Abstract
The TSK/TSK mutation is embryonic lethal; embryos have been reported to die at 7–8 days of gestational age. Crossing TSK/+, IL-4+/− mice revealed that disrupting one or both IL-4 alleles allowed survival of 29 and 47%, respectively, of TSK/TSK mice. These mice failed to develop cutaneous hyperplasia but did exhibit the emphysema that is found in TSK/+ mice. We showed that IL-4 stimulation of fibroblasts increased the level of transforming growth factor-β (TGF-β) mRNA and that lungs of TSK/+, IL-4−/− mice had substantially less TGF-β mRNA than lungs of TSK/+, IL-4+/+ mice. Thus IL-4 seems to regulate the expression of TGF-β in fibroblasts, providing an explanation for the absence of cutaneous hyperplasia in TSK/+, IL-4Rα−/− and TSK/+, TGF-β+/− mice.
Tight-skin (TSK) mice are heterozygous for a mutation in the fibrillin-1 gene (TSK/+). They develop a scleroderma-like syndrome characterized by cutaneous hyperplasia, emphysema, cardiac hypertrophy, and autoantibodies specific for topoisomerase I, RNA polymerase I, and fibrillin-1 (1, 2). TSK/+ mice also develop CD4+ T helper (Th)1 cells specific for peptides derived by digestion of the lung with elastase (3). TSK homozygosity results in embryonic lethality at days 7–8 of gestation (1).
Both IL-4 and transforming growth factor-β (TGF-β) seem to play important roles in the pathogenesis of the fibrosis that characterizes the scleroderma-like syndrome of TSK/+ mice. Both cytokines have been shown to induce collagen synthesis by fibroblasts, and fibroblasts from TSK/+ mice are hyperresponsive to IL-4 and TGF-β (4). The importance of IL-4 in the fibrosis of TSK/+ mice is emphasized by the observation that targeted mutations in either the signaling chain of the IL-4 receptor or Stat6, one of the key signaling intermediates of the IL-4 receptor, prevents the cutaneous hyperplasia seen in TSK/+ mice (4, 5). IL-4-deficient mice fail to develop skin hyperplasia, and administration of anti-IL-4 antibody to neonatal TSK/+ mice also prevents skin fibrosis (5, 6). CD4+ T cells seem essential for the development of skin hyperplasia, because CD4−/− TSK/+ mice fail to develop such hyperplasia (7). Furthermore, TSK/+ mice transgenic for a T cell receptor Vβ8.2 segment fail to develop cutaneous hyperplasia, suggesting that the T cells that mediate this aspect of the TSK syndrome use distinct T cell antigen receptors (5). These results indicate that T cell production of IL-4 is critical to the development of cutaneous hyperplasia in TSK/+ mice. Interestingly, IL-4-producing cells have been found in skin biopsies from patients with scleroderma, and these patients have been reported to exhibit elevated levels of serum IL-4 (8–10).
TSK/+ mice that are heterozygous for a disrupted TGF-β gene also fail to develop cutaneous hyperplasia (4). This observation could be accounted for either through the requirement of the joint action of IL-4 and TGF-β in the stimulation of collagen biosynthesis by fibroblasts or by the two cytokines being in a “pathway” in which one is responsible for the production of the other, and it is the second cytokine that induces fibrosis.
Here we address issues regarding the embryonic lethality of TSK homozygosity and the possible role of IL-4 as an inducer of TGF-β, thus explaining its profibrotic role in TSK mice.
Material and Methods
Mice.
BALB/c IL-4−/−tm2Nt mice and TSK/+ mice were purchased from The Jackson Laboratory. F1 and F2 mice were generated in the animal facilities of Mount Sinai School of Medicine.
Fibroblast Lines.
C57BL/6 pa/pa and TSK/+ primary fibroblast lines were maintained in DMEM supplemented with 100 units/ml penicillin/100 μg/ml streptomycin/10% FCS (Mediatech, Herndon, VA).
Reagents.
Recombinant murine IL-4, highly purified bacterial collagenase, and 5,6-dichloro-1-β-d-ribofuranosyl-benzimidazole (DRB) were purchased from Sigma. Recombinant human TGF-β1 and purified monoclonal anti-murine-TGF-β1 antibodies were purchased from R & D systems.
Northern Blotting Analysis.
Total RNA was extracted from normal or TSK/+ fibroblasts or from the lungs of F2 mice by using RNeasy kits (Qiagen, Chatsworth, CA) according to the manufacturer's instructions. Total RNA (20 μg) was separated on 1% agarose gels containing 2.2 M formaldehyde and transferred to Hybond N+ nylon membrane (Amersham Pharmacia) unless otherwise noted. The membranes were hybridized with fibrillin-1 or TGF-β1 32P-labeled DNA probes as described (11). The membrane was stripped and rehybridized with 32P-labeled β-actin or c-myc cDNA probes.
DNA Probes.
The probe for fibrillin-1 was described previously (11). Probes for TGF-β and c-myc were made by reverse transcription–PCR using placental cDNA as a template. PCR-generated cDNA was cloned in a pGEM-T vector (Promega). Selected clones were confirmed as containing a cDNA fragment corresponding to the appropriate gene by sequencing in an ABI3700 automated sequencer and were used as templates for PCR reactions with the same set of primers. PCR products made by reamplification were purified with a PCR-purification kit (Qiagen) and used as DNA probes. The primers used were: forward (5′-ggactactatgctaaagaggtcac-3′) and reverse (5′-ctgtattccgtctccttggttcagc-3′) for TGF-β and forward (5′-atccaggactgtatgtggag-3′) and reverse (5′-tctcacgagagattccagct-3′) for c-myc. DNA probes were labeled with [32P] by using the random-primed method (Roche, Indianapolis, IN) according to manufacturer instructions.
PCR.
Genomic DNA was extracted from tails, and 100 ng was amplified to detect the wild-type and disrupted IL-4 alleles as described (12). The PCR product was visualized by ethidium bromide staining. The primers used were sense (5′-gtgagcagatgacatggggc-3′) antisense (5′-cttcaagcatggagttttccc-3′).
Estimation of the Half-Life of TGF-β Transcripts in Fibroblast.
The half-life of TGF-β mRNA in fibroblasts was determined with DRB according to a technique described previously (13). Fibroblasts grown to confluence in DMEM supplemented with 10% FCS were serum-starved for 24 h and incubated for an additional 12 h with or without 5 ng/ml IL-4. After washing out IL-4, cells were treated for 2, 4, and 8 h with 100 μM DRB. DRB inhibits RNA polymerase II and effectively reduces transcription by 90% or greater in most cells (14). Total RNA was extracted from 2 × 106 cells that were used for Northern blotting as described above. The c-Myc mRNA was used as a control to check the efficacy of DRB treatment, because the c-Myc transcript has a short half-life (13).
Measurement of Collagen Synthesis.
Collagen production was measured by using a modified version of the protocol described by Peterkofsky and Diegelmann (15). Briefly, fibroblasts were grown to confluence in 75-cm2 flasks, detached via trypsinization, and plated at a density of 105 cells per well in 24-well plates. The cells were washed with PBS, and the medium was replaced with proline-free DMEM supplemented with [3H]proline [3μCi (1 Ci = 37 GBq) per well, Amersham Pharmacia]/50 μg/ml ascorbic acid/β-aminopropionitrile and with 5 ng/ml murine IL-4 or 10 ng/ml human recombinant TGF-β1 or 1 μg/ml anti-TGF-β antibody. After 24 h of incubation, the media were collected for determination of collagen synthesis, and the cell numbers were counted. To measure collagen synthesis, 100-μl aliquots of media from labeled fibroblasts were incubated at 37°C for 18 h in either the presence or absence of highly purified bacterial collagenase at a concentration of 200 units per aliquot. The samples then were dialyzed extensively in microdialysis chambers (Pierce) against PBS at 4°C to remove unincorporated [3H]proline and digested collagen. The total counts per sample then were determined by using a liquid scintillation counter. Biosynthetic labeling of collagen was estimated by subtracting the cpm of the collagenase-digested aliquot from the cpm of the aliquot without collagenase added.
Histological Examination.
Skin samples (4 cm2) were removed from the dorsal side immediately below the neck in a manner that minimized stress. Samples were fixed for 16 h in buffered saline containing formalin, cut into 2–3-mm-wide longitudinal strips, dehydrated, embedded in paraffin, and stained with hematoxylin/eosin according to routine histological methods as described (4). The thickness of the skin was determined by measuring it from the top of the granular layer to the junction between the dermis and s.c. fat on hematoxylin/eosin-stained sections.
Inflated lungs were fixed and processed by the same methods described above. The extent of airspace dilation and cellular infiltration in the interstitium were analyzed on hematoxylin/eosin-stained sections as described (4).
Results
Disrupting IL-4 Allows Survival of TSK/TSK Mice.
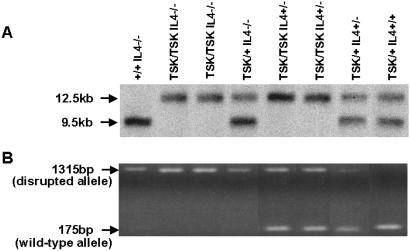
We first analyzed the role of IL-4 in embryonic lethality of TSK in its homozygous form. TSK/+, IL-4+/− F1 mice were crossed to generate F2 mice. Genotyping of F2 mice was carried out by Northern blotting for the TSK mutation and PCR analysis for the disrupted IL-4 allele. The TSK mutation is a duplication of exons 17–40 of the fibrillin-1 gene (16). The wild-type fibrillin-1 gene encodes a transcript of 9.5 kb, whereas the mutant gene encodes an mRNA of 12.5 kb (11). The wild-type IL-4 gene yields a PCR product of 175 bp, whereas the disrupted allele give a product that is 1,315 bp in size. Fig. 1 illustrates typical examples of genotyping for the TSK mutation by Northern blotting (Fig. 1A) and for the wild-type or disrupted IL-4 genes by PCR (Fig. 1B).
Figure 1.
Genotyping mice by Northern blotting and PCR. (A) Autoradiograph of Northern blots hybridized with a fibrillin-1 probe. RNA (20 μg) extracted from lung was electrophoresed, and the blots were hybridized with a 3′-end [32P]fibrillin probe (8,401–8,601 bp). The size of the wild-type fibrillin-1 gene transcript is 9.5 kb, whereas the transcript of the mutated gene is 12.5 kb. (B) PCR for genotyping mice bearing wild-type or disrupted IL-4 genes. Tail-extracted genomic DNA was amplified by using forward and reverse primers as described in Material and Methods. The disrupted allele yields a PCR product of 1,351 bp, whereas the wild-type allele yields a product of 175 bp.
Nine different genotypes are expected among the F2 mice. Table 1 shows the expected and observed frequency of the genotypes among the 308 F2 mice studied. As can be seen, no TSK/TSK, IL-4+/+ mice were identified. This observation is in agreement with previous findings that the homozygous TSK mutation is lethal (1). By contrast, significant numbers of TSK/TSK mice with one or both disrupted IL-4 alleles survived. TSK/TSK, IL-4+/− mice and TSK/TSK, IL-4−/− mice were obtained in frequencies that were 29 and 47%, respectively, of those expected. If the expected number is recalculated taking into account the failure of any TSK/TSK, IL-4+/+ mice to survive, the percentage survival of TSK/TSK mice fell slightly to 27% for IL-4+/− and 43% for IL-4−/− mice.
Table 1.
Expected and observed frequency of genotypes of 308 F2 mice obtained from brother-sister breeding of TSK/+, IL-4+/− F1 mice
| Genotype | Expected
|
Observed
|
FX† | ||
|---|---|---|---|---|---|
| % | no.* | % | no.* | ||
| TSK/TSK IL-4+/+ | 6.3 | 19 | 0 | 0 | 0 |
| TSK/TSK IL-4+/− | 12.5 | 38 | 3.6 | 11 | 0.29 |
| TSK/TSK IL-4−/− | 6.3 | 19 | 2.9 | 9 | 0.47 |
| TSK/+ IL-4+/+ | 12.5 | 38 | 18.5 | 57 | |
| TSK/+ IL-4+/− | 25 | 77 | 28.9 | 89 | |
| TSK/+ IL-4−/− | 12.5 | 38 | 16.5 | 51 | |
| +/+ IL-4+/+ | 6.3 | 19 | 6.8 | 21 | |
| +/+ IL-4+/− | 12.5 | 38 | 14.2 | 44 | |
| +/+ IL-4−/− | 6.3 | 19 | 8.4 | 26 | |
Number of F2 mice genotyped.
FX is calculated as the ratio of the number of mice observed/number of mice expected.
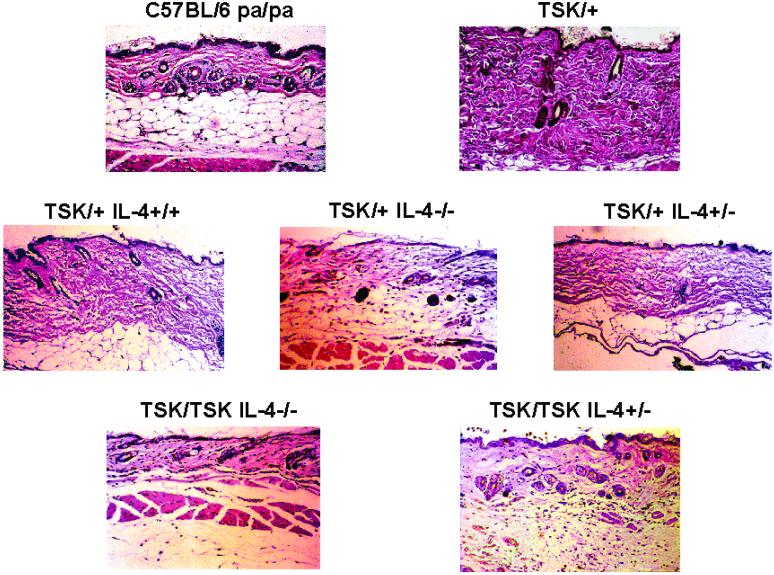
Histological analysis of skin sections shows that in contrast to skin from C57/BL6 pa/pa mice (3-month-old), the skin of age-matched TSK/+, pa/+ mice displays increased fibrosis in the superficial and deep dermis associated with expanded fascia and lamellar architecture of collagenous material. The connective tissue hyperplasia frequently extended to the panniculus carnosus (Fig. 2). Similar abnormalities were also observed in the skin of F2 TSK/+, IL-4+/+ mice. There was a considerable reduction of collagen in both the superficial and deep dermis in 3-month-old F2 TSK/+ and TSK/TSK mice with both disrupted IL-4 alleles. The skin thickness of C57BL/6 pa/pa mice was comparable to that of other mouse strains (17).
Figure 2.
Histopathology of the skin from mice exhibiting various genotypes. Representative examples of skin sections stained with hematoxylin/eosin are shown (magnification ×20).
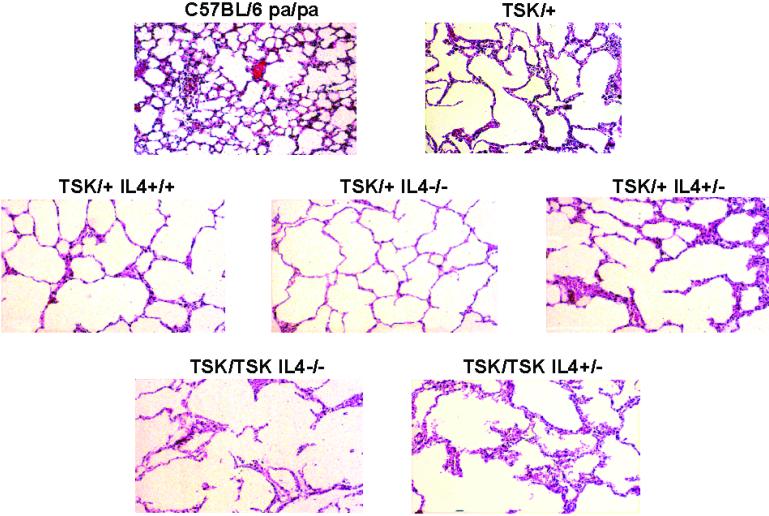
In TSK/+ mice, emphysema is characterized by alveoli that are 3–4 times larger than those in normal mice. The presence of emphysema was determined by measuring the diameter of at least 100 alveoli. Fig. 3 shows that in all TSK/+ and F2 mice exhibiting the TSK mutation, the diameter of alveoli was enlarged significantly compared with C57BL/6 pa/pa mice. Disruption of one or both IL-4 alleles in F2 TSK/+ or TSK/TSK mice did not prevent the development of emphysema (Fig. 3).
Figure 3.
Histopathology of the lung from mice exhibiting various genotypes. Representative examples of lung sections stained with hematoxylin/eosin are shown (magnification ×20).
Table 2 summarizes these observations. There was a significant increase in skin thickness and frequency of lung emphysema in TSK/+ and F2 TSK/+, IL-4+/+ mice compared with C57BL/6 pa/pa mice; by contrast, no increase in skin thickness was observed in TSK/TSK or TSK/+ mice with both disrupted IL-4 alleles or TSK/+, IL-4−/− mice. These results clearly demonstrate that IL-4 is critical not only in the early death of TSK/TSK mice but also in the cutaneous hyperplasia of the TSK syndrome. By contrast, IL-4 is not essential for the development of emphysema in TSK mice.
Table 2.
Skin thickness and frequency of lung emphysema in F2 mice obtained from brother-sister breeding of TSK/+, IL-4+/− F1 mice
| Mice genotype | N | Skin thickness, μm | P* | Emphysema† |
|---|---|---|---|---|
| C57BL/6 pa/pa | 6 | 118.3+/−21.4 | — | 0/5 |
| TSK/+ pa/+ | 13 | 231.5+/−89.0 | 0.008 | 13/13 |
| TSK/TSK IL-4−/− | 5 | 138.5+/−19.2 | 0.128 | 5/5 |
| TSK/TSK IL-4+/− | 5 | 162.4+/−23.0 | 0.09 | 5/5 |
| TSK/+ IL-4−/− | 27 | 142.5+/−42.0 | 0.19 | 21/24 |
| TSK/+ IL-4+/− | 12 | 204.5+/−90.6 | 0.04 | 11/12 |
| TSK/+ IL-4+/+ | 6 | 326.5+/−81.2 | 0.001 | 6/6 |
P value between C57BL/6pa/pa and other mice calculated by Student's t test.
Number of mice exhibiting lung emphysema/total number of mice studied.
IL-4 Induces Expression of TGF-β by Fibroblasts.
Both IL-4 and TGF-β have been shown to enhance the expression of collagen in fibroblasts. IL-4 has been reported to play a role in the differentiation of naive CD4 cells to a TGF-β-producing phenotype and the induction of so-called Th3 cells that secrete TGF-β (18, 19). Furthermore, ΤGF-β synthesis by human eosinophils has been shown to be IL-4-dependent (20), and mice transgenic for the IL-4 homolog, IL-13, have been reported to induce increased macrophage TGF-β1 expression (21). This information raises the possibility that IL-4 may control fibroblast collagen production through induction of TGF-β in these cells.
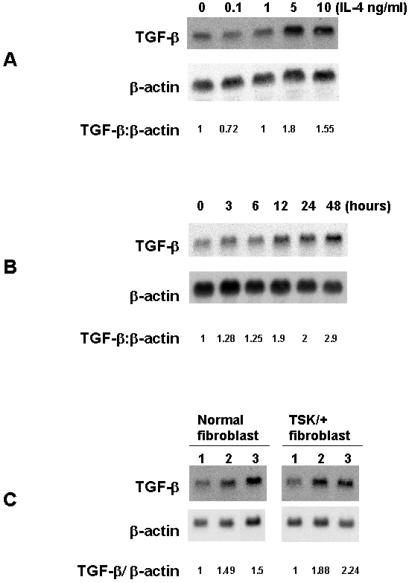
To test this hypothesis, C57BL/6 fibroblasts were treated with 0.1–10 ng/ml IL-4. A significant increase in TGF-β mRNA expression at 48 h, as assessed by Northern blotting, was observed when 5 or 10 ng of IL-4/ml was used (Fig. 4A). The increase in TGF-β mRNA was evident within 12 h of the addition of IL-4 (Fig. 4B). Furthermore, the increase in TGF-β mRNA was detected 48 h after fibroblasts had been treated with IL-4 for 12 h (Fig. 4C). It is unlikely that fibroblasts themselves are the source of the IL-4 that induces TGF-β, because no IL-4 transcripts could be detected by reverse transcription–PCR in fibroblast cultures regardless of whether they were treated with IL-4 (data not shown).
Figure 4.
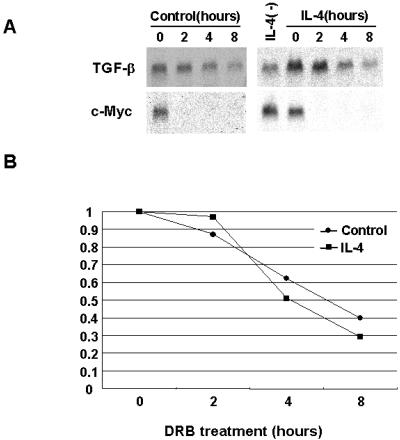
Autoradiograph of Northern blots hybridized with TGF-β and β-actin probes. (A) TGF-β mRNA in fibroblasts treated with various concentrations of murine rIL-4. C57BL/6 pa/pa fibroblasts were serum-starved for 24 h and then incubated for 48 h in medium alone or medium supplemented with IL-4. RNA (20 μg) was electrophoresed and transferred to a nylon membrane, and the blots were hybridized with [32P]TGF-β probe. After stripping, the blots were hybridized with [32P]β- actin probe. The results were expressed as the ΤGF-β/β-actin ratio comparing the fibroblasts incubated with IL-4 to those incubated in medium alone. The intensity of bands was determined by using NIH IMAGE software. This experiment was repeated five times. In each experiment, 10 ng of IL-4 induced a maximal response. (B) The time course of increase in ΤGF-β transcript in C57BL/6 pa/pa fibroblasts incubated with IL-4. Fibroblasts were serum-starved for 24 h and then incubated for various intervals of time with 5 ng/ml IL-4 and processed as described for Fig. 4A. (C) Persistence of TGF-β transcript in C57BL/6 pa/pa and TSK/+ fibroblasts pulsed for 12 h with 5 ng/ml rIL-4. Fibroblasts were serum-starved for 24 h and then incubated for 12 h in DMEM alone (lane 1), for 12 h with 5 ng/ml IL-4 (lane 2), or for 12 h with 5 ng/ml IL-4 and for an additional 48 h without IL-4 (lane 3). Shown is one representative experiment of three.
The persistence of increased TGF-β transcripts after IL-4 stimulation could reflect stabilization of TGF-β mRNA by IL-4. However, the half-life of the TGF-β transcript (4 h) was the same in fibroblasts treated or not with IL-4 (Fig. 5 B and C) as determined by the DRB assay. As a control, we determined the half-life of the c-myc transcript; our result was similar to the reported half-life of 2 h (13). It is noteworthy that in all experiments, a low level of TGF-β transcript was detected in resting fibroblasts. This result may be related to autocrine activity of the TGF-β gene, which has two distinct regions in the promoter that are responsive to autoregulation (22).
Figure 5.
Half-life of TGF-β transcript in fibroblasts exposed to IL-4. (A) C57BL/6 pa/pa fibroblasts were serum-starved for 24 h and incubated for 12 h with or without IL-4. After extensive washing, cells were incubated with 100 μM DRB as described in Materials and Methods. RNA was extracted from 2 × 106 cells at various time points (2–8 h). The presence of RNA transcript was determined as was described for Fig. 4A. (B) Quantitative distribution of the results of Northern blotting assay shown in A.
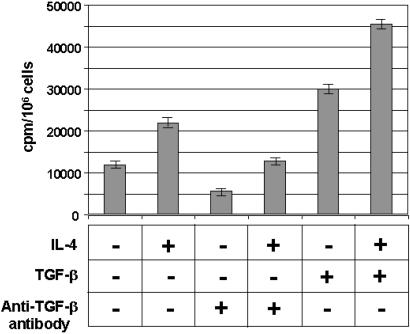
Exposure of fibroblasts to IL-4 increased TGF-β mRNA levels and also enhanced collagen synthesis. This increase was blocked by anti-TGF-β antibodies (Fig. 6). TGF-β also increased collagen synthesis. Interestingly, addition of the two cytokines caused the largest increase in collagen synthesis. This effect was observed when the fibroblasts were exposed to 10 ng of IL-4 and various concentrations of TGF-β ranging from 1 pg to 10 ng (data not shown).
Figure 6.
Synthesis of collagen by normal fibroblast after incubation with rIL-4 and TGF-β. C57BL/6 pa/pa fibroblasts were serum-starved for 24 h and incubated in medium alone (first bar), 5 μg/ml IL-4 (second, fourth, and sixth bars), 1 μg/ml anti-TGF-β antibody (third and fourth bars), or 10 ng/ml TGF-β (fifth and sixth bars). The effect of anti-TGF-β antibody on collagen synthesis was studied by preincubation for 2 h with anti-TGF-β antibody. The bars represent the mean value of triplicate samples ± 1 SD. This experiment is representative of three independent experiments.
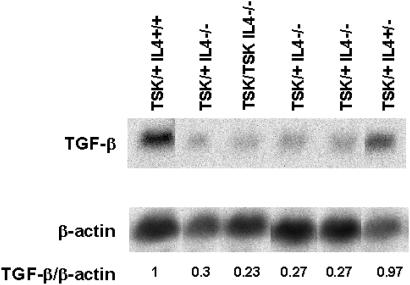
Because the addition of IL-4 induces TGF-β mRNA expression in fibroblasts, we compared the levels of TGF-β mRNA in lungs of TSK/+ or TSK/TSK F2 mice in which the IL-4 gene was intact or disrupted to determine whether the presence of IL-4 controlled TGF-β expression in vivo. TGF-β transcript levels were significantly lower in TSK/TSK or TSK/+ mice with disrupted IL-4 genes than in TSK/+, IL-4+/+ mice (Fig. 7). The lack of alteration of TGF-β message in TSK/+, IL-4+/− mice correlated with the increased skin thickness observed in these mice (204 ± 90 μm in TSK/+, IL-4+/− versus 118 ± 21 μm in C57/BL6 pa/pa mice) as well as with the previously reported increased skin thickness in TSK/+, IL-4Rα+/− mice (4). These results indicate that the expression of TGF-β is modulated by IL-4, and IL-4 and TGF-β are in an epistatic relationship with IL-4 inducing TGF-β and TGF-β inducing collagen biosynthesis in fibroblasts. However, the increase in collagen biosynthesis in response to IL-4 and TGF-β suggests that IL-4 also directly enhances fibrosis.
Figure 7.
Autoradiograph of Northern blots of lung RNA extracted from F2 mice with various genotypes and hybridized with a TGF-β probe. Lung RNA (10 μg) was used for Northern blotting analysis as described for Fig. 4A.
Discussion
It has been demonstrated previously that IL-4 plays a critical role in the cutaneous hyperplasia of TSK mice, because this dermal abnormality was prevented by disruption of the IL-4Rα, IL-4, or STAT6 genes (4–6). Furthermore, repeated injection of a plasmid encoding IL-12 also diminished skin hyperplasia in TSK/+ mice, suggesting that biasing immune responses in adult animals to a Th1 (non-IL-4-producing) polarity inhibits the development of skin pathology in TSK/+ mice (23). The simplest explanation for these results is that IL-4 produced by T cells plays a critical role in the skin manifestations of the scleroderma-like syndrome of TSK mice. By contrast, Siracusa et al. (24) reported that the disruption of the Rag2 gene does not influence the length of stretchable skin in TSK mice. It was proven that the generation of TSK/+, Rag2−/−mice was difficult, because both TSK and Rag2 genes are on chromosome 2, ≈6.5 cM apart. In a similar type of breeding, Kasturi et al. (11) did not obtain viable TSK/+, RAG2−/− F2 mice in the breeding of the TSK/+, RAG2+/− F1 mice. Thus, with one caveat, the weight of evidence seems to favor a role for IL-4 produced postnatally in skin hyperplasia and strongly suggests that CD4+ T cells of the Th2 phenotype are important in this process (7).
Lee et al. (21) reported recently that mice that transgenically overexpress IL-13 have increased lung fibrosis and increased levels of TGF-β1. They further demonstrated that increased TGF-β1 levels were inhibited by treatment with soluble IL-13 receptor, implying that IL-13 mediated the increase in TGF-β1. Treatment with aprotinin, which blocks TGF-β1 activation, blocked the fibrosis observed in IL-13 transgenic mice, suggesting that IL-13 mediated its profibrotic effects through TGF-β1. They noted further that TGF-β1 mRNA was elevated in alveolar macrophages in IL-13 transgenic mice, suggesting that macrophages were the source of the TGF-β1 that mediated lung fibrosis.
Our experiments show that IL-4, which uses the same receptor as IL-13 (25), can act directly on fibroblasts, particularly fibroblasts from TSK/+ and TSK/TSK donors, to induce TGF-β1. Furthermore, the increase in collagen biosynthesis caused by IL-4 could be inhibited by anti-TGF-β. In IL-4 knockout mice, TGF-β1 mRNA was diminished substantially in lungs of TSK/TSK and TSK/+ mice, and the skin fibrosis normally seen in TSK/+ mice was prevented, establishing a pathophysiologic role for IL-4 in fibrosis and strongly suggesting that the IL-4 effect was mediated at least in part through the induction of TGF-β1. Whether in vivo fibrosis is caused by IL-4 acting on fibroblasts to induce TGF-β1 was not directly established. It is possible that that in IL-4−/− mice, IL-13 production was diminished substantially, because IL-13 is generally a product of Th2 cells, and in many circumstances Th2 differentiation depends on the action of IL-4 (26). However, in our prior work (4) we found that IL-4 was more effective than IL-13 in inducing collagen biosynthesis in TSK/+ fibroblasts. It is notable that collagen biosynthesis was maximal in the presence of IL-4 and TGF-β, suggesting that IL-4 also may have a profibrotic activity that is mediated independent of the induction of TGF-β. Why TSK mice respond to IL-4 and TGF-β with cutaneous hyperplasia whereas +/+ mice do not is unclear.
Green et al. (1) reported that embryos homozygous for TSK mutation die on days 7–8 of gestation. The survival of TSK/TSK mice in the absence of IL-4 and before the appearance of conventional lymphocytes is an unexpected observation. Makino et al. (27) have reported that Vα14, CD3ɛ, and Rag1 transcripts could be detected early during embryonic development. Furthermore, Vα14 transcripts containing a paternal polymorphism were detected in embryos from Rag−/− mothers. Because Vα14 is expressed in the form of an invariant Vα14Jα281 transcript by natural killer T (NKT) cells, these results were interpreted to indicate that NKT cells may be present as early as 9.5 days of gestation. Because NKT cells in young mice produce IL-4 immediately after challenge to anti-CD3 (28) or to their cognate ligand (α-Gal-Cer associated with CD1; ref. 29), these cells are a potential source of IL-4 in the embryos. Whether NKT cells contribute to the death of TSK/TSK, IL-4+/+ mice and whether they are what stimulates their IL-4 production remains to be determined.
In addition, Cooke et al. (30) were able to detect IL-4 in the decidua and placenta at 8.5 days of gestational age and in the embryo at day 10.5. All the mothers in our experiments were TSK/+, IL-4+/−. Thus, maternal IL-4 could contribute to the death of TSK/TSK mice but cannot be sufficient on its own, because the elimination of one embryonic IL-4 allele allowed 27% survival, and elimination of both embryonic alleles allowed 47% survival.
The role of IL-4 in embryogenesis could be related to up-regulation of the expression of adhesion molecules affecting cell migration during morphogenesis (31). Thus, a lack of IL-4 may allow the migration pattern to revert to a normal phenotype. Alternatively, IL-4 may influence development indirectly via TGF-β, because TGF-β is an important regulator of cellular migration and proliferation of fibroblasts during morphogenesis (32).
In summary, our results show that the disruption of one or both IL-4 alleles rescues homozygous TSK/TSK mice that normally die by day 9 of gestation. IL-4 seems to mediate fibrosis at least in part by increasing the expression of the TGF-β mRNA in fibroblasts.
Acknowledgments
This work was supported in part by National Institute of Allergy and Infectious Diseases-National Institutes of Health Grant PO1AI24671-13 and a grant from the Scleroderma Foundation.
Abbreviations
- TSK
tight skin
- Th
T helper
- TGF-β
transforming growth factor-β
- DRB
5,6-dichloro-1-β-d-ribofuranosyl-benzimidazole
References
- 1.Green M C, Sweet H O, Bunker L E. Am J Pathol. 1976;82:493–507. [PMC free article] [PubMed] [Google Scholar]
- 2.Bona C, Rothfield N. Curr Opin Immunol. 1994;6:931–937. doi: 10.1016/0952-7915(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 3.DeLustro F A, Mackel A M, Le Roy E C. Cell Immunol. 1983;81:175–179. doi: 10.1016/0008-8749(83)90223-x. [DOI] [PubMed] [Google Scholar]
- 4.McGaha T, Saito S, Phelps R G, Gordon R, Noben-Trauth N, Paul W E, Bona C. J Invest Dermatol. 2001;116:136–143. doi: 10.1046/j.1523-1747.2001.00217.x. [DOI] [PubMed] [Google Scholar]
- 5.Ong C J, Ip S, Teh S J, Wong C, Jirik F R, Grusby M, Teh H S. Cell Immunol. 1999;196:60–68. doi: 10.1006/cimm.1999.1537. [DOI] [PubMed] [Google Scholar]
- 6.Ong C, Wong C, Roberts C R, Teh H S, Jirik F R. Eur J Immunol. 1998;128:2619–2629. doi: 10.1002/(SICI)1521-4141(199809)28:09<2619::AID-IMMU2619>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.Wallace V A, Kondo S, Kono T, Xing Z, Timms E, Furlonger C, Keystone E, Gauldie J, Sander D N, Mak T W, Paige C J. Eur J Immunol. 1994;124:1463–1466. doi: 10.1002/eji.1830240634. [DOI] [PubMed] [Google Scholar]
- 8.Salmon-Ehr V, Serpier H, Nawrocki B, Gillery P H, Clavel C, Kalis B, Biermbaut P, Maquart F X. Arch Dermatol. 1996;132:802–806. [PubMed] [Google Scholar]
- 9.Nedeleman B W, Wigley F M, Stair R W. Arthritis Rheum. 1992;35:479–482. doi: 10.1002/art.1780350111. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa M, Fujimoto M, Kikuki K, Takehara K. J Rheumatol. 1997;24:328–332. [PubMed] [Google Scholar]
- 11.Kasturi K N, Hatakeyama A, Murai C, Gordon R, Phelps R G, Bona C A. J Autoimmun. 1997;10:505–517. doi: 10.1006/jaut.1997.0158. [DOI] [PubMed] [Google Scholar]
- 12.Noben-Trauth H, Kropf P, Muller I. Science. 1996;271:987–990. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- 13.Andreou E R, Prockipap R D. Biochem Biophys. 1998;357:137–146. doi: 10.1006/abbi.1998.0792. [DOI] [PubMed] [Google Scholar]
- 14.Harrold S, Genovese C, Kobrin B, Morrison S L, Milcarek C A. Anal Biochem. 1991;198:19–29. doi: 10.1016/0003-2697(91)90500-s. [DOI] [PubMed] [Google Scholar]
- 15.Peterkofsky B, Diegelmann R. Biochemistry. 1991;10:988–993. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- 16.Siracusa L D, McGrath R, Ma Q, Moskow J J, Manne J, Christner P J, Buchberg A M, Jimenez S A. Genome Res. 1996;6:300–313. doi: 10.1101/gr.6.4.300. [DOI] [PubMed] [Google Scholar]
- 17.Hatakeyama A, Kasturi K, Wolf I M, Phelps R G, Bona C A. Cell Immunol. 1997;167:135–140. doi: 10.1006/cimm.1996.0017. .I. M. [DOI] [PubMed] [Google Scholar]
- 18.Seder R A, Marth T, Sieve M C, Strober W, Letterio J J, Roberts A, Kelsall B. J Immunol. 1998;160:5719–5728. [PubMed] [Google Scholar]
- 19.Inobe J, Slavin A J, Komagata Y, Chen Y, Liu L, Weiner H L. Eur J Immunol. 1998;28:2780–2790. doi: 10.1002/(SICI)1521-4141(199809)28:09<2780::AID-IMMU2780>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 20.Elovic A E, Ohyama H, Sauty A, McBride J, Tsuji T, Nagai M, Weller P F, Wong D T. J Immunol. 1999;160:6121–6127. [PubMed] [Google Scholar]
- 21.Lee C G, Homer R J, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley J M, Gotwals P, Noble P, Chen Q, Senior R M, Elias J A. J Exp Med. 2001;194:809–822. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S J, Jeang K T, Glick A B, Sporn M B, Roberts A B. J Biol Chem. 1989;264:7041–7045. [PubMed] [Google Scholar]
- 23.Tsuji-Yamada J, Nakazawa M, Takahashi K, Iijima K, Hattori S, Okuda K, Minami M, Ikezawa Z, Sasaki T. Biochem Biophys Res Commun. 2001;280:707–712. doi: 10.1006/bbrc.2000.4171. [DOI] [PubMed] [Google Scholar]
- 24.Siracusa L D, McGrath R, Fisher J K, Jimenez S A. Mamm Genome. 1998;9:907–909. doi: 10.1007/s003359900894. [DOI] [PubMed] [Google Scholar]
- 25.Obiri N I, Leland P, Leonard W J, Puri R K. J Biol Chem. 1995;270:8797–8804. doi: 10.1074/jbc.270.15.8797. [DOI] [PubMed] [Google Scholar]
- 26.LeGros G, Ben-Sasson S Z, Seder R, Finkelman F D, Paul W E. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makino Y, Kanno R, Koseki H, Taniguchi M. Proc Natl Acad Sci USA. 1996;93:6516–6520. doi: 10.1073/pnas.93.13.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimoto T, Paul W E. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuda J L, Naidenko O V, Gapin L, Nakayama T, Taniguchi M, Wang C R, Koezuka Y, Kronenberg M. J Exp Med. 2000;192:741–753. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooke J E, Heasman J, Wylie C C. Dev Biol. 1996;174:14–21. doi: 10.1006/dbio.1996.0047. [DOI] [PubMed] [Google Scholar]
- 31.Thornhill M H, Willcoms S M, Mahiouz D L, Lanchbury J S, Kyan-Aung U, Haskard D O. J Immunol. 1991;146:592–598. [PubMed] [Google Scholar]
- 32.Massague J. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]