Abstract
The gene for cystathionine β-synthase (CBS) is located on chromosome 21 and is overexpressed in children with Down syndrome (DS), or trisomy 21. The dual purpose of the present study was to evaluate the impact of overexpression of the CBS gene on homocysteine metabolism in children with DS and to determine whether the supplementation of trisomy 21 lymphoblasts in vitro with selected nutrients would shift the genetically induced metabolic imbalance. Plasma samples were obtained from 42 children with karyotypically confirmed full trisomy 21 and from 36 normal siblings (mean age 7.4 years). Metabolites involved in homocysteine metabolism were measured and compared to those of normal siblings used as controls. Lymphocyte DNA methylation status was determined as a functional endpoint. The results indicated that plasma levels of homocysteine, methionine, S-adenosylhomocysteine, and S-adenosylmethionine were all significantly decreased in children with DS and that their lymphocyte DNA was hypermethylated relative to that in normal siblings. Plasma levels of cystathionine and cysteine were significantly increased, consistent with an increase in CBS activity. Plasma glutathione levels were significantly reduced in the children with DS and may reflect an increase in oxidative stress due to the overexpression of the superoxide dismutase gene, also located on chromosome 21. The addition of methionine, folinic acid, methyl-B12, thymidine, or dimethylglycine to the cultured trisomy 21 lymphoblastoid cells improved the metabolic profile in vitro. The increased activity of CBS in children with DS significantly alters homocysteine metabolism such that the folate-dependent resynthesis of methionine is compromised. The decreased availability of homocysteine promotes the well-established “folate trap,” creating a functional folate deficiency that may contribute to the metabolic pathology of this complex genetic disorder.
Introduction
Down syndrome (DS [MIM 190685]), or trisomy 21, is a complex metabolic and genetic disorder that stems from the failure of chromosome 21 to segregate normally during meiosis (Epstein 1995). It is the first clinically defined syndrome shown to be chromosomal in origin and has been the prototype for clinical, cytogenetic, and molecular investigation into mechanisms and management of human aneuploid conditions. The origin of the extra chromosome 21 has been shown to be maternal in ∼93% of cases, with ∼7% of cases due to paternal nondisjunction and a very small proportion of cases due to postzygotic mitotic nondisjunction (mosaics) (Hassold and Sherman 2000). DS is the most common genetic cause of human mental retardation and occurs with a prevalence of ∼1/700–800 live births (Krivchenia et al. 1993). In addition to mental deficiency, 40%–50% of children with DS have congenital heart defects (primarily endocardial cushion defects), ∼5% have gastrointestinal anomalies, and there is a 15–20-fold–increased risk of transient myelodysplasia and childhood leukemia (Korenberg et al. 1994; Torfs and Christianson 1998). In addition, immunologic disorders, such as celiac disease, thyroid dysfunction, and diabetes, are prevalent in children with DS (Jannson and Johansson 1995). The excessive synthesis of multiple gene products derived from overexpression of the genes present on chromosome 21 is thought to underlie both the dysmorphic features and the pathogenesis of the neurological, immunologic, endocrine, and biochemical abnormalities that are characteristic of DS. The successful management of the clinical problems and unique pharmacological sensitivities of these children is a major medical challenge and depends on an understanding of the unique metabolic imbalance induced by overexpression of the constitutively expressed genes on chromosome 21.
The sequence and gene catalog for chromosome 21 have recently been published, with 97.7% coverage of 21q (Hattori et al. 2000). This analysis has revealed 127 known genes, 98 predicted genes, and 59 pseudogenes. Although there has been early speculation that the phenotypic abnormalities in individuals with DS were caused by a 50% overexpression of all genes on chromosome 21, recent evidence suggests that this view is too simplistic and that certain tissues and gene products are more sensitive than others to gene dosage effects (Greber-Platzer et al. 1999; Tassone et al. 1999; Hattori et al. 2000). The presence of tissue-specific epigenetic and biochemical adaptive responses to overexpressed genes greatly complicates the interpretation and impact of gene overdosage in trisomic individuals. DNA sequence analysis is an essential first step toward identification of the trisomic genes (Yu et al. 1997; Hattori et al. 2000), and the subsequent measurement of mRNA transcript level provides an indication of the extent of gene overexpression (Brodsky et al. 1997; Tassone et al. 1999). However, the elucidation of tissue-specific biochemical and metabolic imbalance is likely to be more useful in the clinical management of children with DS (Lejeune 1990; Peeters et al. 1993). Although the perception of DS as a metabolic disease is not yet prevalent, the overexpression of genes coding for specific enzymes translates directly into biochemical aberrations that affect multiple interacting metabolic pathways and that culminate in cellular dysfunction and contribute to the unique pathogenesis of DS.
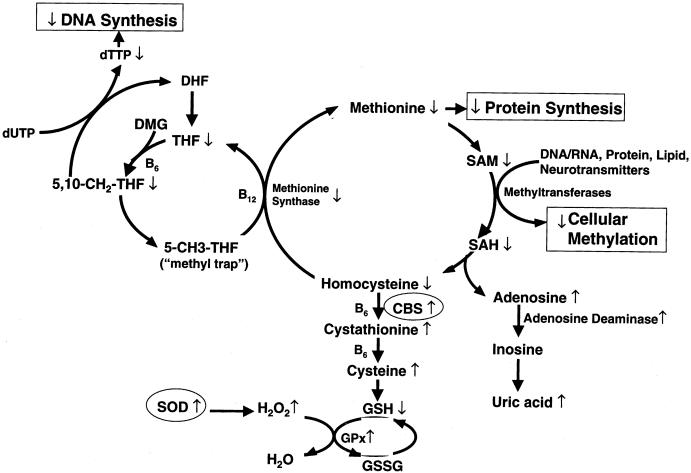
In the present study, we focused on a single gene on chromosome 21 coding for the enzyme cystathionine β-synthase (CBS) and on its indirect impact on one-carbon metabolism, in children with DS. We hypothesized that the plasma profile of the metabolites in the methionine/homocysteine pathway in children with DS would be consistent with a functional folate deficiency secondary to overexpression of the CBS gene. The specific metabolites and metabolic pathways affected by CBS are summarized in figure 1. A 157% increase in CBS enzyme activity has been previously documented in individuals with DS and has been associated with reduced levels of homocysteine (Chadefaux et al. 1985, 1988). The one-way CBS reaction condenses homocysteine with serine, to effectively remove homocysteine from the methionine cycle and to commit it to the transsulfuration pathway for cysteine and glutathione synthesis (see fig. 1). An increase in the transsulfuration pathway via CBS overexpression indirectly deprives the methionine synthase reaction of one of its precursors, homocysteine, while, at the same time, it promotes the accumulation of its other precursor, 5-methyltetrahydrofolate (5-MTHF) (Ueland et al. 1990). The decrease in the methionine synthase activity thus creates the well-established “methyl trap,” as a consequence of the one-way kinetics of 5-MTHF synthesis (fig. 1). More important, a decrease in methionine synthase activity reduces the conversion of 5-MTHF to tetrahydrofolate (THF), the metabolically active form of folate, required for de novo synthesis of nucleotides for RNA and DNA synthesis. Because of the methyl trap, an intracellular functional folate deficiency can exist in the presence of normal, or even elevated, serum folate and B12 levels.
Figure 1.
Overview of interactive and interdependent reactions involved in cellular one-carbon metabolism, with emphasis on the two major metabolic functions of these pathways: normal DNA synthesis/repair and normal cellular methylation reactions. These two major functions intersect at the folate/B12–dependent methionine synthase reaction, which regenerates methionine from homocysteine and, at the same time, generates metabolically active THF for DNA/RNA nucleotide synthesis. Two genes (CBS and SOD) on chromosome 21 that are overexpressed in individuals with DS are shown in circles. Arrows indicate direct and indirect alterations in metabolites, induced by CBS overexpression in individuals with DS.
To define the specific imbalances induced by CBS overexpression, plasma levels of relevant metabolites and lymphocyte DNA methylation status were measured in children with DS and were compared to those from normal siblings. The potential to correct the observed metabolic imbalance via the in vitro addition of selected nutrients to cultured human trisomy 21 lymphoblastoid cells was also examined.
Subjects, Material, and Methods
Subjects and Blood Collection
Participants were 42 children with karyotypically confirmed full trisomy 21, as cases, and 36 normal siblings, as controls. The mean ± SD age of the children was 7.4±4.2 years. Exclusion criteria included antifolate medications, leukemia, and recent surgery. Written, informed consent, approved by the Research Involving Human Subjects Committee of the Food and Drug Administration and by the University of Arkansas for Medical Sciences Human Research Advisory Committee, was obtained from the parents of all participants. A questionnaire was completed by each mother to indicate whether, at the time of blood draw, the child was taking either an over-the-counter vitamin pill or a commercially available nutritional formula. Of the 42 children with DS, 27 were taking a nutritional supplement, and 15 were not on any form of nutritional supplement; thus, the study population was nutritionally heterogeneous. Fasting blood samples were collected into EDTA Vacutainer tubes, immediately chilled on ice, and centrifuged at 400 g for 15 min at 4°C. Aliquots of the plasma layer were transferred into cryostat tubes and were stored at −80°C until analysis. Aliquots were thawed for determination of plasma methionine, S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), adenosine, homocysteine, cystathionine, cysteine, and glutathione, by high-performance liquid chromatography (HPLC).
Sample Preparation for Measurement of Plasma Methionine, SAM, SAH, Adenosine, Homocysteine, Cysteine, Cystathionine, and Glutathione
For determination of total homocysteine (tHcy), methionine, cystathionine, cysteine, and total glutathione, 50 μl of freshly prepared 1.43-M sodium borohydride solution containing 1.5 μM EDTA, 66 mM NaOH, and 10 μl of n-amyl alcohol were added to 200 μl of plasma to reduce all sulfhydryl bonds. To precipitate proteins, 250 μl of ice-cold 10% metaphosphoric acid was added, and the sample was incubated on ice for 10 min. After centrifugation at 18,000 g for 15 min at 4°C, the supernatant was filtered through a 0.2-μm filter (PGC Scientific), and a 20-μl aliquot was injected into the HPLC system. For determination of SAM and SAH, 40 μl of 40% trichloroacetic acid were added to 200 μl of plasma to precipitate protein, the solution was mixed well and incubated on ice for 30 min. After centrifugation for 15 min at 18,000 g at 4°C, supernatants containing SAM, SAH, and adenosine were passed through a 0.2-μm filter, and a 20-μl aliquot was injected into the HPLC system.
HPLC with Coulometric Electrochemical Detection
The methodological details for metabolite elution and electrochemical detection have been described elsewhere (Melnyk et al. 1999, 2000). The analyses were accomplished by HPLC with a Shimadzu solvent-delivery system (model 580; ESA) and a reverse-phase C18 column (5 μm, 4.6×150 mm; MCM) obtained from ESA. A 20-μl aliquot of plasma extract was directly injected onto the column by a Beckman autosampler (model 507E). All plasma metabolites were quantified by a Coulochem II electrochemical detector (model 5200A; ESA) equipped with a dual analytical cell (model 5010) and a guard cell (model 5020). The concentrations of plasma metabolites were calculated from peak areas and standard calibration curves, by HPLC software.
Cytosine-Extension Assay to Assess Lymphocyte Global DNA Methylation
Lymphocytes were isolated in a random subset of children by Histopaque-1077 reagent (Sigma Diagnostics). Genomic DNA was extracted via standard phenol/chloroform procedures (Ausebel et al. 1989, pp. 2.3.1.–2.3.3). In brief, the cytosine-extension assay for global DNA methylation measures the extent of radiolabel incorporation into DNA, after treatment with a methylation-sensitive endonuclease that leaves a single guanine overhang at unmethylated CpG sites. Details of the cytosine-extension assay for DNA methylation have been described elsewhere (Pogribny et al. 1999). The [3H]-dCTP hybridizes to the exposed guanine, in proportion to the number of unmethylated (cleaved) sites in DNA. Thus, an increase in radiolabel incorporation reflects a relative increase in global DNA hypomethylation, and, conversely, lower radiolabel incorporation reflects relative hypermethylation. Results are expressed as the relative [3H]-dCTP/0.5 μg of DNA.
Trisomy 21 Cell Line and Cell-Culture Reagents
A human trisomy 21 lymphoblastoid cell line was obtained from Coriell Cell Repositories. Cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 growth medium (Gibco BRL), 10% fetal bovine serum (Hyclone Laboratories), and 1% penicillin-streptomycin (Gibco BRL). Purified methionine, folinic acid, SAM, thymidine, and dimethylglycine (DMG) were obtained from Sigma-Aldrich. Methyl-B12 was a generous gift from Enzymatic Therapy. Because differences in cell growth rates could potentially affect metabolite concentrations, pilot studies were conducted to confirm that all cells would be in log-phase growth at the time of harvest.
Supplementation, with Specific Nutrients, of Trisomy 21 Lymphoblastoid Cells
Human trisomy 21 lymphoblasts were seeded, at 0.2×106 cells/ml, in folic acid–free RPMI 1640 medium adjusted to a final concentration of 50 ng of folic acid/ml and 10 μmol of methionine/liter. The concentrations of the nutrients added to the culture medium in six replicate wells were as follows: folinic acid (100 μM), methyl-B12 (2 μg/liter), thymidine (20 μM), methionine (100 μM), and DMG (50 μM). In order to maintain a constant level of nutrients until harvest at day 7, the top half of the medium in each well was carefully aspirated and replaced, without disturbing the cells at the bottom. Control wells contained trisomy 21 lymphoblastoid cells cultured in RPMI growth medium without nutrient addition.
Statistics
Data are presented as the mean ± SD. Statistical differences between means were calculated with Student’s t-test and SigmaStat software (Jandel Scientific).
Results
Plasma Concentrations of Methionine, tHcy, Cystathionine, Cysteine, and Glutathione, in Children with DS and in Controls
The children with DS had significantly altered plasma levels of each of the metabolites, in the methionine/homocysteine pathway and also in the CBS-mediated transsulfuration pathway (table 1). Plasma tHcy was 76% of that of the control children, and plasma methionine level was only 53% of that for the controls (P<.001). The observed 3.8-fold increase in plasma cystathionine represents the first demonstration that the product of the CBS reaction is significantly elevated, despite the presence of adaptive mechanisms to down-regulate gene expression and enzyme activity. The significant increase in cysteine in the children with DS confirms previous observations (Lejeune et al. 1992) and may reflect a bottleneck at the rate-limiting step for glutathione synthesis. Finally, the significant decrease in plasma glutathione in the children with DS is consistent with previous reports of intracellular oxidative-stress increase due to overexpression of the Cu-Zn superoxide dismutase gene (SOD), which is also located on chromosome 21 (Iannello et al. 1999).
Table 1.
Plasma Levels of tHcy, Methionine, Cystathionine, Cysteine, and Glutathione, in Children with DS and in Controls
|
Mean ± SD Plasma Level(μmol/liter) |
|||||
| Subjects | tHcy | Methionine | Cystathionine | Cysteine | Glutathione |
| Children with DS (n=42) | 5.1 ± 1.1* | 16.1 ± 3.3* | .7 ± .2* | 233.5 ± 21.7* | 6.9 ± 1.4* |
| Controls (n=36) | 6.7 ± 1.6 | 30.6 ± 6.5 | .2 ± .05 | 203.7 ± 20.0 | 8.0 ± 1.2 |
P<.001.
Plasma Concentrations of SAM, SAH, and Adenosine—and Lymphocyte DNA Methylation Status—in Children with DS and in Controls
Methionine is the precursor for the ATP-dependent synthesis of SAM, the universal methyl donor for essential cellular methylation reactions. In association with the reduced plasma methionine levels, the level of SAM was also significantly reduced in the children with DS, as presented in table 2. In addition, a significant decrease in plasma SAH, the product of SAM-dependent methylation reactions, was observed in the children with DS.
Table 2.
Plasma Levels of SAM, SAH, and Adenosine—and Lymphocyte DNA Methylation Status—in Children with DS and in Controls
|
Mean ± SD Plasma Level(nmol/liter) |
||||
| Subjects | SAM | SAH | Adenosine | Global DNA Methylation(dpm/0.5 μg DNA)a |
| Children with DS | 80.9 ± 13.4 (n = 42)** | 15.7 ± 3.8 (n = 42)** | 1.0 ± .5 (n = 42)* | 4,540 ± 505 (n = 17)* |
| Controls | 98.3 ± 15.7 (n = 36) | 20.0 ± 4.2 (n = 36) | .75 ± .31 (n = 36) | 4,954 ± 542 (n = 12) |
Decrease in dpm indicates relative hypermethylation.
P<.04.
P<.001.
The decrease in both SAM and SAH is consistent with a generalized depression in cellular methylation capacity in these children. Interestingly, plasma adenosine levels were significantly elevated in the children with DS compared to those in the controls. The increase in adenosine is consistent with an adaptive up-regulation of adenosine deaminase and with the increase in uric acid levels, both of which have been reproducibly documented in children with DS (Puukka et al. 1986). Finally, the level of lymphocyte DNA hypomethylation was measured, to assess an endpoint that might be negatively affected by reduced levels of SAM and by reduced methylation capacity. Surprisingly, the lymphocyte DNA from the children with DS had decreased radiolabel incorporation, compared to that in the controls, indicating that the DNA was relatively hypermethylated, despite depressed levels of SAM and SAH.
In Vitro Supplementation, with Selected Nutrients, of Trisomy 21 Lymphoblastoid Cells
The choice of nutrients for in vitro supplementation was based on the potential of each nutrient to augment the synthesis of specific metabolites, shown, in table 1, to be depressed in the children with DS. The specific metabolites depleted in the children with DS appear to be the indirect result of the inappropriate increase in CBS activity and of the consequent permanent removal of homocysteine from the folate-dependent homocysteine/methionine cycle. As presented in table 3, the in vitro addition of 100 μM methionine doubled intracellular methionine and significantly increased levels of SAM, SAH, and adenosine. The addition of 100 μM folinic acid significantly increased tHcy, methionine, cysteine, and SAM. Methyl-B12, at a final concentration of 2 μg/liter, increased tHcy, methionine, SAM, SAH, and cysteine. Thymidine and DMG were added, separately, both to support folate-dependent thymidylate and DNA synthesis and to augment one-carbon units available for methionine synthesis. Both methionine and SAM were increased by the addition of 20 μM thymidine. DMG converts THF to 5,10-methylenetetrahydrofolate in mitochondria and should also indirectly spare one-carbon units for the methionine cycle. The addition of 50 μM of DMG significantly increased tHcy, methionine, SAM, SAH, and cysteine in the trisomy 21 lymphoblastoid cells. Taken together, these results suggest that selective nutritional intervention in vitro can shift one-carbon metabolism in trisomy 21 cells.
Table 3.
Intracellular tHcy, Methionine, Cysteine, Glutathione, SAM, and SAH Levels, in Trisomy 21 Lymphoblastoid Cells (AG 09802 Cell Line) Grown Either in Medium Supplemented with Selected Nutrients or in Control RPMI Medium
|
Mean ± SD Intracellular Levela (nmol/mg protein) |
|||||||
| Supplement (Concentration) | tHcy | Methionine | Cysteine | Glutathione | SAM | SAH | Adenosine |
| Methionine (100 μM) | .26 ± .06 | 14.7 ± 2.4* | 31.8 ± 3.2** | 49.1 ± 4.1** | .64 ± .1** | .16 ± .04* | 1.73 ± .7 |
| Folinic acid (100 μM) | .34 ± .06* | 11.3 ± 1.7* | 48.5 ± 3.1** | 47.8 ± 10.5 | .52 ± .1* | .12 ± .04 | 1.07 ± .2 |
| Methyl-B12(2 μg/liter) | .37 ± .06* | 11.4 ± 1.9* | 51.4 ± 3.5** | 49.0 ± 6.9* | .52 ± .1* | .19 ± .05* | 1.56 ± .5 |
| Thymidine (20 μM) | .23 ± .04 | 12.4 ± 2.4* | 39.9 ± 3.1 | 37.0 ± 5.1 | .48 ± .1* | .15 ± .06 | 1.04 ± .2 |
| DMG (50 μM) | .35 ± .07* | 14.3 ± 3.3** | 39.5 ± 7.7* | 39.5 ± 7.7 | .62 ± .1** | .15 ± .02* | 2.00 ± .8* |
| None | .25 ± .04 | 7.4 ± .04 | 35.8 ± 5.0 | 40.3 ± 5.4 | .31 ± .01 | .09 ± .03 | 1.08 ± .2 |
For six wells/nutrient.
P<.04.
P⩽.001.
Discussion
The plasma profile of the metabolites involved in the methionine/homocysteine pathway in children with DS is consistent with a functional folate deficiency secondary to CBS overexpression and the folate trap. Several clinical observations suggest that children with DS may be functionally folate deficient despite normal plasma levels of folate and B12. For example, profound methotrexate sensitivity (Ueland et al. 1990; Peeters et al. 1995), increased mean corpuscular volume (Gericke et al. 1977), and gastrointestinal malabsorption (Jannson and Johansson 1995) are often present in children with DS and have also been associated with clinical folate deficiency. Deoxynucleotide-pool imbalance, increased folate-sensitive fragile sites, and DNA strand breaks are induced by folate deprivation and have been documented in cells from individuals with DS (Gericke et al. 1977; Taub et al. 1996; Smith and Borsatto 1998). Moreover, these lesions are associated with increased risk of neoplastic transformation and may relate to increased risk of leukemia in children with DS (Taub et al. 1996).
The significant decrease in plasma methionine levels observed in the children with DS most likely reflects a decrease in methionine synthase activity, secondary to CBS-mediated removal of its precursor, homocysteine. The 50% decrease in methionine is of particular clinical concern because of the central importance of methionine in maintaining protein synthesis for growth, acute-phase protein/antibody synthesis, and peptide-hormone synthesis. In addition, methionine is the sole precursor for the synthesis of SAM, the primary methyl donor for essential cellular methylation reactions (fig. 1). An adaptive metabolic response to reduced intracellular levels of methionine and SAM is the breakdown of phosphatidylcholine to choline and betaine (trimethylglycine [TMG]). The enzyme betaine-homocysteine methyltransferase (BHMT) transfers a methyl group from betaine to homocysteine and provides an important alternative route for endogenous methionine synthesis when folate is limited (or trapped). A progressive depletion of plasma choline levels via controlled folate deprivation in humans has recently been documented (Jacob et al. 1999). Individuals with DS have increased sensitivity to anticholinergic drugs (Sacks and Smith 1989) and altered membrane phospholipid content and fluidity (Scott et al. 1994), both of which can be explained by increased catabolism and depletion of choline for methionine resynthesis.
The significant decreases, in both SAM and SAH, in the plasma of the children with DS suggest a general depression in cellular methylation capacity in these children. The observed increase in global DNA methylation in lymphocytes of the children with DS was unexpected in the context of reduced SAM and SAH levels. It is possible that the reduced SAM reflects increased methylation demands, as well as reduced synthesis. A recent analysis of the proximal 20 kb of chromosome 21 revealed a high proportion (46%) of interspersed repetitive sequences that are densely methylated (>90%) (Yu et al. 1997). It is possible that the observed increase in genomewide lymphocyte DNA methylation reflects the addition of the third highly methylated chromosome 21. An alternative explanation is that the higher DNA methylation density reflects an adaptive mechanism to down-regulate overexpressed genes on chromosome 21. Gene overdosage in normal females with two copies of the X chromosome is regulated by promoter-region methylation on the inactive X chromosome, resulting in transcriptional silencing of all genes on the inactive X chromosome. Consistent with an increase in de novo methylation as a mechanism to silence overexpressed genes, the promoter region of the h2-calponin gene on chromosome 21 was recently shown to be hypermethylated and down-regulated in individuals with DS (Kuromitsu et al. 1997). Of additional relevance to methylation dysregulation in DS is the recent discovery of a new de novo DNA methyltransferase gene, known as “DNMT3L,” located on chromosome 21, although its functional significance is not yet known (Aapola et al. 2000). Further evidence for protein-methylation dysfunction is provided by the observation that individuals with DS synthesize reduced levels of methylnicotinamide after a nicotinamide load, indicating a reduced protein-methylation capacity (Gershoff and Hegsted 1958). Finally, evidence of delayed myelination of DS neurons is consistent with reduced methylation of myelin basic protein (Wisniewski and Schmidt-Sidor 1989).
The increase in plasma adenosine levels in the children with DS is consistent with previous observations. The increase in plasma adenosine and in adenosine deaminase activity is strongly correlated with hyperuricemia in individuals with DS (Puukka et al. 1986). It has been suggested that hyperuricemia in individuals with DS is due to excessive purine synthesis resulting from overexpression of the gene for de novo purine synthesis, GARS-AIRS-GART, which is located on chromosome 21 (Peeters et al. 1993). Although increased mRNA transcripts for this gene have been documented both prenatally and during the early postnatal period (Brodsky et al. 1997), evidence for an increase in enzyme activity is lacking. The observations in the present study suggest an alternative explanation for increased uric acid production in DS. The overexpression of CBS would result in an enhanced flux of methionine metabolites down the transsulfuration pathway, resulting in excess production of adenosine from SAH hydrolysis (fig. 1). In addition, the methyl trap in individuals with DS would reduce the availability of folate for de novo synthesis of the purine ring (Ueland et al. 1990). Therefore, we suggest that the hyperuricemia in DS reflects increased adenosine production secondary to CBS overexpression and is not the result of increased de novo purine synthesis.
The reduced plasma glutathione observed in the children with DS most likely reflects an adaptive antioxidant response to chronic oxidative stress, resulting from SOD overexpression (fig. 1). The 3.8-fold increase in intracellular cystathionine is the first documentation that the product of the CBS reaction is highly elevated in the children with DS. Elevated cystathionine is not likely to have pathological consequences, however, since children with an inactivating mutation in cystathionine γ-lyase exhibit cystathioninuria but no associated pathology (Mudd et al. 1995). The presence of both CBS and SOD on chromosome 21 may be fortuitous, since the end product of CBS transsulfuration, glutathione, serves as a major antioxidant, by detoxifying H2O2, the product of SOD. The increase in plasma cysteine is significant but not excessive and provides substrate for glutathione synthesis.
The modulation of the metabolites in the homocysteine/methionine cycle, via in vitro addition of selected nutrients, is not surprising. Nutritional intervention with high doses of folic acid and B12 in patients with homocysteinemia has proved to be a successful clinical intervention to modulate these pathways and to restore normal homocysteine levels and one-carbon homeostasis (Brattström et al. 1998). Because individuals with DS have low homocysteine levels and a high probability of a folate trap (Ueland et al. 1990), intervention with folinic acid (5-formyltetrahydrofolate) has several advantages over intervention with folic acid. This form of folate is more efficiently absorbed as the reduced metabolite, is rapidly polyglutamated, and is more readily available for folate-dependent reactions (Priest et al. 1991). Although DMG successfully increased methionine levels in the cultured trisomy 21 lymphoblastoid cells, intervention with betaine, or TMG, would have the additional advantage of providing the folate-independent mechanism for methionine resynthesis from homocysteine. In the present study, DMG, rather than TMG, was used because lymphoid cells do not express the BHMT gene. The BHMT gene is primarily expressed in liver and kidney, and betaine has been used successfully to treat children with congenital defects in homocysteine metabolism (Wilcken et al. 1983). The increase in SAH levels that occurs with in vitro nutritional supplementation suggests that cellular methylation reactions were stimulated in these cells. On the basis of both (a) these encouraging in vitro findings and (b) successful clinical trials with these nutrients in patients with homocysteinemia, clinical intervention with folinic acid and betaine is worthy of investigation.
Acknowledgments
We would like to thank the mothers and the children who participated in this study. We are indebted to Laurette Janak for many helpful discussions. This work was supported by the Food and Drug Administration Office of Women’s Health and by Friends of Trisomy 21 Research, Inc.
Electronic-Database Information
The accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for DS [MIM 190685])
References
- Aapola U, Shibuya K, Scott HS, Ollila J, Vihinen M, Heino M, Shintani A, Kawasaki K, Minoshima S, Krohn K, Antonarakis SE, Shimizu N, Kudoh J, Peterson P (2000) Isolation and initial characterization of a novel zinc finger gene, DNMT3L, on 21q22.3, related to the cytosine-5-methyltransferase 3 gene family. Genomics 65:293–298 [DOI] [PubMed] [Google Scholar]
- Ausebel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA (eds) (1989) Current protocols in molecular biology. Wiley-Interscience, New York [Google Scholar]
- Brattström L, Landgren F, Israelsson B, Lindgren A, Hultberg B, Andersson A, Cuskelly G, McNulty H, Strain SS, McPartlin J, Weir DG, Scott JM, Den Heijer M, Brouwer IA, Blom HJ, Bos GM, Spaans A, Rosendaal FR, Thomas CM, Haak HL, Wijermans PW, Gerrits WB, Naurath HJ, Joosten E (1998) Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. BMJ 316:894–8989569395 [Google Scholar]
- Brodsky G, Barnes T, Bleskan J, Becker L, Cox M, Patterson D (1997) The human GARS-AIRS-GART gene encodes two proteins which are differentially expressed during human brain development and temporally overexpressed in cerebellum of individuals with Down syndrome. Hum Mol Genet 6:2043–2050 [DOI] [PubMed] [Google Scholar]
- Chadefaux B, Ceballos I, Hamet M, Poissonnier M, Kamon P, Allard D (1988) Is absence of atheroma in Down syndrome due to decreased homocysteine levels? Lancet 2:741 [DOI] [PubMed] [Google Scholar]
- Chadefaux B, Rethore MO, Raoul O, Ceballos I, Poissonnier M, Gilgenkranz S, Allard D (1985) Cystathionine beta synthase: gene dosage effect in trisomy 21. Biochem Biophys Res Commun 128:40–44 [DOI] [PubMed] [Google Scholar]
- Epstein CJ (1995) Down syndrome (trisomy 21). In: Stansbury JB, Wyngarden JB, Fredrickson DS (eds) The metabolic and molecular bases of inherited disease, 3d ed. McGraw-Hill, New York, pp 749–795 [Google Scholar]
- Gericke GS, Hesseling PB, Brink S, Tiedt FC (1977) Leucocyte ultrastructure and folate metabolism in Down's syndrome. S Afr Med J 51:369–374 [PubMed] [Google Scholar]
- Gershoff SN, Hegsted DM (1958) Metabolic studies of mongoloids. Am J Clin Nutr 6:526–530 [DOI] [PubMed] [Google Scholar]
- Greber-Platzer S, Schatzmann-Turhani D, Wollenek G, Lubec G (1999) Evidence against the current hypothesis of “gene dosage effects” of trisomy 21: ets-2, encoded on chromosome 21 is not overexpressed in hearts of patients with Down syndrome. Biochem Biophys Res Commun 254:395–399 [DOI] [PubMed] [Google Scholar]
- Hassold T, Sherman S (2000) Down syndrome: genetic recombination and origin of the extra chromosome 21. Clin Genet 57:95 [DOI] [PubMed] [Google Scholar]
- Hattori M, Fujiyama A, Taylor TD, Watanabe H, Yada T, Park HS, Toyoda A, et al (2000) The DNA sequence of human chromosome 21. Nature 405:311–319 [DOI] [PubMed] [Google Scholar]
- Ianello RC, Crack PJ, de Haan JB, Kola I (1999) Oxidative stress and neural dysfunction in Down syndrome. J Neural Transm 57:257–267 [DOI] [PubMed] [Google Scholar]
- Jacob RA, Jenden DJ, Allman-Farinelli MA, Swendseid ME (1999) Folate nutriture alters choline status of women and men fed low choline diets. J Nutr 129:712–717 [DOI] [PubMed] [Google Scholar]
- Jannson U, Johansson C (1995) Down syndrome and celiac disease. J Pediatr Gastroenterol Nutr 21:443–445 [DOI] [PubMed] [Google Scholar]
- Korenberg JR, Chen XN, Schipper R, Sun Z, Gonsky R, Gerwehr S, Carpenter N, Daumer C, Dignan P, Disteche C (1994) Down syndrome phenotypes: the consequences of chromosomal imbalance. Proc Natl Acad Sci USA 91:4997–5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivchenia E, Hether CA, Edmonds LD, May DS, Guckenberger S (1993) Comparative epidemiology of Down syndrome in two United States populations. Am J Epidemiol 137:815–828 [DOI] [PubMed] [Google Scholar]
- Kuromitsu J, Yamashita H, Kataoka H, Takahara T, Muramatsu M, Sekine T, Okamoto N, Furuichi Y, Hayashizaki Y (1997) A unique downregulation of h2-calponin gene expression in Down syndrome: a possible attenuation mechanism for fetal survival by methylation at the CpG island in the trisomic chromosome 21. Mol Cell Biol 17:707–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune J (1990) Pathogenesis of mental deficiency in trisomy 21. Am J Med Genet 7:20–30 [DOI] [PubMed] [Google Scholar]
- Lejeune J, Rethore MO, de Blois MC, Peeters M, Naffah J, Megarbane A, Cattaneo F, Mircher C, Rabier D, Parvy P (1992) Amino acids and trisomy 21. Ann Genet 35:8–13 [PubMed] [Google Scholar]
- Melnyk S, Pogribna M, Pogribny I, Hine RJ, James SJ (1999) A new HPLC method for the simultaneous determination of oxidized and reduced plasma aminothiols using coulometric electrochemical detection. J Nutr Biochem 10:490–497 [DOI] [PubMed] [Google Scholar]
- Melnyk S, Pogribna M, Pogribny IP, Yi P, James SJ (2000) Measurement of plasma and intracellular S-adenosylmethionine and S-adenosylhomocysteine utilizing coulometric electrochemical detection. Clin Chem 46:265–272 [PubMed] [Google Scholar]
- Mudd SH, Levy HL, Skoby F (1995) Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 7th ed. McGraw-Hill, New York, pp 1279–1327 [Google Scholar]
- Peeters MA, Megarbane A, Cattaneo F, Rethore MO, Lejeune J (1993) Differences in purine metabolism in patients with Down's syndrome. J Intellect Disabil Res 37:491–505 [DOI] [PubMed] [Google Scholar]
- Peeters MA, Rethore MO, Lejeune J (1995) In vivo folic acid supplementation partially corrects in vitro methotrexate toxicity in patients with Down syndrome. Br J Haematol 89:678–680 [DOI] [PubMed] [Google Scholar]
- Pogribny I, Yi P, James SJ (1999) A sensitive new method for rapid detection of abnormal methylation patterns in global DNA and within CpG islands. Biochem Biophys Res Commun 262:624–628 [DOI] [PubMed] [Google Scholar]
- Priest DG, Schmitz JC, Bunni MA, Stuart RK (1991) Pharmacokinetics of leukovorin metabolites in human plasma as a function of dose administered orally and intravenously. J Natl Cancer Inst 83:1806–1812 [DOI] [PubMed] [Google Scholar]
- Puukka R, Puukka M, Perkkila L, Kouvalainen K (1986) Levels of some purine metabolizing enzymes in lymphocytes from patients with Down's syndrome. Biochem Med Metab Biol 36:45–50 [DOI] [PubMed] [Google Scholar]
- Sacks B, Smith S (1989) People with Down's syndrome can be distinguished on the basis of cholinergic dysfunction. J Neurol Neurosurg Psychiatry 52:1294–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RB, Collins JM, Hunt PA (1994) Alzheimer's disease and Down syndrome: leukocyte membrane fluidity alterations. Mech Ageing Dev 75:1–10 [DOI] [PubMed] [Google Scholar]
- Smith MA, Borsatto B (1998) Down's syndrome, ageing and fragile sites. Mech Ageing Dev 101:167–173 [DOI] [PubMed] [Google Scholar]
- Tassone F, Lucas R, Slavov D, Kavsan V, Crnic L, Gardiner K (1999) Gene expression relevant to Down syndrome: problems and approaches. J Neural Transm 57:179–195 [DOI] [PubMed] [Google Scholar]
- Taub JW, Matherly LH, Stout ML, Buck SA, Gurney JG, Ravindranath Y (1996) Enhanced metabolism of 1-β-d-arabinofuranosylcytosine in Down syndrome cells: A contributing factor to the superior event free survival of Down syndrome children with acute myeloid leukemia. Blood 87:3395–3403 [PubMed] [Google Scholar]
- Torfs CP, Christianson RE (1998) Anomalies in Down syndrome individuals in a large population-based registry. Am J Med Genet 77:431–438 [PubMed] [Google Scholar]
- Ueland PM, Refsum H, Christensen B (1990) Methotrexate sensitivity in Down's syndrome: a hypothesis. Cancer Chemother Pharmacol 25:384–386 [DOI] [PubMed] [Google Scholar]
- Wilcken DEL, Wilcken B, Dudman NPB, Tyrell PA (1983) Homocysinuria: the effects of betaine treatment in the patients not responsive to pyridoxine. N Engl J Med 309:448–453 [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Schmidt-Sidor B (1989) Postnatal delay of myelin formation in brains from Down syndrome infants and children. Clin Neuropathol 8:55–62 [PubMed] [Google Scholar]
- Yu J, Tong S, Shen Y, Kao F-T (1997) Gene identification and DNA sequence analysis in the GC-poor 20 megabase region of human chromosome 21. Proc Natl Acad Sci USA 94:6862–6867 [DOI] [PMC free article] [PubMed] [Google Scholar]