Abstract
Summer-diapause and winter-diapause pupae of the onion maggot, Delia antiqua (Diptera: Anthomyiidae), were significantly more cold hardy than nondiapause, prediapause, and postdiapause pupae. Moreover, cold acclimation of nondiapause pupae conferred strong cold hardiness comparable with that of diapause pupae. Differential display analysis revealed that the expression of a gene encoding TCP-1 (the t-complex polypeptide–1), a subunit of chaperonin CCT, in D antiqua (DaTCP-1) is upregulated in the pupae that express enhanced cold hardiness. Quantitative real-time polymerase chain reaction analyses showed that the levels of DaTCP-1 messenger RNA in pupal tissues, brain, and midgut in particular, are highly correlated with the cold hardiness of the pupae. These findings suggest that the upregulation of DaTCP-1 expression is related to enhanced cold hardiness in D antiqua. The upregulation of CCT in response to low temperature in an organism other than the yeast is newly reported.
INTRODUCTION
Chaperonins are molecular chaperones responsible for the folding and assembly of newly synthesized proteins and refolding of denatured proteins in cells (Sonna et al 2002). Chaperonins are classified into 2 groups (I and II): group I includes bacterial GroEL (Georgopoulos et al 1973) and the rubisco subunit–binding protein in higher plant chloroplasts (Hemmingsen et al 1988). Group II includes CCT (chaperonin containing the t-complex peptide–1 [TCP-1]) (Lewis et al 1992) and thermophilic factor 55 (Trent et al 1991).
CCT is a hetero-oligomeric chaperonin composed of 8 to 9 different subunit species of approximately 60 kDa (Rommelaere et al 1993) and has a central cavity that mediates the folding of polypeptides (Gómez-Puertas et al 2004). Each subunit species of CCT is encoded by an independent gene, and although they have common adenosine triphosphate (ATP)–binding motifs (SLGPVG, TI/ VTNDG, and GDGTT) (Kim et al 1994), their amino acid sequence identities are only approximately 30% (Yokota et al 1999; Valpuesta et al 2002). Whereas GroEL in group I takes various substrates (Georgopoulos et al 1973), only actin and tubulin are known as the major substrates of CCT in vivo (Sternlicht et al 1993), although α-transducin and firefly luciferase are folded by CCT in vitro (Farr et al 1997; Gebauer et al 1998).
Despite the importance of molecular chaperones in stress response, the role of CCT is not clearly understood. We have long been interested in the unusually strong cold hardiness of the diapause pupae of the onion maggot, Delia antiqua (Diptera: Anthomyiidae). To explore the molecular mechanisms underlying the strong cold hardiness in D antiqua, we sought differentially expressed genes in the diapause pupae. As a consequence, the expression of DaTCP-1, which encodes a subunit species of CCT, was found to be upregulated in the cold hardy pupae. This study reports the expression levels of DaTCP-1 in the Malpighian tubules, brain, fat body, and midgut of the pupae in different physiological states with varying cold hardiness and examines the relationship between cold hardiness and DaTCP-1 expression.
MATERIALS AND METHODS
Insects
The onion maggots were originally collected at Sapporo, Japan, and their offspring have been reared on an artificial diet (Ishikawa et al 1983) for about 40 generations in the laboratory. Adults were reared in fabric screen cages (30 × 30 × 30 cm) with a supply of water, sugar, and yeast extract (Dried Yeast Extract D-3, Wako Pure chemicals, Kyoto, Japan). The cages were maintained in an environmental chamber controlled at 23 ± 1.0°C, under a 16 hour light:8 hour dark photoperiod (16L:8D) and 50– 70% relative humidity. A plastic dish (8 × 5 cm diameter) containing damp glass beads and several pieces of onion was placed in the cage as the oviposition substrate. Eggs were inoculated on a piece of artificial diet placed on moistened fine sand in a plastic container (13 × 8 cm diameter). To obtain pupae with varied cold hardiness, larvae were reared under the conditions described below (Nomura and Ishikawa 2001). In this study, the age of pupae is expressed by the days after pupariation, with the day of pupariation being day 0.
Cold-acclimated nondiapause pupae
Larvae were reared on the artificial diet at 20 ± 0.2°C and 16L:8D. After pupariation, the nondiapause pupae were maintained at 17°C ± 0.2°C and 16L:8D for 5 days (ND5). ND5 pupae were then housed in a Styrofoam box and placed in an incubator adjusted to 5.0 ± 0.2°C for 0, 2, 8, or 32 days (CA0, CA2, CA8, or CA32). Because the lower threshold temperature for pupal development in D antiqua is 5.7°C (Nomura and Ishikawa 2000), no development was considered to have occurred during acclimation. Hence, all cold-acclimated pupae were regarded as ND5, irrespective of the duration of acclimation.
Summer-diapause pupae
Larvae were reared at 25 ± 0.2°C and 16L:8D. Under these conditions, more than 95% of pupae entered summer diapause (SD). Puparia were maintained under the same conditions. For obtaining pupae that have terminated summer diapause (SDT), the 10-day-old SD pupae (SD10) were transferred to 17 ± 0.2°C and 16L:8D for 10 days. In more than 95% of pupae, the diapause was terminated with this treatment.
Winter diapause
Larvae were reared at 15 ± 0.2°C and 12L:12D. Under these conditions, more than 95% of pupae entered winter diapause (WD). Puparia were maintained under the same conditions.
Cold-hardiness assay
The cold hardiness of the cold-acclimated nondiapause pupae (CA0, CA2, CA8, and CA32), WD pupae (WD3, WD10, and WD50), SD pupae (SD3, SD20), and the SDT pupae were evaluated from the survival rate after exposure to −20 ± 0.2°C for 5 days (Kayukawa and Ishikawa 2005).
Dissection and total RNA extraction
The thin outermost shell of the puparia (puparial case) was removed using fine forceps, and the pupa was dissected in phosphate-buffered saline (137 mM NaCl, 8 mM Na2HPO4·12H2O, 2.7 mM KCl, and 1.5 mM KH2PO4, pH 7.4) on ice. The brain, Malpighian tubules, fat body, and midgut were isolated from 25 to 30 animals and snap frozen in liquid nitrogen. Total RNA was extracted from these tissues using an RNeasy Mini Kit (QIAGEN, Valencia, CA, USA) with RNase-free DNase I (QIAGEN).
Differential display analysis
Differential display (DD) was performed with a Gene Fishing DEG Kit (Seegene, Seoul, South Korea). The total RNA of the brain from CA0, CA2, CA32, and WD50 pupae was reverse-transcribed using an RNA PCR Kit (AMV) (V3.0, TAKARA BIO, Otsu, Shiga, Japan) and dT-ACP1 primer (Seegene; 5′-CTGTGAATGCTGCGACTACGATXXXXX(T)18-3′). Using the first-strand complementary DNA (cDNA) as the template, polymerase chain reactions (PCRs) were performed with combinations of an arbitrary ACP (ACP01-ACP20) and dT-ACP2 (5′-CTGTGAATGCTGCGACTACGATXXXXX(T)15-3′) at 94°C for 5 minutes, 50°C for 3 minutes, and 72°C for 1 minute, 40 cycles of 94°C for 40 seconds, 65°C for 40 seconds, and 72°C for 40 seconds, and 72°C for 5 minutes. The PCR products were separated by 2% agarose gel electrophoresis and stained with SYBR™ Green I (Molecular Probes, Eugene, OR, USA). One of the differentially expressed gene fragments, the DaTCP-1 fragment, was extracted from the agarose gel, reamplified by PCR (94°C for 3 minutes, 35 cycles of 94°C for 40 seconds, 65°C for 40 seconds, and 72°C for 40 seconds, and 72°C for 5 minutes) with 5′ and 3′ universal primers (Seegene), and sequenced using a BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) and 5′ universal primer on an ABI PRISM 377HN Sequencer (Applied Biosystems).
Cloning of full-length cDNA by RACE
The 5′ and 3′ ends of the DaTCP-1 cDNA were obtained by a modified rapid amplification of cDNA ends (RACE) method using a FirstChoice® RLM RACE Kit (Ambion, Austin, TX, USA). The template first-strand cDNA was synthesized from the total RNA extracted from the brain of WD50 pupae. The first PCR in the 5′ RACE was performed using 5′ RACE outer primer (Ambion, Austin, TX, USA) and SP-R1 (5′-AGAAGCAGCAGCTCTGGCTTTAG-3′). The first and second nested PCRs were performed using 5′ RACE inner primer and SP-R2 (5′-GCAGCACCAGTTGCCTTAGC-3′) and the inner primer and SP-R3 (5′-AATTCACGGGCACGGATACC-3′), respectively. The first PCR in the 3′ RACE was performed using 3′ RACE outer primer and SP-F1 (5′-TCGCAACAGATGCCCAAGA-3′). The nested PCRs were performed using 3′ RACE inner primer and SP-F2 (5′-CGGCGGTGTTGATGATTTGT-3′), and the inner primer and SP-F3 (5′-GCTGCTGAGGTGGCTCAAGA-3′). The PCR products obtained by 5′ and 3′ RACE were subcloned using pGEM®-T Easy Vector System I (Promega, Madison, WI, USA) and sequenced.
Quantitative real-time PCR
The complementary DNAs (cDNAs) prepared from the Malpighian tubules, brain, fat body, and midgut using the SYBR® RT-PCR Kit (TAKARA BIO) were diluted to 1/50 for detection of DaTCP-1 detection and 1/1000 for detection of the 18S ribosomal RNA (rRNA) gene. The primers used for quantitative real-time PCR (qr-PCR) of DaTCP-1 were SP-F2 and SP-R2. The primers for the 18S rRNA gene were 5′-TTAAGCCATGCATGTCTAAGTAC-3′ and 5′-TCTCAGGCTCCCTCTCCGGAATCG-3′. The DaTCP-1 transcript was quantified on an ABI PRISM® 7700 Sequence Detection System (Applied Biosystems). The qr-PCR was carried out in a 20-μL reaction volume containing 1 μL of template cDNA, SYBR Premix Ex Taq, ROX Reference Dye (TAKARA BIO), and 0.2 μM of each primer. Shuttle PCR conditions were 95°C for 10 seconds followed by 40 cycles of 95°C for 5 seconds and 60°C for 30 seconds. The level of DaTCP-1 messenger RNA (mRNA) was normalized to that of 18S rRNA.
RESULTS
Cold hardiness
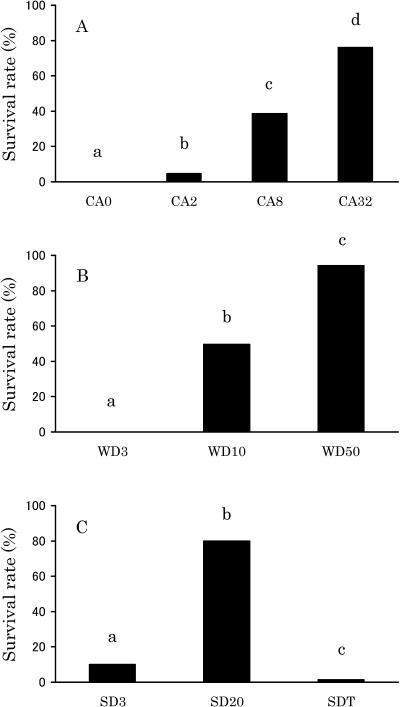
Nondiapause (CA0) pupae of D antiqua could not survive a 5-day exposure to −20°C (Fig 1A). However, the survival rate increased as the period of cold acclimation was extended (Fig 1A). Most prediapause pupae (WD3 and SD3) were not able to survive the −20°C treatment; however, the pupae in the midst of diapause (WD10, WD50, and SD20) had marked cold hardiness (Fig 1 B,C). Moreover, the SDT pupae could not survive the −20°C treatment (Fig 1C).
Fig 1.
Cold hardiness of Delia antiqua pupae assessed by survival after a 5-day treatment at −20°C. After the −20°C treatment, pupae were maintained at 17°C. (A) Nondiapause pupae acclimated at 5°C for 0, 2, 8, and 32 days (CA0, CA2, CA8, and CA32) (n = 150). (B) The pupae obtained under winter diapause–inducing conditions were maintained under the same conditions for 3, 10, and 50 days (WD3, WD10, and WD50) (n = 200). (C) Pre–summer diapause (SD) pupae (SD3), SD pupae (SD20), and pupae that had terminated summer diapause (SDT) (n = 150). Bars with the same letter are not significantly different (Ryan's multiple comparison test of proportions, P < 0.05)
Differential display
The cDNAs prepared from CA0, CA2, CA32, and WD50 pupae were subjected to DD analysis. DD analyses using 20 arbitrary primers revealed 3 bands that appear in association with the increase in cold hardiness (data not shown). In this study, only the band that showed the clearest change (Fig 2) was analyzed further. Analysis of the sequence of the DD product by BLAST (Altschul et al 1997) for the nucleotide and for the deduced amino acid sequence showed high homology to TCP-1s in Drosophila and Anopheles.
Fig 2.
Differential display of messenger RNA derived from the brain of CA0, CA2, CA32, and WD50 pupae. The arrow indicates a band corresponding to the DaTCP-1 complementary DNA fragment, the size of which is 1.5 kbp
Full-length cDNA
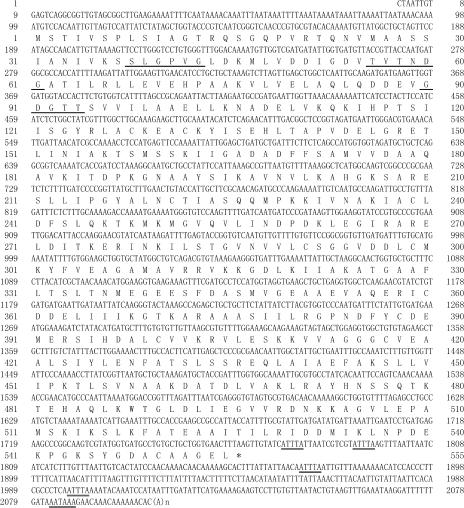
The full-length cDNA (DaTCP-1) has a predicted ORF encoding a protein of 555 amino acids (Fig 3). The protein is estimated to have a molecular weight of 59.4 kDa and a pI of 6.45. In the 3′ UTR, 4 ATTTA-motifs specific to mRNAs that are susceptible to degradation were observed. In addition, 3 characteristic ATP-binding motifs are present in DaTCP-1 (SLGPVG, TVTNDG, and GDGTT) (Fig 3). The homology analyses showed 93% amino acid identity to D melanogaster TCP-1–α (GenBank accession no. P12613), 78% identity to Apis mellifera (XP_392660), 74% identity to Homo sapiens TCP-1 (AAH00665), and 61% identity to Neurospora crassa (XP_323801).
Fig 3.
Nucleotide and deduced amino acid sequences of DaTCP-1 complementary DNA. The underlined amino acids represent adenosine triphosphate–binding motifs. Double-underlined nucleotides represent a presumable polyadenylation signal (AATAAA) and RNA instability motif (ATTTA). This sequence has been deposited in the GenBank database (Accession no. AB194475)
Expression of DaTCP-1 mRNA
The changes in levels of DaTCP-1 mRNA in different tissues of the pupae that occur in association with cold acclimation, SD, and WD were analyzed by qr-PCR (Fig 4). The expression of DaTCP-1 was increased in the brain, midgut, and Malpighian tubules as the period of cold acclimation was extended (Fig 4A). In contrast, the expression in the fat body was decreased to half after a 32-day cold acclimation (Fig 4A). In WD pupae, the expression levels in all tissues examined were increased 2- to 6-fold (Fig 4B). In SD pupae, levels in all tissues examined were increased 2- to 6-fold in the midst of diapause (SD20). Interestingly, when the SD was completely terminated (SDT), the level decreased to that of the prediapause pupae (SD3) (Fig 4C).
Fig 4.
Quantitative real-time PCR analysis of DaTCP-1 messenger RNA (mRNA) levels. The levels of DaTCP-1 mRNA were normalized to those of the internal standard, 18S ribosomal RNA (3 replicates). DaTCP-1 mRNA levels in cold-acclimated nondiapause pupae (A), winter-diapause pupae (B), and summer-diapause pupae (C). Br, brain; MG, midgut; MP, Malpighian tubules; FB, fat body
DISCUSSION
Cold hardiness in insects has been discussed in terms of cryoprotectants, supercooling point, antifreeze proteins, homeoviscous adaptation, and heat shock proteins (Lee 1991; Storey and Storey 1991; Graether et al 2000; Yocum 2001; Koštál et al 2003). This study clearly demonstrated that expression levels of DaTCP-1 are highly correlated with the cold hardiness of the D antiqua pupae.
The roles of molecular chaperones in response to various stresses have been widely studied in organisms from microbes to plants and animals. GroEL, a chaperonin in group I, was reported to play important roles in response to temperature stress, osmotic stress, and chemical stress (Fayet et al 1989; Ben-Zvi and Goloubinoff 2001; Hennequin et al 2001). In contrast, CCT, a chaperonin in group II, was considered not to be induced by stress until the early 1990s (Ursic and Culbertson 1992; Leroux and Candido 1995). Recently, however, CCT was shown to be induced by heat shock in human cells (Schena et al 1996), chemical stress (CdCl2) in Oxytricha granulifera (Palmedo and Ammermann 1997), and cold shock in the yeast Saccharomyces cerevisiae (Somer et al 2002).
In general, the mechanisms that cause cold injuries in cells are not well understood, although the involvement of phase transitions in the cell membrane lipid and a complex metabolic disorder have been proposed (Drobnis et al 1993; Nedvĕd et al 1998). Recently, depolymerization of actin and tubulin at low temperature has been recognized as an important factor for the occurrence of cold injuries. In a wide range of plant and animal species, the significance of the recrystallization of cytoskeletons in the recovery from cold stress has been reported (Upadhya and Strasberg 1999; Egierszdorff and Kacperska 2001). Pokorná et al (2004) investigated changes in the organization of actin filaments and microtubules in tobacco cells during cold treatment and subsequent recovery and proposed a model for the mode of reorganization of the cytoskeletons. In wheat, an actin-binding protein induced by cold acclimation is suggested to facilitate cytoskeletal rearrangements, resulting in the enhancement of cold tolerance (Ouellet et al 2001). As mentioned in the Introduction, actin and tubulin are the major substrates of CCT. On the basis of the finding that CCT was upregulated in response to low temperature in the yeast S cerevisiae, Somer et al (2002) hypothesized that upregulation of CCT expression is related to the reorganization of actin and tubulin monomers during the recovery from cold stress. In view of these findings, the upregulation of DaTCP-1 expression in D antiqua is likely to be related to the recrystallization or rearrangement of actin and tubulin and hence contribute to the reinforcement of cold hardiness.
D antiqua is known to pass the coldest and hottest seasons as diapause pupae. WD and SD pupae as well as cold-acclimated nondiapause pupae have significantly higher tolerance to both low and high temperatures than nondiapause pupae (Nomura and Ishikawa 2001). However, the ecological significance of cold tolerance in SD pupae and heat tolerance in WD pupae has been unclear. We suggest that diapause pupae acquire both low- and high-temperature tolerance because common mechanisms underlie these 2 kinds of tolerance. Although the conditions that induce SD and WD are different (Nomura and Ishikawa 2001), it is likely that common mechanisms are activated in the 2 diapauses. Actually, in addition to DaTCP-1, the expressions of other chaperones, Hsp70 and Hsp90, are also upregulated in both SD and WD pupae (B. Chen et al, unpublished data). A curious finding in this study is that the expression of DaTCP-1 in the fat body was decreased by extended cold acclimation. This finding strongly suggests that cold acclimation and diapause confer tolerance to pupae through different processes.
Acknowledgments
We thank Dr K. T. Yoshida of the Laboratory of Conservation Ecology, The University of Tokyo, for technical advice and Drs S. Tatsuki and S. Hoshizaki of our laboratory for their encouragement during the study.
REFERENCES
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389.0305-1048(1997)025[3389:GBAPAN]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi AP, Goloubinoff P. Review: mechanisms of disaggregation and refolding of stable protein aggregates by molecular chaperones. J Struct Biol. 2001;135:84–93. doi: 10.1006/jsbi.2001.4352.1047-8477(2001)135[0084:RMODAR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Drobnis EZ, Crowe LM, Berger T, Anchordoguy TJ, Overstreet JW, Crowe JH. Cold shock damage is due to lipid phase transitions in cell membranes: a demonstration using sperm as a model. J Exp Zool. 1993;265:432–437. doi: 10.1002/jez.1402650413.0022-104X(1993)265[0432:CSDIDT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Egierszdorff S, Kacperska A. Low temperature effects on growth and actin cytoskeleton organization in suspension cells of winter oilseed rape. Plant Cell Tiss Organ Cult. 2001;40:17–25.0167-6857(2001)040[0017:LTEOGA]2.0.CO;2 [Google Scholar]
- Farr GW, Scharl EC, Schumacher RJ, Sondek S, Horwich AL. Chaperonin-mediated folding in the eukaryotic cytosol proceeds through rounds of release of native and nonnative forms. Cell. 1997;89:927–937. doi: 10.1016/s0092-8674(00)80278-0.0092-8674(1997)089[0927:CFITEC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fayet O, Zieggelhoffer T, Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989;171:1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989.0021-9193(1989)171[1379:TGAGHS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer M, Melki R, Gehring U. The chaperone cofactor Hop/ p60 interacts with the cytosolic chaperonin-containing TCP-1 and affects its nucleotide exchange and protein folding activities. J Biol Chem. 1998;273:29475–29480. doi: 10.1074/jbc.273.45.29475.0021-9258(1998)273[29475:TCCPIW]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Georgopoulos CP, Hendrix RW, Casjens SR, Kaiser AD. Host participation in bacteriophage lambda assembly. J Mol Biol. 1973;76:45–60. doi: 10.1016/0022-2836(73)90080-6.0022-2836(1973)076[0045:HPIBLA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gómez-Puertas P, Martín-Benito J, Carrascosa JL, Willison KR, Valpueasta JM. The substrate recognition mechanisms in chaperonins. J Mol Recognit. 2004;17:85–94. doi: 10.1002/jmr.654.0952-3499(2004)017[0085:TSRMIC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Graether SP, Kuiper MJ, Gagne SM, Walker VK, Jia Z, Sykes BS, Davies PL. β-Helix structure and ice-binding properties of a hyperactive antifreeze protein from an insect. Nature. 2000;406:325–328. doi: 10.1038/35018610.0028-0836(2000)406[0325:HSAIPO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hemmingsen SM, Wollford C, van der Vies SM, Tilly K, Dennis DT, Georgopoulos CP, Nendrix RW, Ellis RJ. Homologous plant and bacterial proteins chaperone oligomeric assembly. Nature. 1988;333:330–334. doi: 10.1038/333330a0.0028-0836(1988)333[0330:HPABPC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hennequin C, Collignon A, Karjalainen T. Analysis of expression of GroEL (HSP60) of Clostridium difficile in response to stress. Microb Pathog. 2001;31:255–260. doi: 10.1006/mpat.2001.0468.0882-4010(2001)031[0255:AOEOGH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Mochizuki A, Ikeshoji T, Matsumoto Y. Mass-rearing of the onion and seed-corn flies, Hylemya antiqua and H. platura (Diptera: Anthomyiidae) on a artificial diet with antibiotics. Appl Entomol Zool. 1983;18:62–69.0003-6862(1983)018[0062:MOTOAS]2.0.CO;2 [Google Scholar]
- Kayukawa T, Ishikawa Y. 2005. Detection of chill injuries in the pupae of the onion maggot, Delia antiqua (Diptera: Anthomyiidae) Appl Entomol Zool. 40:193–198. [Google Scholar]
- Kim S, Willison KR, Horwich AL. Cytosolic chaperonin subunits have a conserved ATPase domain but diverged polypeptide-binding domains. Trends Biochem Sci. 1994;19:543–548. doi: 10.1016/0968-0004(94)90058-2.0376-5067(1994)019[0543:CCSHAC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Koštál V, Berková P, Šimek P. Remodelling of membrane phospholipids during transition to diapause and cold-acclimation in the larvae of Chymomyza coatata (Drosophilidae) Comp Biochem Physiol B. 2003;135:407–419. doi: 10.1016/s1096-4959(03)00117-9.1096-4959(2003)135[0407:ROMPDT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lee RE 1991 Principles of insect low temperature tolerance. In: Insects at Low Temperature, ed Lee RE, Denlinger DL. Chapman and Hall, New York, NY, 17–46. [Google Scholar]
- Leroux MR, Candido EPM. Molecular analysis of Caenorhabiditis elegans TCP-1, a gene encoding a chaperonin protein. Gene. 1995;156:241–246. doi: 10.1016/0378-1119(95)00025-2.0378-1119(1995)156[0241:MAOCET]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lewis VA, Hynes GM, Dong Z, Saibil H, Willison K. TCP1 is a subunit of a heteromeric particle in the eukaryotic cytosol. Nature. 1992;358:249–252. doi: 10.1038/358249a0.0028-0836(1992)358[0249:TIASOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nedvĕd O, Lavy D, Verhoef HA. Modelling the time–temperature relationship in cold injury and effect of high-temperature interruptions on survival in a chill-sensitive collembolan. Funct Ecol. 1998;12:816–824.0269-8463(1998)012[0816:MTTRIC]2.0.CO;2 [Google Scholar]
- Nomura M, Ishikawa Y. Biphasic effect of low temperature on completion of winter diapause in the onion maggot, Delia antiqua. J Insect Physiol. 2000;46:373–377. doi: 10.1016/s0022-1910(99)00120-1.0022-1910(2000)046[0373:BEOLTO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nomura M, Ishikawa Y. Dynamic changes in cold hardiness, high-temperature tolerance and trehalose content in the onion maggot, Delia antiqua (Diptera: Anthomyiidae), associated with the summer and winter diapause. Appl Entomol Zool. 2001;36:443–449.0003-6862(2001)036[0443:DCICHH]2.0.CO;2 [Google Scholar]
- Ouellet F, É Carpen, Jamie M, Cope MJTV, Monroy AF, Sarhan F. Regulation of a wheat actin-depolymerizing factor during cold acclimation. Plant Physiol. 2001;125:360–368. doi: 10.1104/pp.125.1.360.0032-0889(2001)125[0360:ROAWAF]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmedo G, Ammermann D. Cloning and characterization of the Oxytricha granulifera chaperonin containing tailless complex polypeptide 1 γ gene. Eur J Biochem. 1997;247:877–883. doi: 10.1111/j.1432-1033.1997.00877.x.0014-2956(1997)247[0877:CACOTO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pokorná J, Schwarzerová K, Zelenková S, Petrášek J, Janotová I, Čapková V, Opatrný Z. Sites of actin filament initiation and reorganization in cold-treated tobacco cells. Plant Cell Environ. 2004;27:641–653.0140-7791(2004)027[0641:SOAFIA]2.0.CO;2 [Google Scholar]
- Rommelaere H, Vantroys M, Gao YJ, Melki R, Cowan NJ, Vandekerckhove J, Ampe C. Eukaryotic cytosolic chaperonin contains t-complex polypeptide 1 and seven related subunits. Proc Natl Acad Sci U S A. 1993;90:11975–11979. doi: 10.1073/pnas.90.24.11975.0027-8424(1993)090[11975:ECCCTP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena M, Shalon D, Heller R, Chai A, Brown PO, Davis RW. Parallel human genome analysis: microarray-based expression monitoring of 1000 genes. Proc Natl Acad Sci U S A. 1996;93:10614–10619. doi: 10.1073/pnas.93.20.10614.0027-8424(1996)093[10614:PHGAME]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somer L, Shmulman O, Dror T, Hashmueli S, Kashi Y. The eukaryote chaperonin CCT is a cold shock protein in Saccharomyces cerevisiae. Cell Stress Chaperones. 2002;7:47–54. doi: 10.1379/1466-1268(2002)007<0047:teccia>2.0.co;2.1466-1268(2002)007[0047:TECCIA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonna LA, Fujita J, Gaffin SL, Lilly CM. Molecular biology of thermoregulation: effects of heat and cold stress on mammalian gene expression. J Appl Physiol. 2002;92:1725–1742. doi: 10.1152/japplphysiol.01143.2001.8750-7587(2002)092[1725:MBOTEO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sternlicht H, Farr GW, Sternlicht ML, Driscoll JK, Willison K, Yaffe MB. The t-complex polypeptide 1 complex is a chaperonin for tubulin and actin in vivo. Proc Natl Acad Sci U S A. 1993;90:9422–9426. doi: 10.1073/pnas.90.20.9422.0027-8424(1993)090[9422:TTPCIA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey KB, Storey JM 1991 Biochemistry of cryoprotectants. In: Insects at Low Temperature, ed Lee RE, Denlinger DL. Chapman and Hall, New York, NY, 64–93. [Google Scholar]
- Trent JD, Nimmesgern E, Wall JS, Hartl FU, Horwich AL. A molecular chaperone from a thermophilic archaebactcrium is related to the eukaryotic protein-t-complex polypeptide-1. Nature. 1991;354:249–252. doi: 10.1038/354490a0.0028-0836(1991)354[0249:AMCFAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Upadhya GA, Strasberg SM. Evidence that actin disassembly is a requirement for matrix metalloproteinase secretion by sinusoidal endothelial cells during cold preservation in the rat. Hepatology. 1999;30:169–176. doi: 10.1002/hep.510300130.0270-9139(1999)030[0169:ETADIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ursic D, Culbertson MR. Is yeast TCP-1 a chaperonin? Nature. 1992;356:392. doi: 10.1038/356392a0.0028-0836(1992)356[0392:IYTAC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Valpuesta JM, Martín-Benito J, Gómez-Puertas P, Carrascosa JL, Willison KR. Structure and function of a protein folding machine: the eukaryotic cytosolic chaperonin CCT. FEBS Lett. 2002;529:11–16. doi: 10.1016/s0014-5793(02)03180-0.0014-5793(2002)529[0011:SAFOAP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yocum GD. Differential expression of two HSP70 transcripts in response to cold shock, thermoperiod, and adult diapause in the Colorado potato beetle. J Insect Physiol. 2001;47:1139–1145. doi: 10.1016/s0022-1910(01)00095-6.0022-1910(2001)047[1139:DEOTHT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yokota S, Yanagi H, Yura T, Kubota H. Cytosolic chaperonin is up-regulated during cell growth. J Biol Chem. 1999;267:1658–1664. doi: 10.1074/jbc.274.52.37070.0021-9258(1999)267[1658:CCIUDC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]