Abstract
The pheochromocytomas are an important cause of secondary hypertension. Although pheochromocytoma susceptibility may be associated with germline mutations in the tumor-suppressor genes VHL and NF1 and in the proto-oncogene RET, the genetic basis for most cases of nonsyndromic familial pheochromocytoma is unknown. Recently, pheochromocytoma susceptibility has been associated with germline SDHD mutations. Germline SDHD mutations were originally described in hereditary paraganglioma, a dominantly inherited disorder characterized by vascular tumors in the head and the neck, most frequently at the carotid bifurcation. The gene products of two components of succinate dehydrogenase, SDHC and SDHD, anchor the gene products of two other components, SDHA and SDHB, which form the catalytic core, to the inner-mitochondrial membrane. Although mutations in SDHC and in SDHD may cause hereditary paraganglioma, germline SDHA mutations are associated with juvenile encephalopathy, and the phenotypic consequences of SDHB mutations have not been defined. To investigate the genetic causes of pheochromocytoma, we analyzed SDHB and SDHC, in familial and in sporadic cases. Inactivating SDHB mutations were detected in two of the five kindreds with familial pheochromocytoma, two of the three kindreds with pheochromocytoma and paraganglioma susceptibility, and 1 of the 24 cases of sporadic pheochromocytoma. These findings extend the link between mitochondrial dysfunction and tumorigenesis and suggest that germline SDHB mutations are an important cause of pheochromocytoma susceptibility.
Introduction
The pheochromocytomas are catecholamine-producing, chromaffin tumors that arise in the adrenal medulla in 90% of cases; in the remaining 10% of cases, they develop in extra-adrenal sympathetic ganglia and may be referred to as “paraganglioma.” Pheochromocytoma usually presents with hypertension. Approximately 10% of pheochromocytoma is hereditary, and it is a well-recognized feature of von Hippel–Lindau (VHL) disease (MIM 193300), of multiple-endocrine neoplasia type 2 (MIM 164761), and, rarely, of neurofibromatosis type 1 (MIM 162200) (Maher and Eng 2000). Nonsyndromic familial pheochromocytoma also occurs in some cases, and, although a proportion of nonsyndromic familial cases have germline VHL mutations, the molecular basis of most nonsyndromic familial cases is unknown (Crossey et al. 1995; Neumann et al. 1995; Hofstra et al. 1996; Woodward et al. 1997).
The hereditary paragangliomas belong to a group of dominantly inherited disorders characterized by the development of highly vascularized, nonchromaffin tumors arising in parasympathetic ganglia. Paragangliomas usually develop in the head and the neck, most commonly at the bifurcation of the carotid artery (i.e., at the carotid body). Up to 50% of paragangliomas are familial, and three paraganglioma-susceptibility loci are described herein: PGL1 (MIM 168000), which results from SDHD mutations at 11q23; PGL2 (MIM 601650), which maps to 11q13, although the causative gene has not been identified (Mariman et al. 1995); and PGL3 (MIM 605373), which results from SDHC mutations at 1q21 (Baysal et al. 2000; Niemann and Muller 2000). SDHC and SDHD are the two subunits of mitochondrial complex II, or succinate dehydrogenase (SDH), that anchor the other two subunits, SDHA and SDHB, to the inner-mitochondrial membrane. SDHA mutations are associated with juvenile encephalopathy (Bourgeron et al. 1995), and the phenotypic consequences of SDHB mutations have not been defined.
The occurrence of pheochromocytoma in some kindreds with hereditary paragangliomas, as well as the occasional reports of familial and isolated cases of both pheochromocytoma and carotid-body tumors (MIM 115310) (Pritchett 1982; Jensen et al. 1991), have suggested a possible etiological link. Recently, we described germline SDHD mutations in patients presenting with familial pheochromocytoma and in patients presenting with isolated pheochromocytoma (Gimm et al. 2000; Astuti et al. 2001). These findings prompted us to investigate familial and sporadic cases of pheochromocytoma and of paraganglioma, for mutations in the genes encoding the SDHB and the SDHC components of SDH.
Subjects, Material, and Methods
Subjects and Tumor Material
In familial cases, blood was obtained from living affected relatives available for investigation. Diagnoses of familial cases were made according to the following criteria: (a) the occurrence of pheochromocytoma in two first-degree relatives, (b) the occurrence of pheochromocytoma and head and neck paraganglioma in first-degree relatives, and (c) the occurrence of pheochromocytoma in three relatives in a single family. Previous investigations had excluded germline mutations in VHL, in SDHD, and in MEN 2–associated regions of RET in the familial cases (Woodward et al. 1997; Astuti et al. 2001). Twenty-four cases of sporadic pheochromocytoma were analyzed for mutations in SDHB and in SDHC. In each case, tumor tissue and either corresponding peripheral blood or adjacent normal adrenal tissue were obtained at the time of surgery. Genomic DNA was then extracted from tumor tissue and from corresponding normal tissue, according to standard procedures. Ethical approval was obtained from the South Birmingham Ethics Committee and the appropriate institutional review boards, and informed consent was received from each patient.
SDHB and SDHC Mutation Analysis
For SDHC, the primer pairs for exon amplification were as follows: 1F (5′-AAA ACA ACC AGC AAA CCA GC-3′) and 1R (5′-CTC CCA GTC CCA CTG AAG TC-3′), 2F (5′-TAC TTT TAA TCT ATC CCT TCA C-3′) and 2R (5′-TCT CCA GAC TTA GAA ACT TA-3′), 3F (5′-TTA TGC AAA ATA TTA AAC CAA GTT-3′) and 3R (5′-CTT ACC TGT AGA TAG TAA TGT GGG-3′), 4F (5′-TTA AAA TTG TCT TTG TGT GTT TCT-3′) and 4R (5′-AAG AGA CTT ACT GTT CCC TCT AAA-3′), 5F (5′-GGG GTC CCA GTT TTA TGT ATC A-3′) and 5R (5′-CCT TCA CAG AGA AAA TGT GCA A-3′), and 6F (5′- TGT TAA TGT CCT ATT TAC TGA A-3′) and 6R (5′-TAA ACA AAT AAG GAG AAC TTT T-3′). Primers were designed on the basis of the genomic sequences. The amplicon sizes for SDHC exons 1, 2, 3, 4, 5, and 6 are 209, 178, 166, 215, 278, and 263 bp, respectively (GenBank accession numbers AF039589, AF039590, AF039591, AF039592, AF039593, and AF039594, respectively).
For SDHB, the primer pairs for exon amplification were as follows: 1F (5′-GCC GCT ACT GCG CTA TTG-3′) and 1R (5′-GCT TTC CTG ACT TTT CCC-3′), 2F (5′-TTT TTC CTT TTT GTG AAC TTT-3′) and 2R (5′-AAG CAT GTC CCT AAA TCA AA-3′ ), 3F (5′-GAA CTT TAC ATA AAT ACC ACT GGA-3′) and 3R (5′-CTA TCA GCT TTG GCC AGC-3′), 4F (5′-ATG GGT GAG GTG TGT TAA TG-3′) and 4R (5′-TGC AAA TAA AAA CAA AAC CA-3′), 5F (5′-TGA TGA TGG AAT CTG ATC CT-3′) and 5R (5′-CAG ATT GAA ACA ATA AAT AGG GA-3′), 6F (5′-CCT CTC TTT TCT CCC CAT AC-3′) and 6R (5′-CAG CAA TCT ATT GTC CTC TTG-3′), 7F (5′-AGC TAA TCA TCC CTG GTT TT-3′) and 7R (5′-TTG TGA GCA CAT GCT ACT TC-3′), and 8F (5′-GTG GGT TTT CCC TTT CAG TT-3′) and 8R (5′-CGG CAA GTA AAG GAA CAG GT-3′). Primers were designed on the basis of the genomic sequences. The amplicon sizes for SDHB exons 1, 2, 3, 4, 5, 6, 7, and 8 are 320, 226, 200, 210, 175, 206, 216, and 319 bp, respectively (GenBank accession numbers U17296, U17880, U17881, U17882, U17883, U17884, U17885, and U17886, respectively; the GenBank accession number for SDHB cDNA is U17248).
By use of HotStar Taq (Qiagen), 50–100 ng of genomic DNA was amplified in Omn-E thermal cyclers (Hybaid). Amplification conditions were as follows: initial denaturation at 95°C for 10 min; then 35 cycles of 30 s of denaturation at 95°C, 30 s of annealing at 55–60°C, and 30 s of extension at 72°C; and a final extension at 72°C for 5 min.
SSCP analysis was performed as described elsewhere (Crossey et al. 1995). Aberrant amplicons were purified using the QiaQuick gel extraction kit (Qiagen) before being analyzed on the ABI377 DNA sequencer (Applied Biosystems). For the 24 sporadic cases, both tumor and blood DNA was analyzed by SSCP.
Haplotype and Loss of Heterozygosity (LOH) Analysis
The two polymorphic markers used for haplotype and LOH analysis, D1S407 and D1S2647, flank SDHB (D1S407 is ∼2.7 Mb telomeric, and D1S2647 is ∼1.8 Mb centromeric). Both markers were amplified using standard PCR conditions. Amplicons were separated on 6% polyacrylamide denaturing gels, were visualized by silver staining, and were exposed on an automatic-processor–compatible film (Promega). LOH was determined by comparing the intensities of the alleles in heterozygous samples of matched tumor and normal DNA. A tumor was considered to have LOH in one of the alleles if the ratio of the signal intensity in the tumor samples differed at least twice as much as the signal intensity in the corresponding normal sample. LOH analysis was not performed for familial cases, because suitable material was not available.
Results
Overall, we found four germline SDHB mutations, in eight families segregating pheochromocytoma with or without head and neck paraganglioma, and one occult germline SDHB mutation, in 24 unrelated patients with isolated pheochromocytoma. No pathogenic mutations were identified in SDHC.
Familial Pheochromocytoma and Paraganglioma
We analyzed eight probands from kindreds with either familial pheochromocytoma only (n=5) or familial pheochromocytoma with head and neck paraganglioma (n=3). SSCP analysis of six exons constituting the coding sequence of SDHC revealed only intronic single-nucleotide polymorphisms that were also detected in controls. Thus, there was no evidence of germline SDHC mutations in the kindreds with familial pheochromocytoma and paraganglioma.
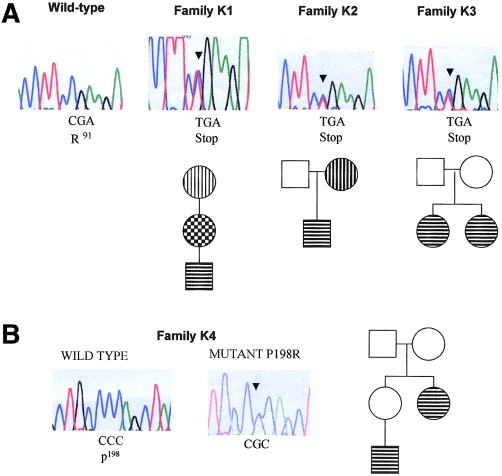
For SDHB mutations, we performed SSCP analysis of the eight exons (281 amino acids) comprising the coding sequence. First, we detected a C→T transition at nucleotide 402 in exon 3, in a Swedish three-generation family, K1, with both extra-adrenal pheochromocytoma and cervical paraganglioma, described elsewhere (Sköldberg et al. 1998) (see figs. 1A and 2). The 402C→T mutation changes an arginine to a termination codon (R91X) and was predicted to result in a truncated SDHB protein lacking the C-terminal 191 amino acids; the R91X mutation was not detected in 200 control chromosomes. The R91X (402C→T) mutation was also detected in a British family, K2, in which the proband presented with extra-adrenal pheochromocytoma at the age of 10 years and in which the proband's mother had a cervical paraganglioma (i.e., a glomus jugiulare tumor) removed at the age of 45 years; there was no other relevant family history (see fig. 1A). An identical R91X (402C→T) mutation was identified in another family, K3 (see fig. 1A), in which two siblings developed early-onset pheochromocytoma (i.e., onset at age <30 years).
Figure 1.
Electropherograms, showing germline SDHB mutations (arrowheads), and simplified pedigrees for families K1–K3 (A) and family K4 (B). The affected codon and the amino acids are depicted below the electropherograms. In pedigrees, symbols with horizontal bars indicate pheochromocytoma (intra- or extra-adrenal), symbols with vertical bars indicate cervical paraganglioma, and symbols with checkered pattern indicate both pheochromocytoma and paraganglioma.
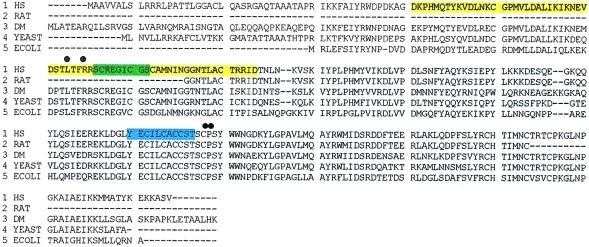
Figure 2.
Locations of germline mutations and missense substitution, detected in the highly conserved SDHB coding sequence (HS = Homo sapiens; DM = Drosophila melanogaster). Multiple-sequence alignment was performed by the ClustalW program, available from BCM Search Launcher. The mutations are shown with respect to the functional domains of the protein, as predicted by the InterPro program. The black dots represent the four germline mutations described in the text. The yellow screen indicates the predicted ferredoxin domain (54–119 amino acids; GenBank accession number NP_002991), the green screen indicates the 2Fe-2S ferredoxin domain (93–101 amino acids), and the blue screen indicates the 4Fe-4S ferredoxin iron-sulfur–binding domain (186–197 amino acids).
Because the 402C→T mutation occurred at a hypermutable CpG dinucleotide in three apparently unrelated kindreds, we investigated the possibility of a founder mutation, by haplotype analysis. Allelotyping at microsatellite markers D1S407 and D1S2647, flanking SDHB, did not show any shared alleles at D1S2647 (K1 included two alleles, K2 included three or five alleles, and K3 included four alleles) and did not show any common allele at D1S407 (K1 included one or three alleles, K2 included two or three alleles, and K3 included two alleles), suggesting multiple de novo origins for the R91X mutation.
We also identified a germline SDHB mutation in another kindred, K4, a large family containing three individuals with familial extra-adrenal pheochromocytoma and without evidence of cervical paragangliomas (see figs. 1B and 2). An exon 6 SSCP bandshift was detected in two affected members of K4. Sequence analysis demonstrated a C→G transversion at nucleotide 724, causing a proline-to-arginine missense mutation (P198R) (fig. 1B); this change was not detected in 200 control chromosomes. This proline is conserved throughout all living species analyzed—from human to rat, Drosophila, yeast, and Escherichia coli (fig. 2).
Analysis of Sporadic Pheochromocytoma
Analysis of SDHB in matched blood and tumor DNA from 24 cases of sporadic pheochromocytoma led to the identification of two heterozygous sequence variants. In case 1 (S1), an exon 6 frameshift deletion (725delC) was present in blood and tumor DNA of a 55-year-old female with a single adrenal pheochromocytoma and without other significant personal or family history; this truncating mutation was not observed in 200 control chromosomes. Analysis of tumor DNA in S1 did not demonstrate allele loss at either D1S407 or D1S2647. The second sequence variant (394T→C, which causes an L88S missense substitution) was detected in 1 of 200 control chromosomes and is therefore of uncertain significance. No somatic or germline SDHC mutations were detected in the 24 cases of sporadic pheochromocytoma analyzed.
Discussion
We have identified germline SDHB mutations in (a) individuals with familial pheochromocytoma, (b) individuals with familial pheochromocytoma and paraganglioma of the head and the neck, and (c) apparently sporadic cases of pheochromocytoma. The phenotypic consequences of germline mutations in each of the four components of SDH have now been defined. It is interesting that, whereas heterozygous mutations in SDHB (i.e., the gene for the iron-sulfur–protein subunit) and in both SDHC and SDHD (i.e., the genes for the integral membrane–protein subunits) are associated with neoplasia (Baysal et al. 2000; Niemann and Muller 2000; present study), autosomal recessive homozygous SDHA (i.e., the gene for the flavoprotein subunit) mutations are associated with Leigh syndrome (MIM 600857) (Bourgeron et al. 1995; Parfait et al. 2000).
The discovery of SDHB mutations in paraganglioma and in pheochromocytoma further strengthens the link between tumorigenesis and mitochondrial dysfunction. Nevertheless, the precise mechanism by which mutations in SDHB, in SDHC, and in SDHD predispose to tumors derived from the autonomic nervous system is uncertain. Germline mutations in the tumor-suppressor gene VHL may predispose patients with mitochondrial dysfunction to a variety of hypervascular tumors, including pheochromocytoma (Kaelin and Maher 1998). The product of the VHL gene has a critical role in the regulation of hypoxia-responsive genes. Thus, VHL inactivation, both in human cancers and in mouse models, results in up-regulation of angiogenic growth factors, such as vascular endothelial growth factor, secondary to a defect in ubiquitylation of the α-subunits of the HIF-1 and the HIF-2 transcription factors (Maxwell et al. 1999; Cockman et al. 2000; Ohh et al. 2000). However, although head and neck paragangliomas are hypervascular, and although mitochondria have been implicated in both oxygen sensing and regulation of HIF-1 expression, it is not certain that this can explain the tumor susceptibility in patients with SDHB and SDHC mutations. Interestingly, germline VHL mutations that predispose patients to pheochromocytoma do not impair HIF-1α ubiquitylation (Clifford et al. 2001; Hoffman et al. 2001). Another possible explanation for the association between SDH-subunit mutations and neoplasia is the role of mitochondria in apoptosis—that is, its role in the failure of apoptosis in autonomic-nervous-system progenitor cells, which may result in the development of paragangliomas and pheochromocytoma (Cavalli and Liang 1998; Green and Reed 1998).
If failure of apoptosis in autonomic-nervous-system progenitor cells is a relevant mechanism of SDHB-, SDHC-, and SDHD-associated tumorigenesis, it might be hypothesized that germline mutations in SDHB, in SDHC, and in SDHD would be more frequent than somatic mutations. Interestingly, in 62 cases of pheochromocytoma, we identified only one somatic SDHD mutation (Gimm et al. 2000; Astuti et al. 2001; Aguiar et al., in press), and we did not identify any somatic SDHB mutations. However, although somatic mutations in SDHB and in SDHD are infrequent in cases of pheochromocytoma, SDHD allele loss is frequent; epigenetic inactivation by promoter methylation has not been investigated. Similarly, SDHB maps to 1p36, a region of frequent allele loss in pheochromocytoma, neuroblastoma, and other tumors, suggesting that further analysis of SDHB in these tumor types is needed (Benn et al. 2000; Nomoto et al. 2000).
Familial paragangliomas caused by SDHD mutations demonstrate genomic-imprinting effects, and, consequently, a disease phenotype is manifested only after paternal transmission. However, examination of the phenotypic expression of (a) germline SDHB mutations, reported herein, and (b) SDHC mutations, reported by Niemann and Muller (2000), does not reveal parent-of-origin effects, which would be clinical evidence for genomic imprinting of these genes. To date, no genotype-phenotype correlations that would explain the variable phenotype of germline mutations in SDHB and in SDHD are apparent, and genetic or environmental modifiers may be implicated. We have detected occult germline mutations in SDHB and in SDHD, in patients with apparently isolated pheochromocytoma; ∼8% of isolated cases of pheochromocytoma that have been tested have had unsuspected germline mutations in one of these two genes, although frequency estimates are based on small numbers. These findings have important implications for the management of patients with pheochromocytoma (because of the risk of further intra- and extra-adrenal pheochromocytomas and cervical paragangliomas, in susceptible individuals) and suggest that routine genetic screening may be warranted.
Acknowledgments
We thank the British Heart Foundation and the National Cancer Institute (grant P30CA16058) for financial support, and we are grateful to the families and to Prof. Olle Kämpe and other clinicians who assisted in this study.
Electronic-Database Information
- BCM Search Launcher, Baylor College of Medicine HGSC, http://searchlauncher.bcm.tmc.edu:9331/multi-align/Options/clustalw.html (for multiple-sequence alignment)
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for ferredoxin domain [54–119 amino acids] [accession number NP_002991], SDHB cDNA [accession number U17248] and exons 1, 2, 3, 4, 5, 6, 7, and 8 [accession numbers U17296, U17880, U17881, U17882, U17883, U17884, U17885, and U17886, respectively], and SDHC exons 1, 2, 3, 4, 5, and 6 [accession numbers AF039589, AF039590, AF039591, AF039592, AF039593, and AF039594, respectively])
- InterPro, http://www.ebi.ac.uk/interpro/scan.html (for protein domains)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for carotid-body tumors [MIM 115310], multiple-endocrine neoplasia type 2 [MIM 164761], neurofibromatosis type 1 [MIM 162200], PGL1 [MIM 168000], PGL2 [MIM 601650], PGL3 [MIM 605373], and VHL disease [MIM 193300])
References
- Aguiar RCT, Cox G, Pomeroy S, Dahia PLM. Analysis of the SDHD gene, the susceptibility gene for familial paraganglioma (PGL1), in pheochromocytomas. J Clin Endocrinol Metab (in press) [DOI] [PubMed] [Google Scholar]
- Astuti D, Douglas F, Ball S, Lennard L, Aliaganis I, Woodward ER, Evans DGR, Latif F, Maher ER (2001) Germline SDHD mutation in familial phaeochromocytoma. Lancet 357:1181–1182 [DOI] [PubMed] [Google Scholar]
- Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, Richard CW III, Cornelisse CJ, Devilee P, Devlin B (2000) Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287:848–851 [DOI] [PubMed] [Google Scholar]
- Benn DE, Dwight T, Richardson AL, Delbridge L, Bambach CP, Stowasser M, Gordon RD, Marsh DJ, Robinson BG (2000) Sporadic and familial pheochromocytomas are associated with loss of at least two discrete intervals on chromosome 1p. Cancer Res 60:7048–7051 [PubMed] [Google Scholar]
- Bourgeron T, Rustin P, Chretien D, Birch-Machin M, Bourgeois M, Viegas-Pequignot E, Munnich A, Rotig A (1995) Mutation of a nuclear succinate dehydrogenase gene results in mitochondrial respiratory chain deficiency. Nat Genet 11:144–149 [DOI] [PubMed] [Google Scholar]
- Cavalli LR, Liang BC (1998) Mutagenesis, tumorigenicity, and apoptosis: are the mitochondria involved? Mutat Res 398:19–26 [DOI] [PubMed] [Google Scholar]
- Clifford SC, Cockman ME, Smallwood AC, Mole DR, Woodward ER, Maxwell PH, Ratcliffe PJ, Maher ER (2001) Contrasting effects on HIF-1α regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel-Lindau disease. Hum Mol Genet 10:1029–1038 [DOI] [PubMed] [Google Scholar]
- Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH (2000) Hypoxia inducible factor-α binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem 275:25733–25741 [DOI] [PubMed] [Google Scholar]
- Crossey PA, Eng C, Ginalska-Malinowska M, Lennard TWJ, Wheeler DC, Ponder BAJ, Maher ER (1995) Molecular genetic diagnosis of von Hippel-Lindau disease in familial phaeochromocytoma. J Med Genet 32:885–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimm O, Armanios M, Dziema H, Neumann HP, Eng C (2000) Somatic and occult germ-line mutations in SDHD, a mitochondrial complex II gene, in nonfamilial pheochromocytoma. Cancer Res 60:6822–6825 [PubMed] [Google Scholar]
- Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312 [DOI] [PubMed] [Google Scholar]
- Hoffman MA, Ohh M, Yang H, Klco JM, Ivan M, Kaelin WG Jr (2001) von Hippel-Lindau protein mutants linked to type 2C VHL disease preserve the ability to downregulate HIF. Hum Mol Genet 10:1019–1027 [DOI] [PubMed] [Google Scholar]
- Hofstra RM, Stelwagen T, Stulp RP, de Jong D, Hulsbeek M, Kamsteeg EJ, van den Berg A, Landsvater RM, Vermey A, Molenaar WM, Lips CJ, Buys CH (1996) Extensive mutation scanning of RET in sporadic medullary thyroid carcinoma and of RET and VHL in sporadic pheochromocytoma reveals involvement of these genes in only a minority of cases. J Clin Endocrinol Metab 81:2881–2884 [DOI] [PubMed] [Google Scholar]
- Jensen JC, Choyke PL, Rosenfeld M, Pass HI, Keiser H, White B, Travis W, Linehan WM (1991) A report of familial carotid body tumors and multiple extra-adrenal pheochromocytomas. J Urol 145:1040–1042 [DOI] [PubMed] [Google Scholar]
- Kaelin WG, Maher ER (1998) The VHL tumour suppressor gene paradigm. Trends Genet 14:423–425 [DOI] [PubMed] [Google Scholar]
- Maher ER, Eng C (2000) Genetics of phaeochromocytoma. In: Thakker R (ed) Genetics of endocrine and metabolic disorders. Chapman & Hall, New York [Google Scholar]
- Mariman EC, van Beersum SE, Cremers CW, Struycken PM, Ropers HH (1995) Fine mapping of a putatively imprinted gene for familial non-chromaffin paragangliomas to chromosome 11q13.1: evidence for genetic heterogeneity. Hum Genet 95:56–62 [DOI] [PubMed] [Google Scholar]
- Maxwell P, Wiesener M, Chang G-W, Clifford SC, Vaux E, Cockman M, Wykoff C, Pugh C, Maher ER, Ratcliffe PJ (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–275 [DOI] [PubMed] [Google Scholar]
- Neumann HPH, Eng C, Mulligan L, Glavac D, Ponder BAJ, Crossey PA, Maher ER, Brauch H (1995) Consequences of direct genetic testing for germline mutations in the clinical management of families with multiple endocrine neoplasia type 2. JAMA 274:1149–1151 [PubMed] [Google Scholar]
- Niemann S, Muller U (2000) Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet 26:268–270 [DOI] [PubMed] [Google Scholar]
- Nomoto S, Haruki N, Tatematsu Y, Konishi H, Mitsudomi T, Takahashi T, Takahashi T (2000) Frequent allelic imbalance suggests involvement of a tumor suppressor gene at 1p36 in the pathogenesis of human lung cancers. Genes Chromosomes Cancer 28:342–346 [DOI] [PubMed] [Google Scholar]
- Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG (2000) Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol 2:423–427 [DOI] [PubMed] [Google Scholar]
- Parfait B, Chretien D, Rotig A, Marsac C, Munnich A, Rustin, P (2000) Compound heterozygous mutations in the flavoprotein gene of the respiratory chain complex II in a patient with Leigh syndrome. Hum Genet 106:236–243 [DOI] [PubMed] [Google Scholar]
- Pritchett JW (1982) Familial concurrence of carotid body tumor and pheochromocytoma. Cancer 49:2578–2579 [DOI] [PubMed] [Google Scholar]
- Sköldberg F, Grimelius L, Woodward ER, Rorsman F, Van Schothorst EW, Winqvist O, Karlsson FA, Akerstrom G, Kampe O, Husebye ES (1998) A family with hereditary extra-adrenal paragangliomas without evidence for mutations in the von Hippel-Lindau disease or RET genes. Clin Endocrinol (Oxf) 48:11–16 [DOI] [PubMed] [Google Scholar]
- Woodward ER, Eng C, McMahon R, Voutilainen R, Affara NA, Ponder BAJ, Maher ER (1997) Genetic predisposition to phaeochromocytoma: analysis of candidate genes GDNF, RET, and VHL. Hum Mol Genet 6:1051–1056 [DOI] [PubMed] [Google Scholar]