Abstract
Although >55 CTNS mutations occur in patients with the lysosomal storage disorder cystinosis, no regulatory mutations have been reported, because the promoter has not been defined. Using CAT reporter constructs of sequences 5′ to the CTNS coding sequence, we identified the CTNS promoter as the region encompassing nucleotides −316 to +1 with respect to the transcription start site. This region contains an Sp-1 regulatory element (GGCGGCG) at positions −299 to −293, which binds authentic Sp-1, as shown by electrophoretic-mobility–shift assays. Three patients exhibited mutations in the CTNS promoter. One patient with nephropathic cystinosis carried a −295 G→C substitution disrupting the Sp-1 motif, whereas two patients with ocular cystinosis displayed a −303 G→T substitution in one case and a −303 T insertion in the other case. Each mutation drastically reduced CAT activity when inserted into a reporter construct. Moreover, each failed either to cause a mobility shift when exposed to nuclear extract or to compete with the normal oligonucleotide’s mobility shift. The CTNS promoter region shares 41 nucleotides with the promoter region of an adjacent gene of unknown function, CARKL, whose start site is 501 bp from the CTNS start site. However, the patients’ CTNS promoter mutations have no effect on CARKL promoter activity. These findings suggest that the CTNS promoter region should be examined in patients with cystinosis who have fewer than two coding-sequence mutations.
Introduction
Cystinosis (MIM 219800), a rare autosomal recessive storage disease, results from impaired transport of the disulfide amino acid cystine out of cellular lysosomes (Gahl et al. 1982). The subsequent accumulation of cystine, which crystallizes in a host of cells, destroys various tissues at different rates (Gahl et al. 2001). In classic nephropathic cystinosis, patients experience growth retardation and renal tubular Fanconi syndrome in infancy, renal failure by 10 years of age, photophobia, hypothyroidism, and a variety of other complications that generally occur after renal transplantation (Gahl and Kaiser-Kupfer 1987; Theodoropoulos et al. 1993; Gahl et al. 2001). Symptomatic treatment includes replacement of renal losses and thyroid-hormone supplementation. However, cystine-depleting therapy with oral cysteamine (Cystagon) serves as the primary therapy, enhancing growth, retarding renal glomerular deterioration (Gahl et al. 1987; Markello et al. 1993), and obviating the need for thyroid-hormone replacement (Kimonis et al. 1995). In addition, cysteamine eyedrops dissolve the corneal crystals responsible for photophobia (Kaiser-Kupfer et al. 1987; Gahl et al. 2000).
Although ∼95% of patients with cystinosis exhibit the classic, infantile nephropathic type described above, two other categories exist. Patients with intermediate or adolescent cystinosis develop renal failure during the 2d or 3d decades of life, whereas individuals with ocular or non-nephropathic cystinosis have only photophobia due to corneal crystals and never develop renal disease (Gahl et al. 2001). The variants of cystinosis can often be differentiated on the basis of the cystine levels found in polymorphonuclear leukocytes or cultured fibroblasts (Gahl et al. 2001): 5–23 nmol half-cystine/mg protein in nephropathic cystinosis, 2–5 nmol half-cystine/mg protein in intermediate cystinosis, and 1–2 nmol half-cystine/mg protein in ocular cystinosis (normal range, 0–0.2 nmol half cystine/mg protein).
Some correlations have been noted between the clinical phenotypes of patients with cystinosis and the mutations that they carry in the cystinosis gene, CTNS. This 12-exon gene is transcribed into an mRNA of ∼2.6 kb (Town et al. 1998). The encoded protein, named “cystinosin,” consists of 367 amino acids and appears to be an integral membrane protein, most likely functioning as the lysosomal cystine transporter (Gahl et al. 1982). To date, >55 mutations in the coding sequence have been reported (Shotelersuk et al. 1998; Town et al. 1998; Anikster et al. 1999b; Attard et al. 1999; McGowan-Jordan et al. 1999), and the three variants of cystinosis have proved to be allelic, each displaying causative mutations in CTNS (Attard et al. 1999; Thoene et al. 1999; Anikster et al. 2000). Among patients with nephropathic cystinosis, the most prevalent mutation is a 57,257-bp deletion removing the first nine exons of CTNS plus a large amount of upstream sequence (Shotelersuk et al. 1998; Town et al. 1998; Anikster et al. 1999a; Forestier et al. 1999), recently found to include a new gene called “CARKL” (MIM 605060), for “carbohydrate kinase-like” (Touchman et al. 2000). In a series of 70 European patients (Town et al. 1998), 33% were homozygous for the 57-kb deletion, compared with 44% in an American cohort (Shotelersuk et al. 1998). In the latter group of 108 patients with nephropathic cystinosis, 2 had a smaller major deletion, 11 were homozygous and 3 were heterozygous for a 753 G→A transition (W138X), 24 exhibited 1 of 21 other mutations, and several affected individuals had no identifiable coding-sequence mutation. Recent studies have demonstrated that patients with intermediate (Thoene et al. 1999) or non-nephropathic cystinosis (Anikster et al. 2000) have one severe CTNS mutation (e.g., the 57-kb deletion or the W138X mutation) and one mild mutation (e.g., a 928 G→A transition [G197R] or a splicing mutation).
Despite extensive mutation analysis of patients with cystinosis, the molecular regulation of CTNS, specifically involving its promoter and nuclear-transcription factors, has not been elucidated. This void assumes greater import because CTNS and CARKL are separated by only 501 bp (fig. 1a), and this GC-rich region may contain the promoters for both genes. In addition, some of the patients with cystinosis in whom coding-sequence mutations are lacking may have promoter mutations. In this article, we identify the promoter regions for CTNS and CARKL, two adjacent genes that are aligned on separate DNA strands in different directions. We demonstrate that a region of the CTNS promoter binds the transcription factor Sp-1 and that other portions of the CTNS/CARKL promoter area have sequence homology to AP-4, AP-2, and NF-1 regulatory elements. Finally, using both functional assays and mutation analysis, we identify three patients with cystinosis who have promoter mutations as the cause of their disease.
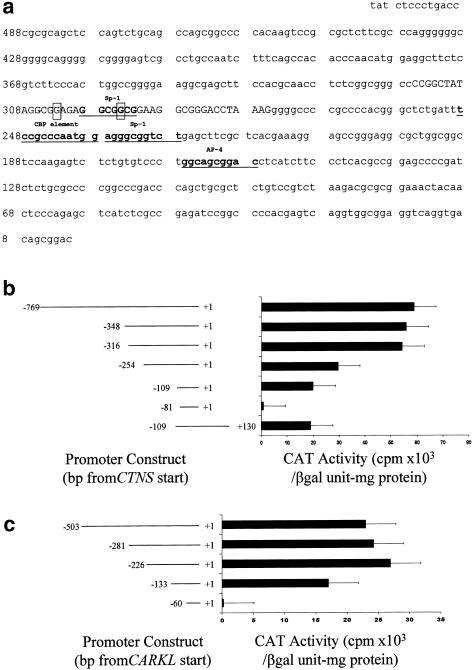
Figure 1.
Promoter region and its activity. a, Nucleotides within the 501-bp intergene region separating the start sites of CTNS and CARKL. The number on the left shows the distance from the CTNS start site. The Sp-1, AP-4, and CAAT-binding protein (CBP) motifs are underlined and in boldface. The mutations in patients 1 and 2 are boxed. The 41 nucleotides in uppercase font may contribute to both expression of CTNS and expression of CARKL. b, CAT activities of CTNS promoter constructs. Nucleotide +1 represents the transcription start site. The constructs portrayed on the left were inserted upstream of pbCAT. The pSV40—that is, the promoterless CAT control—plasmid, which contains both an SV40 enhancer and an SV40 promoter, served as a positive control, and pbCAT served as a negative control. CAT activity was determined for each construct after transient transfection into HeLa cells. A vector carrying the gene for β-galactosidase was cotransfected as a control, for transfection efficiency. The bars represent means ± SD for three determinations of CAT activity. c, CAT activities of CARKL promoter constructs. Nucleotide +1 represents the transcription start site of CARKL.
Patients and Methods
Patients
All patients were enrolled in a protocol approved by the National Institute of Child Health and Human Development's institutional review board. The diagnosis of cystinosis was made on the basis of a typical clinical course combined with elevated leukocyte cystine levels and the presence of corneal crystals on slit-lamp examination. A clinical-severity score was used to assess the phenotype of each patient (Shotelersuk et al. 1998). The severity of the renal tubular reabsorption defect was gauged by a Fanconi syndrome index—that is, a measure of the daily urinary excretion of 21 amino acids per kilogram of body weight (Charnas et al. 1991). In addition, age at presentation, leukocyte cystine value, age at renal failure, and age at development of nonrenal complications served as other measurable parameters. According to this scale, a value of 1 is extremely mild and a value of 3 is the most severe; typical patients with nephropathic cystinosis, including those homozygous for the 57-kb deletion in CTNS, have a value of 2.0 (Shotelersuk et al. 1998).
Promoter Constructs
Promoter sequences of different lengths were constructed by use of forward primers containing HindIII restriction sites and reverse primers containing BamHI restriction sites (table 1). Normal control genomic DNA was used as template for amplification of the constructs by use of PCR beads (Amersham Pharmacia Biotech). After an initial 4-min incubation at 96°C, amplification was performed, with denaturation at 94°C for 1 min, annealing at 60°C for 30 s, and extension at 72°C for 1 min. A further 10-min extension at 72°C was performed after completion of 35 cycles. The PCR product was purified by a GeneClean Kit (Bio101). PCR products were cloned into a TA vector (TOPO TA Cloning; Invitrogen) and were digested with the appropriate restriction enzymes. DNA was ligated into a promoterless and enhancerless CAT vector, pbCAT, by use of a T4 DNA Ligase kit (Roche). All the promoter constructs were sequenced to confirm the orientation and the accuracy of the structure. Automated sequencing was performed on a Beckman CEQ 2000, by use of a CEQ Dye Terminator Cycle Sequencing Kit, according to the manufacturer’s protocol (Beckman-Coulter). For constructs of mutated promoters, each patient’s genomic DNA served as a template for PCR amplification using the primer set containing nucleotides −348 to +1 (table 1, upper section).
Table 1.
Primers for PCR Amplification of CTNS Promoter and CARKL Promoter
|
Primer |
||
| Construct | Sense | Antisense |
|
CTNS |
||
| −769 to +1 | 5′-CATGACCAAGCTTAGACTTCATTGCGGGAAGGGC-3′ | 5′-CGTCTAGAGGTCCGCTGTCACCTGACC-3′ |
| −348 to +1 | 5′-GCATGACCAAGCTTAGGCGAGCTTCCACGCAACCT-3′ | 5′-CGTCTAGAGGTCCGCTGTCACCTGACC-3′ |
| −316 to +1 | 5′-GACCAAGCTTCGGCTATAGGCGGAGAGGCG-3′ | 5′-CGTCTAGAGGTCCGCTGTCACCTGACC-3′ |
| −254 to +1 | 5′-GACCAAGCTTCTAAAGGGGGCCCCGCCCCAC-3′ | 5′-CGTCTAGAGGTCCGCTGTCACCTGACC-3′ |
| −109 to +1 | 5′-GACCAAGCTTCCAGCTGCGCTCTGTCCGTC-3′ | 5′-CGTCTAGAGGTCCGCTGTCACCTGACC-3′ |
| −81 to +1 | 5′-GACCAAGCTTGCGGAAACTACAACTCCCAG-3′ | 5′-CGTCTAGAGGTCCGCTGTCACCTGACC-3′ |
| −109 to +130 | 5′-GACCAAGCTTCCAGCTGCGCTCTGTCCGTC-3′ |
5′-CATGACCAAGCTTGAGGCCGCGTCCGCCTCTCAC-3′ |
|
CARKL |
||
| −503 to +1 | 5′-CATGACCAAGCTTGAGGCCGCGTCCGCCTCTCAC-3′ | 5′-CGTCTAGATTATCTCCCTGACCCGCGCAGC-3′ |
| −281 to +1 | 5′-GCATGACCAAGCTTAAGCTCAGACCGCCCTCCATT-3′ | 5′-CGTCTAGATTATCTCCCTGACCCGCGCAGC-3′ |
| −226 to +1 | 5′-GACCAAGCTTTTTAGGTCCCGCCTTCCGCC-3′ | 5′-CGTCTAGATTATCTCCCTGACCCGCGCAGC-3′ |
| −133 to +1 | 5′-GACCAAGCTTAAGACGAGAAGCCTCCATGT-3′ | 5′-CGTCTAGATTATCTCCCTGACCCGCGCAGC-3′ |
| −60 to +1 | 5′-GACCAAGCTTAGAGCGCGGACTTGTGGGGC-3′ | 5′-CGTCTAGATTATCTCCCTGACCCGCGCAGC-3′ |
Mutation Analysis of CTNS in Patients with Cystinosis
Detection of the 57-kb deletion in the heterozygous state was accomplished by use of primers flanking the deletion. This gave a 423-bp product if the deletion was present and gave no product if the deletion was absent (Anikster et al. 1999a). To detect other mutations in CTNS, genomic DNA of each patient was isolated from whole blood and was screened by direct DNA sequencing of exons 3–12, by use of primer conditions reported elsewhere (Shotelersuk et al. 1998; Town et al. 1998). Automated sequencing was performed as described above.
Transfections and Chloramphenicol Acetyltransferase (CAT) Assays
HeLa cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, glutamine (2 mM), penicillin (100 units/ml), and streptomycin (100 μg/ml). The cells were grown in six-well plates, to ∼70%–80% confluence. Transient transfections were performed by use of 1 μg of CAT reporter promoter construct (Promega); pSV40 β-galactosidase plasmid (Promega) served as a control for transfection efficiency. Lipofectamine (Life Technologies) was used as a transfection reagent. After 48 h, the cells were rinsed with phosphate-buffered saline (PBS) and were harvested into 200 μl of lysis buffer (Promega). CAT activity was analyzed by incubation of cell extracts (110 μl) with [14C]-chloramphenicol (0.225 μCi, specific activity 56.2 mCi/mmol or 2,079.4 MBq/mmol; NEN) and n-butyryl coenzyme A (Sigma) at 37°C for 20 min, followed by xylene extraction and liquid scintillation counting (Rackbeta 1219; Perkin Elmer Life Science). Each experiment included three wells per construct. β-Galactosidase activity was assayed in the cell extract (10 μl) by measurement of absorbance at 420 nm, according to the instructions provided by Promega. One unit was defined as the activity that hydrolyzes 1 μM o-nitrophenyl-β-d-galactopyranoside to o-nitrophenol and galactose per minute, at pH 7.5 and 37°C. The cell extract (25 μl) was also assayed for protein, by the standard bicinchoninic acid method (BioRad; Hercules). CAT activity was expressed as counts per minute (cpm), of acetylated chloramphenicol, produced in 20 min, per β-galactosidase unit per milligram of protein.
Electrophoretic-Mobility–Shift Assays (EMSAs)
For EMSAs, two sets of oligonucleotide probes were used. The first set contained nucleotides −308 to −279 (5′-AATTAGGCGGAGAGGCGGCGGAAGGCGGGACCTA) of the normal, wild-type CTNS promoter and nucleotides −308 to −279 of the CTNS promoter in patient 1, containing a G→C conversion at nucleotide −295. The second set contained nucleotides −327 to −300 (5′-AATTTCTCGGCGGGCCCCGGCTATAGGCGGAGA) of the wild-type promoters and nucleotides −308 to −279 of the CTNS promoters in patients 2 and 3—that is, the sets contained either the −303 G→T transversion or the −303 T insertion. These oligonucleotides were labeled with α[32P]-dATP and the Klenow fragment of DNA polymerase I (Amersham Pharmacia), by standard methods, according to the manufacturer's recommendations. Radiolabeled probe (∼2,000 cpm) and 1 μg of HeLa-cell nuclear extract (NE) (Santa Cruz Biotechnology) were incubated for ∼20 min in 20 μl reaction buffer (Bandshift Kit; Amersham Pharmacia). Sp-1 studies were performed by use of a Sp-1 Nushift Kit supplied by Geneka Biotechnology, according to the manufacturer’s instructions. The reaction mixture was electrophoresed in 0.5 × TBE buffer (45 mM Tris-borate, 1 mM EDTA) on precast nondenaturing 6% polyacrylamide DNA-retardation gels (Novex). The gels were dried and exposed on Biomax MR x-ray film (Kodak), for different periods of time.
Results
Characterization of the CTNS Promoter
We first generated a CTNS promoter construct containing the 769 bp upstream from the transcription start site of CTNS and transfected this construct into HeLa cells. An SV-driven β-galactosidase reporter plasmid was cotransfected so that, on the basis of the β-galactosidase activity, CAT activity (in cpm/mg protein) also could be normalized for transfection efficiency. The −769 to +1 construct gave mean ± SD CAT activities of 5.5 ± 0.2 × 104 cpm/β-galactosidase unit–mg protein. A construct of nucleotides −769 to −360 yielded virtually no CAT activity. Both a −348 to +1 construct and a −316 to +1 construct gave CAT activities indistinguishable from that for the −769 to +1 construct (fig. 1b). Consecutive truncations of the complete promoter sequence gave progressively decreased CAT activities; the activity was reduced to 60% when a −254 to +1 construct was used and to 35% when a −109 to +1 construct was used. When the construct was further deleted, to −81 to +1, the CAT activity diminished to baseline. These data indicate that the DNA region encompassing nucleotides −316 to +1 contains the basal CTNS promoter and that DNA regions encompassing nucleotides −316 to −255, nucleotides −254 to −110, and nucleotides −109 to −82 contain cis-acting elements that positively regulate CTNS expression (fig. 1b). Sequence analysis predicts both the presence of Sp-1 motifs at nucleotides −299 to −293 and −237 to −228 and an AP-4 binding site at nucleotides −167 to −159 (fig. 1a). A −109 to +130 construct, extending into exon 1, gave CAT activity comparable to that given by the −109 to +1 construct (fig. 1b), indicating that this portion of the 5′ UTR of CTNS does not contain an activating element.
Characterization of the CARKL Promoter
A complete CARKL promoter construct, extending from −503 to +1 with respect to the CARKL transcription start site, gave mean ± SD CAT activity of ∼2.6 ± 0.5 × 104 cpm/β-galactosidase unit–mg protein. Constructs of −281 to +1 bp and −226 to +1 bp gave comparable CAT activities (fig. 1c). When a construct was further truncated, to −133 to +1, CAT activity was reduced by 40%, and a −60 to +1 construct gave virtually no CAT activity. Therefore, the basal CARKL promoter is located between nucleotides −226 and +1, a region that contains activating elements at nucleotides −226 to −134 and at nucleotides −133 to −61. Sequence analysis indicates that the region encompassing nucleotides −133 to −60 is predicted to bind to transcription factors AP-2 and NF-1.
Promoter Mutations in Patients with Cystinosis
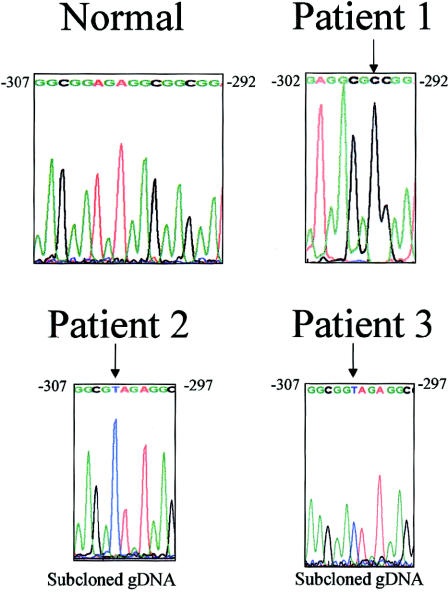
To determine whether some patients with cystinosis have the disease because of mutations in the promoter region of CTNS, we screened a total of 62 patients in whom 93 CTNS alleles had no 57-kb deletion. Each patient’s genomic DNA in the region encompassing nucleotides −348 to +1 of the CTNS promoter was amplified, and the sequence compared with that of 20 control alleles. Three patients, each heterozygous for a known coding-sequence mutation, displayed a promoter mutation in the other allele (fig. 2). Patient 1, who had nephropathic cystinosis, had a G→C change at position −295, involving the Sp-1 regulatory element. Patient 2, who has ocular cystinosis, had a G→T transversion at position −303, and patient 3, who also had ocular cystinosis, had a T insertion after position −303. In each patient, the allele with the promoter mutation was shown to have an entirely normal coding sequence.
Figure 2.
CTNS promoter mutations. The normal sequence is given for nucleotides −311 to −292. Arrows indicate the mutated bases—that is, a G→C change at position −295 in patient 1, a G→T change at position −303 in patient 2, and a T insertion after position −303 in patient 3.
Functional Assessment of CTNS Promoter Mutations
Each of the patients’ three promoter mutations was tested for its effect on promoter activity, by generation of −348 to +1 CTNS-CAT constructs that contained entirely normal sequence except for the mutation under investigation. After being transfected into HeLa cells, the mutant promoters in patients 1, 2, and 3 produced 19%, 5%, and 16%, respectively, of the wild-type CAT activity.
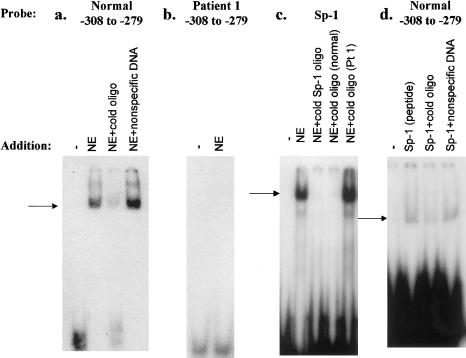
EMSAs were performed to verify binding of nuclear proteins to the CTNS promoter’s putative regulatory elements and to confirm the deleterious effect that each patient’s mutation had on factor binding. A putative regulatory-element sequence, containing nucleotides −308 to −279 and including the Sp-1 site, was radiolabeled to serve as a probe, and a protein-DNA complex was formed with HeLa-cell NE (fig. 3a). The formation of the complex was efficiently blocked by an excess of nonradioactive target DNA but not by nonspecific DNA (fig. 3b). When target DNA (nucleotides −308 to −279) containing the −295 G→C mutation in patient 1 was used, no protein-DNA complex was formed with HeLa-cell NEs (fig. 3b). To demonstrate that the wild-type DNA complex had formed by virtue of its Sp-1 sequence, we performed two sets of experiments. First, we showed that an authentic, radiolabeled Sp-1 regulatory-element sequence formed a DNA-protein complex with HeLa-cell NE (fig. 3c). The complex formation was blocked by the addition of either 100-fold excess nonradioactive Sp-1 oligonucleotide or the normal CTNS promoter region encompassing nucleotides −308 to −279 but not by the same region carrying the −295 G→C mutation in patient 1 (fig. 3c). Second, we showed that authentic Sp-1 peptide formed a DNA-protein complex with the normal, radiolabeled CTNS promoter region encompassing nucleotides −308 to −279 (fig. 3d). The complex formation was blocked by 100-fold excess nonradioactive normal oligonucleotide but not by nonspecific DNA (fig. 3d).
Figure 3.
EMSAs of CTNS promoter region bearing −295 G→C mutation in patient 1. a, Results of double-stranded–DNA probe consisting of nucleotides −308 to −279, radiolabeled with α[32P]-dATP. Lane 1, No addition. Lane 2, NE added. Lane 3, NE and 100-fold excess nonradioactive probe added. Lane 4, NE and 30-fold-molar excess nonspecific (calf thymus) DNA added. b, Results of double-stranded–DNA probe consisting of nucleotides −308 to −279, containing the −295 G→C mutation in patient 1, radiolabeled with α[32P]-dATP. Lane 1, No addition. Lane 2, NE added. c, Results of Sp-1 oligonucleotide probe radiolabeled with γ[32P]-ATP. Lane 1, No addition. Lane 2, NE added. Lane 3, NE and 100-fold excess nonradioactive Sp-1 oligonucleotide added. Lane 4, NE and 100-fold excess nonradioactive probe consisting of nucleotides −308 to −279 added. Lane 5, NE and 100-fold excess nonradioactive probe consisting of nucleotides −308 to −279, with the −295 G→C mutation in patient 1, added. d, Results of double-stranded–DNA probe consisting of nucleotides −308 to −279, radiolabeled with α[32P]-dATP. Lane 1, No addition. Lane 2, Authentic Sp-1 peptide added. Lane 3, Sp-1 peptide and nonradioactive probe consisting of nucleotides −308 to −279 added. Lane 4, Sp-1 peptide and 30-fold-molar excess nonspecific (calf thymus) DNA added.
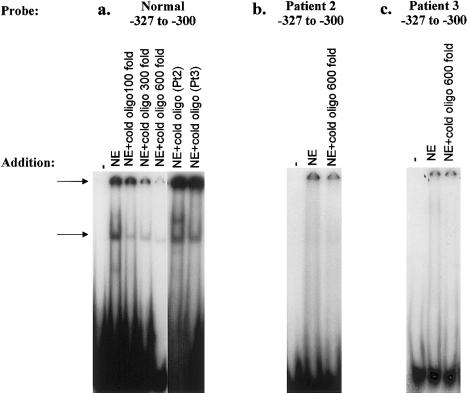
The region encompassing nucleotides −327 to −300 and containing the mutations in patients 2 and 3, was studied in a similar fashion. When this region was used as a radiolabeled probe, two DNA-protein complexes were formed with HeLa-cell NE, and complex formation was blocked by the addition of increasing concentrations of excess nonradioactive probe (fig. 4a). However, neither nonspecific DNA (data not shown) nor the nonradioactive region encompassing nucleotides −327 to −300 and containing the mutations in patients 3 and 4 (fig. 4a) could compete against the formation of complex. In addition, radiolabeled probe consisting of the region encompassing nucleotides −327 to −300 and containing either the −303 G→T mutation in patient 2 (fig. 4b) or the −303 T insertion in patient 3 (fig. 4c) did not undergo a mobility shift when NE was added. The bands at the origin of the gels represented nonspecific binding, since they could not be competed against by 600-fold excess nonradioactive mutant probe (fig. 4b and c).
Figure 4.
EMSAs of CTNS promoter region bearing mutations in patients 2 and 3. a, Results of double-stranded DNA probe consisting of nucleotides −327 to −300, radiolabeled with α[32P]-dATP. Lane 1, No addition. Lane 2, NE added. Lane 3, NE and 100-fold excess nonradioactive probe added. Lane 4, NE and 300-fold excess nonradioactive probe added. Lane 5, NE and 600-fold excess nonradioactive probe added. Lane 6, NE and 600-fold excess nonradioactive probe consisting of nucleotides −327 to −300, containing the −303 G→T mutation in patient 2, added. Lane 7, NE and 600-fold excess nonradioactive probe consisting of nucleotides −327 to −300, containing the −303 T insertion in patient 3, added. Arrows indicate the locations of DNA-protein complexes that can be competed against by the normal but not by the mutant oligonucleotides. b, Results of double-stranded DNA probe consisting of nucleotides −327 to −300, containing the −303 G→T mutation in patient 2, radiolabeled with α[32P]-dATP. Lane 1, No addition. Lane 2, NE added. Lane 3, NE and 600-fold excess nonradioactive mutant probe added. c, Results of double-stranded DNA probe consisting of nucleotides −327 to −300, containing the T insertion after position −303 in patient 3, radiolabeled with α[32P]-dATP. Lane 1, No addition. Lane 2, NE added. Lane 3, NE and 600-fold excess nonradioactive mutant probe added.
None of the three CTNS promoter mutations, when incorporated into a CARKL promoter construct, had any effect on the expression of CAT reporter activity (data not shown).
Genotype/Phenotype Correlations
Patient 1 is a 19-year-old female of Scottish-Irish and Puerto Rican descent who has classic nephropathic cystinosis. At age 8–9 mo, her weight was below the 5th percentile and rickets was evident. At age 4 years, prior to surgical intervention for a tonsillectomy and adenoidectomy, she was found to have renal tubular Fanconi syndrome. At age 7, an eye examination showing corneal crystals prompted the diagnosis of cystinosis. Renal failure required peritoneal dialysis, and a cadaveric renal transplant was performed at age 8 years. The same year, leukocyte cystine was measured as 5.3 nmol half-cystine/mg protein, and oral cysteamine therapy was initiated. The renal function of the allograft gradually deteriorated, and, by age 19 years, the patient was again receiving peritoneal dialysis. On the basis of her early age at disease onset and renal failure and her moderate leukocyte cystine value, the cystinosis-severity score was calculated to be 2.7, clearly within the severe range. The patient is heterozygous for the 57-kb deletion in CTNS and is hemizygous for a −295 promoter mutation.
Patient 2 is an 11-year-old adopted girl of German/Norwegian heritage. Her ocular, non-nephropathic cystinosis was diagnosed after ophthalmic screening revealed corneal crystals. She had no renal tubular or glomerular disease, and her leukocyte cystine value at age 11 years was 1.3 nmol half-cystine/mg protein. On the basis of her lack of symptoms and her low leukocyte cystine level, her severity score was calculated to be 1.0, indicating only ocular involvement. She has a G→A mutation at position 928 in exon 9 of CTNS, resulting in a G197R amino acid change. No other mutation was found in the remainder of the coding sequence. The G197R mutation has been found in several patients with non-nephropathic cystinosis (Anikster et al. 2000) and is considered to be a mild mutation. The patient’s second mutation is the G→T transversion in the promoter region encompassing nucleotide −303.
Patient 3 also has ocular, non-nephropathic cystinosis. She is a 22-year old woman previously reported, as “case 4,” in a study by Anikster et al. (2000). On routine ophthalmic examination at age 20 years, she was noted to have corneal crystals. She had no renal tubular or glomerular disease, and her leukocyte cystine level at age 21 years was 2.3 nmol half-cystine/mg protein. On the basis of her lack of renal symptoms and her low leukocyte cystine value, her severity score was estimated to be 1.0, consistent with ocular cystinosis. Like patient 2, this patient also had a G→A change at position 928 in exon 9 of CTNS, resulting in a G197R substitution, with no other mutations in the entire coding sequence. The patient’s mother is heterozygous for G197R and lacks the proband’s promoter mutation, a T insertion after position −303. However, this mutation was present in the father’s DNA, which lacked the G197R mutation.
Discussion
Lysosomal membranes contain a broad array of integral proteins, ranging from abundant, glycosylated peptides such as LAMP-1 and LAMP-3, which have no proved function, to scarce, poorly glycosylated proteins, which transport small molecules out of lysosomes. Although a wide variety of lysosomal membrane carriers have been described (Thoene 1992), only three—those recognizing cystine, sialic acid, and cobalamin—are associated with a deficiency that causes human disease. Mutations in CTNS, with cystinosin deficiency and defective lysosomal cystine transport, cause cystinosis (Gahl et al. 1982, 2001; Town et al. 1998). Mutations in SLC17A5, with sialin deficiency, abnormal sialic-acid transport, and free-sialic-acid accumulation, cause two allelic disorders, Salla disease and infantile free-sialic-acid–storage disease (Renlund et al. 1986; Tietze et al. 1989; Aula and Gahl 2001). Impaired cobalamin transport, which is due to an undefined gene, results in cobalamin F disease, with homocystinuria and methylmalonic aciduria (Rosenblatt et al. 1985). No promoter region has been identified or characterized for any of these disease-causing genes, or for other genes responsible for lysosomal amino acid or sugar transport, or for the more abundant genes for LAMP proteins.
We now define the CTNS region responsible for regulation of its expression, describing for the first time the features of a promoter for an integral lysosomal membrane protein. The CTNS promoter lacks a canonical TATA-like sequence but is rich in GC sequences, including an Sp-1 motif at nucleotides −299 to −293. The function of this SP-1 site was confirmed by EMSAs showing binding of authentic Sp-1 protein to this region (fig. 4d). The critical nature of this regulatory element is indicated by the 81% reduction, in CAT reporter activity, observed when a single nucleotide (−295) is mutated, as in our patient 1, who has cystinosis.
CTNS resides in close proximity to CARKL, a gene of unknown function. Of the 501 bp separating the CTNS and CARKL start sites, 41 nucleotides in the center (−316 to −276 with respect to the CTNS start site) appear responsible for as much as 65% of the total CTNS-expression–stimulating capacity (fig. 1b). This region may also contribute to CARKL expression, since nucleotides −369 to −276 with respect to CTNS (−226 to −133 with respect to CARKL) contain an activity element responsible for 40% of the total CARKL-expression–stimulating capacity (fig. 1c). However, in the three patients with cystinosis, the specific, 1-bp alterations in this region, which drastically reduced CTNS promoter function, had no effect on CARKL promoter function. This indicates that portions of the shared promoter region display specificity for one gene or the other. To our knowledge, the phenomenon of a single region serving as a promoter for two distinct genes has been reported only once previously—that is, in the case of a bidirectional promoter for poly (ADP-ribose) polymerase 2 and the RNase P RNA subunit (Ame et al. 2001). In addition to the 41 shared nucleotides, the CARKL promoter region encompassing nucleotides −133 to −60 contains predicted transcription-factor binding sites for AP-2 (nucleotides −72 to −61; i.e., 5′-CCCCCCTGGGCG), and NF-1 (nucleotides −103 to −86; i.e., 5′-GATTGGCAGGCGACTCCC).
During the past 3 years, extensive mutation analysis has been performed on the CTNS genes in patients with cystinosis, and >55 different mutations have been reported (Shotelersuk et al. 1998; Town et al. 1998; Anikster et al. 1999b, 2000; Attard et al. 1999; McGowan-Jordan et al. 1999; Thoene et al. 1999). However, for several patients with cystinosis, only a single allele has been found to have a coding-sequence mutation. The cystinosis disease state in some of these cases has been attributed to mutations in the promoter region of CTNS, although no such mutations have been identified. Elucidation of the CTNS promoter now makes possible mutation analysis in this region. In fact, we identified three patients with cystinosis, each of whom is compound heterozygous for a functional promoter mutation and a known coding-sequence mutation.
Patient 1, with classic nephropathic cystinosis, was known to carry the 57-kb deletion on one allele. Her other allele contained a G→C substitution in the −295 position in relationship to the CTNS start site. On the basis of several pieces of evidence, this mutation appeared to cause promoter dysfunction: first, the mutant construct produced only 19% of the control amount of CAT reporter activity when transfected into HeLa cells; second, EMSA experiments demonstrated that an oligonucleotide containing the −295 mutation failed to form a complex with NE containing transcription factors (fig. 3b); finally, an oligonucleotide bearing the −295 mutation failed to compete with the normal Sp-1 regulatory-element oligonucleotide for binding to Sp-1 protein (fig. 3c), indicating that the −295 mutation disrupted an Sp-1 binding consensus sequence. We conclude that the −295 mutation impairs CTNS transcription enough to constitute this patient’s second severe mutation, accounting for the nephropathic nature of her disease. Impaired transcription due to a 1-bp mutation in a promoter region is unusual, and we know of only one report of an Sp-1 regulatory-element mutation altering transcription—that is, a regulatory-element mutation of the LDL-receptor gene (Koivisto et al. 1994). A northern blot showing drastically reduced CTNS message in patient 1 would provide further confirmation that the promoter mutation is pathological, but the patient’s RNA is not available.
Patients 2 and 3 each have ocular, non-nephropathic cystinosis (Anikster et al. 2000), which results from a combination of a “severe” mutation (e.g., the 57-kb deletion or a nonsense mutation) and a “mild” mutation (e.g., a splicing mutation or a missense mutation not involving a transmembrane region). For both patients 2 and 3, the mild mutation was identified as a 928 G→A substitution, already known to confer a mild cystinosis phenotype when associated with a severe CTNS allele (Anikster et al. 2000). Patients 2 and 3 had no mutations in the coding sequences of their second allele, so we examined the promoter regions of their CTNS. In patient 2, a G→T change at position −303 was found (fig. 2), and, on transfection into HeLa cells, a construct containing this mutation yielded only 5% of the normal CAT reporter activity. Furthermore, a normal oligonucleotide containing nucleotides −327 to −300 formed a complex with NE, whereas a similar oligonucleotide, bearing the −303 G→T mutation, did not bind (fig. 4b). The mutant oligonucleotide also could not compete against the normal oligonucleotide sequence for binding to NE (fig. 4a).
For patient 3, a T insertion was identified after position −303 (fig. 2), and a construct containing this mutation resulted in only ∼16% of normal CAT reporter activity when transfected into HeLa cells. As in the case of the mutation in patient 2, the mutation in patient 3 did not permit either binding to NE (fig. 4c) or competition against the normal binding of a −327 to −300 oligonucleotide (fig. 4a). Nevertheless, the formation of two DNA-protein complexes when we used the normal region, encompassing nucleotides −327 to −300, as probe indicates that this region contains at least two transcription-factor binding sites. Furthermore, the specific mutations in patients 2 and 3 are severe enough to account for some degree of clinical disease—that is, ocular cystinosis; if the promoter mutations were “mild,” there would likely be no clinical consequences detectable.
Just as the cystine transporter has served as the prototypical lysosomal membrane carrier (Gahl et al. 1982, 2001), the CTNS promoter might serve as the prototypical promoter for integral lysosomal membrane proteins; like most TATA-less promoters that serve housekeeping genes, it is GC rich and contains common regulatory elements, such as Sp-1 and AP-4. Remarkably, this promoter region overlaps with that of an adjacent gene. Of greater clinical and diagnostic interest, however, is the finding that several patients with cystinosis have the disease because of mutations in the promoter region of CTNS. This calls for diligence in examination of the CTNS promoter region when mutation analysis is performed on patients with any type of cystinosis.
Acknowledgments
Y.A. is a Howard Hughes Medical Institute Physician Postdoctoral Fellow. Isa Bernardini provided excellent technical assistance.
Electronic-Database Information
The accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CTNS [MIM 219800] and CARKL [MIM 605060])
References
- Ame J-C, Schreiber V, Fraulob V, Dolle P, de Murcia G, Niedergang CP (2001) A bidirectional promoter connects the poly(ADP-ribose) polymerase 2 (PARP-2) gene to the gene for RNase P RNA. J Biol Chem 276:11092–11099 [DOI] [PubMed] [Google Scholar]
- Anikster Y, Lucero C, Guo J, Huizing M, Shotelersuk V, Bernardini I, McDowell G, Iwata F, Kaiser-Kupfer MI, Jaffe R, Thoene J, Schneider JA, Gahl WA (2000) Ocular, non-nephropathic cystinosis: clinical, biochemical and molecular correlations. Pediatr Res 47:17–23 [DOI] [PubMed] [Google Scholar]
- Anikster Y, Lucero C, Touchman JW, Huizing M, McDowell G, Shotelersuk V, Green ED, Gahl WA (1999a) Identification and detection of the common 65-kb deletion breakpoint in the nephropathic cystinosis gene (CTNS). Mol Genet Metab 66:111–116 [DOI] [PubMed] [Google Scholar]
- Anikster Y, Shotelersuk V, Gahl WA (1999b) CTNS mutations in patients with cystinosis. Hum Mutat 14:454–458 [DOI] [PubMed] [Google Scholar]
- Attard M, Jean G, Forestier L, Cherqui S, van’t Hoff W, Broyer M, Antignac C, Town M (1999) Severity of phenotype in cystinosis varies with mutations in the CTNS gene: predicted effect on the model of cystinosin. Hum Mol Genet 8:2507–2514 [DOI] [PubMed] [Google Scholar]
- Aula P, Gahl WA (2001) Sialic acid storage diseases. In: Scriver CS, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th ed. McGraw-Hill, New York, pp 5109–5120 [Google Scholar]
- Charnas LR, Bernardini I, Rader D, Hoeg JM, Gahl WA (1991) Clinical and laboratory findings in the oculocerebrorenal syndrome of Lowe, with special reference to growth and renal function. N Engl J Med 324:1318–1325 [DOI] [PubMed] [Google Scholar]
- Forestier L, Jean G, Attard M, Cherqui S, Lewis C, van’t Hoff W, Broyer M, Town M, Antignac C (1999) Molecular characterization of CTNS deletions in nephropathic cystinosis: development of a PCR-based detection assay. Am J Hum Genet 65:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahl WA, Bashan N, Tietze F, Bernardini I, Schulman JD (1982) Cystine transport is defective in isolated leukocyte lysosomes from patients with cystinosis. Science 217:1263–1265 [DOI] [PubMed] [Google Scholar]
- Gahl WA, Kaiser-Kupfer MI (1987) Complications of nephropathic cystinosis after renal failure. Pediatr Nephrol 1:260–268 [DOI] [PubMed] [Google Scholar]
- Gahl WA, Kuehl EM, Iwata F, Lindblad A, Kaiser-Kupfer MI (2000) Corneal crystals in nephropathic cystinosis: natural history and treatment with cysteamine eyedrops. Mol Genet Metab 71:100–121 [DOI] [PubMed] [Google Scholar]
- Gahl WA, Reed GF, Thoene JG, Schulman JD, Rizzo WB, Jonas AJ, Denman DW, Schlesselman JJ, Corden BJ, Schneider JA (1987) Cysteamine therapy for children with nephropathic cystinosis. N Engl J Med 316:971–977 [DOI] [PubMed] [Google Scholar]
- Gahl WA, Thoene J, Schneider JA (2001) Disorders of lysosomal membrane transport. In: Scriver CS, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th ed. McGraw-Hill, New York, pp 5085–5108 [Google Scholar]
- Kaiser-Kupfer MI, Fujikawa L, Kuwabara T, Gahl WA (1987) Removal of corneal crystals by topical cysteamine in nephropathic cystinosis. N Engl J Med 316:775–779 [DOI] [PubMed] [Google Scholar]
- Kimonis VE, Troendle J, Yang ML, Rose SR, Markello TC, Gahl WA (1995) Effects of early cysteamine therapy on thyroid function and growth in nephropathic cystinosis. J Clin Endocrinol Metab 80:3257–3261 [DOI] [PubMed] [Google Scholar]
- Koivisto U-M, Palvimo JJ, Janne OA, Kontula K (1994) A single-base substitution in the proximal Sp1 site of the human low density lipoprotein receptor promoter as a cause of heterozygous familial hypercholesterolemia. Proc Natl Acad Sci USA 91:10526–10530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markello TC, Bernardini IM, Gahl WA (1993) Improved renal function in children with cystinosis treated with cysteamine. N Engl J Med 328:1157–1162 [DOI] [PubMed] [Google Scholar]
- McGowan-Jordan J, Stoddard K., Podolsky L, Orrbine E, McLaine P, Town M, Goodyer P, MacKenzie A, Heick H (1999) Molecular analysis of cystinosis: probable Irish origin of the most common French Canadian mutation. Eur J Hum Genet 7:671–678 [DOI] [PubMed] [Google Scholar]
- Renlund M, Tietze F, Gahl WA (1986) Defective sialic acid egress from isolated fibroblast lysosomes of patients with Salla disease. Science 232:759–762 [DOI] [PubMed] [Google Scholar]
- Rosenblatt DS, Hosack A, Matiaszuk NV, Cooper BA, Laframboise R (1985) Defect in vitamin B12 release from lysosomes: newly described inborn error of vitamin B12 metabolism. Science 228:1319–1321 [DOI] [PubMed] [Google Scholar]
- Shotelersuk V, Larson D, Anikster Y, McDowell G, Lemons R, Bernardini I, Guo J, Thoene J, Gahl W (1998) CTNS mutations in an American-based population of cystinosis patients. Am J Hum Genet 63:1352–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoropoulos DS, Krasnewich D, Kaiser-Kupfer MI, Gahl WA (1993) Classical nephropathic cystinosis as an adult disease. JAMA 270:2200–2204 [PubMed] [Google Scholar]
- Thoene JG (ed) (1992) Pathophysiology of lysosomal transport. CRC Press, Boca Raton, FL [Google Scholar]
- Thoene J, Lemons R, Anikster Y, Mullet J, Paelicke K, Lucero C, Gahl W, Schneider J, Shu SG, Campbell HT (1999) Mutations of CTNS causing intermediate cystinosis. Mol Genet Metab 67:283–293 [DOI] [PubMed] [Google Scholar]
- Tietze F, Seppala R, Renlund M, Hopwood J, Harper GS, Thomas G, Gahl WA (1989) Defective lysosomal egress of free sialic acid in fibroblasts of patients with infantile free sialic acid storage disease. J Biol Chem 264:15316–15322 [PubMed] [Google Scholar]
- Touchman JW, Anikster Y, Dietrich NL, Braden Maduro VV, McDowell G, Shotelersuk V, Bouffard GG, Beckstrom-Sternberg SM, Gahl WA, Green ED (2000) The genomic region encompassing the nephropathic cystinosis gene (CTNS): complete sequencing of a 200-kb segment and discovery of a novel gene within the common cystinosis-causing deletion. Genome Res 10:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, Callen DF, Gribouval O, Broyer M, Bates GP van’t Hoff W, Antignac C (1998) A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet 18:319–324 [DOI] [PubMed] [Google Scholar]
- Verheijen FW, Verbeek E, Aula N, Beerens CEMT, Havelaar AC, Joosse M, Peltonen L, Aula P, Galjaard H, van der Spek PJ, Mancini GMS (1999) A new gene, encoding an anion transporter, is mutated in sialic acid storage diseases. Nat Genet 23:462–465 [DOI] [PubMed] [Google Scholar]