 gene. The latter is not only elevated in response to stress but is also present at basal level in unstressed cells. We found, both by RT-PCR (Fig 3A) and by Western blot analysis (Fig 3B), that ectoine was able to overexpress the hsp70 gene in absence of heat stress. These results are very interesting because they highlight the ability of the molecule to protect cells from occurring stress conditions and to prevent cell damage by maintaining an elevated level of hsp70.
Fig 3.
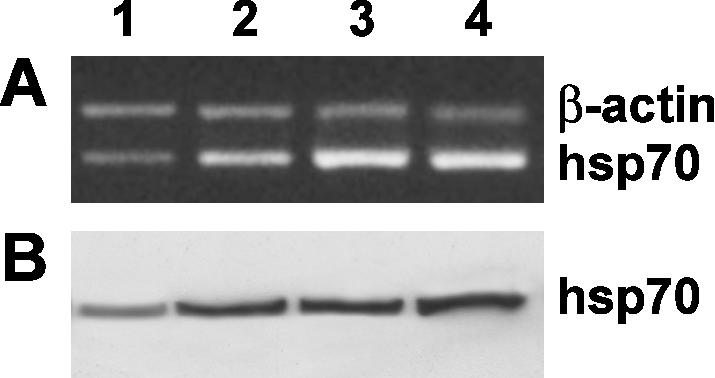
Effect of ectoine treatment on heat shock protein (Hsp)70 gene expression in keratinocyte cells. (A) Reverse transcriptase– polymerase chain reaction analysis using specific primers for hsp70 messenger RNA (mRNA) expression. Lane 1: untreated-cell mRNA; lane 2: mRNA from keratinocytes pretreated for 24 hours with 100 μg/mL ectoine, not heat stressed; lane 3: mRNA from heat-stressed keratinocytes; lane 4: mRNA from keratinocytes pretreated for 24 hours with 100 μg/mL ectoine and, successively, heat stressed. The hsp70:β-actin fluorescence intensity ratios were as follows: lanes 1, 2, 3, and 4, 0.72 ± 0.12, 1.22 ± 0.18, 2.1 ± 0.11, and 2.39 ± 0.21, respectively. The values are the mean of at least 5 independent experiments ± SD (P < 0.05). (B) Western blot analysis for the hsp70 content. Fifty micrograms of cell lysates from each sample was separated by a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions and subjected to Western blot analysis using 1 μg/mL anti-hsp70 monoclonal antibody as a probe
Ectoine prevents the proinflammatory process
To evaluate the ability of ectoine to evoke a proinflammatory process, we analyzed the induction of the proinflammatory cytokines IL-1α, IL-6, IL-8, and TNF-α, in the presence of a well-characterized proinflammatory stimulus (LPS). When keratinocytes were pretreated with ectoine before stimulation with LPS, we did not observe the upregulation of these specific cytokines, as occurred in the LPS-treated cells (Fig 4). However, the sole ectoine treatment did not modify the level of expression for the same cytokines (Fig 4).
Fig 4.
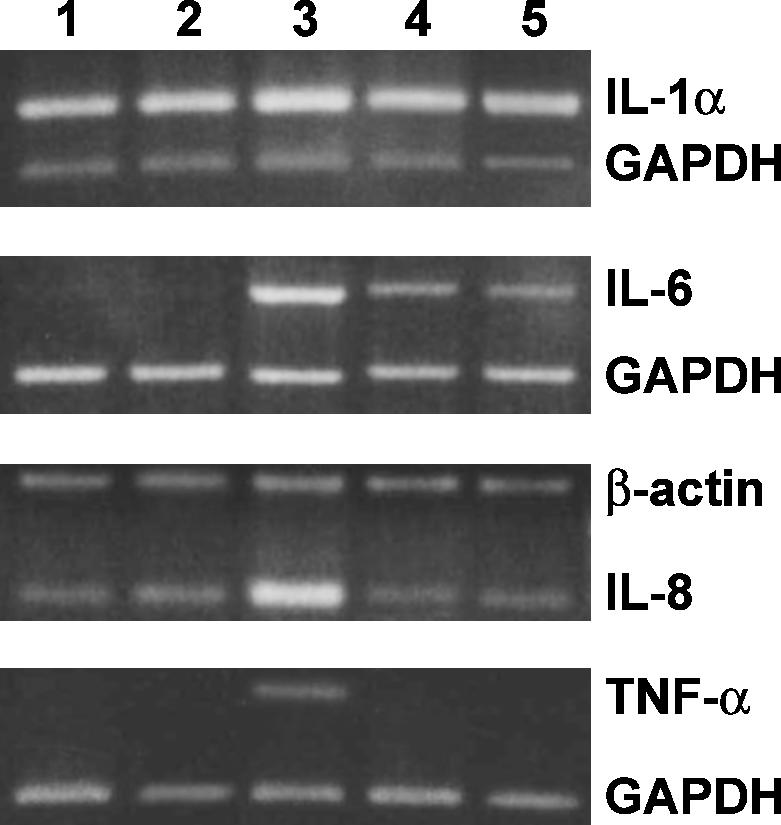
Expression of cytokine messenger RNA (mRNA) in keratinocytes treated with ectoine. Reverse transcriptase–polymerase chain reaction analysis using specific primers for interleukin (IL)-1α, IL-6, IL-8, and tumor necrosis factor–α mRNA expression. Lane 1: untreated cell mRNA; lane 2: mRNA from 100 μg/mL ectoine-treated keratinocytes; lane 3: mRNA from 10 μg/mL lipopolysaccharides (LPS)-treated keratinocytes; lane 4: mRNA from keratinocytes pretreated for 24 hours with 100 μg/mL ectoine, and, successively, incubated with 10 μg/mL LPS; lane 5: mRNA from keratinocytes preincubated for 15 minutes with 30 μM calpain inhibitor I (nuclear factor–kappa B [NF-κB] inhibitor), then incubated with 10 μg LPS for 24 hours. The IL-1α:GAPDH fluorescence intensity (FI) ratios were as follows: lanes 1, 2, 3, 4, and 5, 2.6 ± 0.12, 2.54 ± 0.11, 3.4 ± 0.21, 2.9 ± 0.18, and 2.72 ± 0.15, respectively. The IL-8:β-actin F.I. ratios were as follows: lanes 1, 2, 3, 4, and 5, 1.12 ± 0.18, 1.5 ± 0.11, 3.4 ± 0.19, 0.91 ± 0.21, and 0.97 ± 0.17, respectively. The values are the mean of at least 5 independent experiments ± SD (P < 0.05)
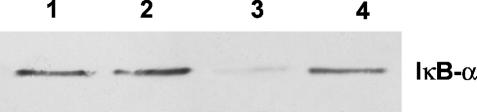
In addition, we evaluated the ability of ectoine to inhibit the activation of NF-κB, through the IκB-α degradation, by Western blot analysis. We demonstrated that ectoine treatment did not induce IκB-α degradation in keratinocyte cells (Fig 5), whereas in LPS-treated keratinocytes, the IκB-α degradation was strongly evident. Moreover, in the keratinocytes preincubated with ectoine, and successively LPS stimulated, the IκB-α levels were comparable to those obtained in the keratinocytes treated with the sole ectoine.
Fig 5.
Western blot analysis for the IκB-α content in keratinocyte cells treated or not for 24 hours with ectoine or lipopolysaccharides (LPS) at the indicated concentrations. Lane 1: untreated cell; lane 2: 100 μg/mL ectoine-treated keratinocytes; lane 3: 10 μg/mL LPS-treated keratinocytes; lane 4: keratinocytes pretreated for 24 hours with 100 μg/mL ectoine, and, successively, incubated with 10 μg/ mL LPS. Fifty micrograms of cell lysates from each sample was separated by a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions and subjected to Western blot analysis using 1 μg/mL anti-IκB-α polyclonal antibody as a probe
As shown in Figure 4, the effect of ectoine was similar to that produced by calpain inhibitor I (NF-κB inhibitor) in the prevention of the inflammatory process; in fact, both avoided IκB-α degradation and induction of the cited cytokines.
Taken together, these data suggest that ectoine does not have the ability to induce a proinflammatory process; moreover, this compatible solute acts as an anti-inflammatory molecule.
DISCUSSION
An important feature of Hsps is their role in the cytoprotection and repair of cells and tissue against the deleterious effects of stress and trauma (Morimoto and Santoro 1998). Overexpression of one or more hsp genes is sufficient to protect skin tissue against otherwise lethal exposures to heat, cytotoxic drugs, toxins, UV radiation, and other environmental stressors such as pollutants. In particular, Simon et al (1995) found that hsp70 overexpression markedly decreases the release of IL-6 induced by UV-A, UV-B, and oxidative stress. As expected in the experiments of this study, Hsp70, the major inducible and cytoprotective Hsp, was constitutively expressed in normal human keratinocyte cells, whereas the expression of hsp70B′ was induced only after heat shock. When keratinocyte cells were treated with ectoine and heat shocked, we observed a marked increase of the constitutive form hsp70 and a significant overexpression of hsp70B′, with respect to the heat-stressed control.
It has been demonstrated that exposure to a number of chemical inducers not only leads to Hsps induction but also to inhibition of NF-κB (Thanoa and Maniatis 1995). NF-κB is an inducible eukaryotic transcription factor that normally exists in an inert cytoplasmic complex bound to inhibitory proteins of the IκB family and is induced by a variety of pathogenic stimuli, including viral and bacterial infection, UV radiation, and inflammation (Thanoa and Maniatis 1995). Activation of NF-κB requires the phosphorylation and degradation of the inhibitory protein IκB-α, which allows translocation of NF-κB to the nucleus where it binds to specific κB sites in a variety of genes encoding signaling proteins. Evaluating the IκB-α degradation, we demonstrated that ectoine did not activate NF-κB in treated keratinocyte cells. Moreover, when keratinocytes were treated with ectoine, before stimulation with LPS, proinflammatory cytokines interleukin (IL)-1α, IL-6, IL-8, and TNF-α were downregulated. These data agree with previous findings showing that the induction of the Hsps inhibited proinflammatory cytokine expressions in vitro (Yoo et al 2000). Yoo et al (2000) also demonstrated that the induction of Hsps blocked nuclear translocation of NF-κB by inhibiting IκB-α degradation. We can argue that the ability of ectoine to induce Hsps and downregulate proinflammatory signals results in a cytoprotective mechanism of keratinocytes.
Here, we propose that a small organic molecule isolated from halophilic microorganisms, might find its applicability in all those skin disorders, characterized by infectious or inflammatory processes (or both), in which Hsp70 overexpression improves the recovery of the patient. In addition, ectoine, increasing the basal level of Hsp70, could represent a potential protective tool allowing a rapid reestablishment of protein synthesis and skin defense function.
Acknowledgments
We gratefully acknowledge Prof Erwin Galinski for providing ectoine. We thank Dr Carmelina Maresca for HPLC analysis. We also thank Dr Manuela Orlando and Dr Anna De Filippis for their technical assistance. This study was supported in part by funds from Ministero dell'Università e della Ricerca scientifica (MURST-PRIN 2003).
REFERENCES
- Boyce ST, Ham RG. Calcium regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum free serial culture. J Investig Dermatol. 1983;81:33–40. doi: 10.1111/1523-1747.ep12540422.0022-202X(1983)081[0033:CRDONH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilising the principle of proteins binding. Anal Biochem. 1976;72:248–256. doi: 10.1006/abio.1976.9999.0003-2697(1976)072[0248:ARASMF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Giannessi D, Caselli C, and Vitale RL. et al. 2003 A possible cardioprotective effect of heat shock proteins during cardiac surgery in pediatric patients. Pharmacol Res. 48:519–529. [DOI] [PubMed] [Google Scholar]
- Laplante A, Moulin V, and Auger FA. et al. 1998 Expression of heat shock proteins in mouse skin during wound healing. J Histochem Cytochem. 46:1291–1301. [DOI] [PubMed] [Google Scholar]
- Leung TKC, Rajendam MY, and Monfries C. et al. 1990 The human heat-shock protein family. Expression of a novel heat-inducible HSP70 (HSP70B′) and isolation of its cDNA and genomic DNA. Biochem J. 267:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippert K, Galinski EA. Enzyme stabilisation by ectoine-type compatible solutes: protection against heating, freezing and drying. Appl Microbiol Biotechnol. 1992;37:61–65.0175-7598(1992)037[0061:ESBECS]2.0.CO;2 [Google Scholar]
- Louis P, Trüper HG, Galinski EA. Survival of Escherichia coli during drying and storage in the presence of compatible solutes. Appl Microbiol Biotechnol. 1994;41:684–688.0175-7598(1994)041[0684:SOECDD]2.0.CO;2 [Google Scholar]
- Morimoto RI, Santoro G. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833.1087-0156(1998)016[0833:SRAHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Simon MM, Reikerstorfer A, and Schwarz A. et al. 1995 Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblasts. Evidence for increased cell viability and suppression of cytokine release. J Clin Investig. 95:926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souil E, Capon A, and Mordon S. et al. 2001 Treatment with 815-nm diode laser induces long-lasting expression of 72-kDa heat shock protein in normal rat skin. Br J Dermatol. 144:260–266. [DOI] [PubMed] [Google Scholar]
- Tavoria M, Gabriele T, Kola I, Anderson RL. A hitchhiker's guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1:23–28. doi: 10.1379/1466-1268(1996)001<0023:ahsgtt>2.3.co;2.1466-1268(1996)001[0023:AHGTTH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanoa D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5.0092-8674(1995)080[0529:NALIFV]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72(4):1063–1081. doi: 10.1152/physrev.1992.72.4.1063.0031-9333(1992)072[1063:MSRCPF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wohlfarth A, Severin J, Galinsky EA. The spectrum of compatible solutes in heterotrophic halophilic eubacteria of the family. Halomonadaceae. J Gen Microbiol. 1990;136:705–712.0022-1287(1990)136[0705:TSOCSI]2.0.CO;2 [Google Scholar]
- Yoo CG, Lee S, and Lee CT. et al. 2000 Anti-inflammatory effect of heat shock protein induction is related to stabilization of IκBα through preventing IκB kinase activation in respiratory epithelial cells. J Immunol. 164:5416–5423. [DOI] [PubMed] [Google Scholar]
- Zügel U, Kaufmann SHE. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19.1098-6618(1999)012[0019:ROHSPI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]