Abstract
We describe a large family in which a combination of chronic mucocutaneous candidiasis (fungal infections of the skin, nails, and mucous membranes) and thyroid disease segregate as an autosomal dominant trait with reduced penetrance. The family includes (a) four members with both candidiasis and thyroid disease, (b) five members, including one pair of phenotype-concordant MZ twins, with candidiasis only, and (c) three members with thyroid disease only. A whole-genome scan using DNA samples from 20 members of the family identified a candidate linkage region on chromosome 2p. By sampling additional individuals and genotyping supplementary markers, we established linkage to a region of ∼15 cM bounded by D2S367 and D2S2240 and including seven adjacent markers consistent with linkage. With a penetrance estimate of .8, which was based on pedigree and affected status, the peak two-point LOD score was 3.70 with marker D2S2328, and the peak three-point LOD score was 3.82. This is the first linkage assignment of a dominant locus for mucocutaneous candidiasis.
Introduction
Chronic mucocutaneous candidiasis (CMC) is a well-recognized clinical syndrome in which patients exhibit increased susceptibility of skin, nails, and mucous membranes to infections by Candida albicans and dermatophyte fungi, mainly of the genera Trichophyton and Microsporum. Most cases of CMC are sporadic. A classification of CMC syndromes, proposed by Kirkpatrick (2001), is based on the location of infections and on the presence of accompanying clinical findings. Although CMC is often secondary to other medical conditions, such as HIV infection, steroid use, or iron deficiency, primary CMC has been reported to have a genetic basis, either dominant or recessive (MIM 114580 and MIM 212050, respectively) (Wells et al. 1972; Kroll et al. 1973; Sams et al. 1979; Loeys et al. 1999). CMC is often accompanied by endocrine or inflammatory disorders, suggesting dysregulation of the immune system. The occurrence and age at onset of fungal infections and other features is variable, even among affected members of a family. Moreover, genetic analysis has not been facilitated by laboratory evaluations; patients with CMC often lack immunologic abnormalities that can be readily diagnosed by routine laboratory tests, although skin tests and in vitro proliferation and cytokine production in response to candida antigens tend to be low or absent.
Starting with the first documented report in the literature (Thorpe and Handley 1929), a high prevalence of endocrinopathies has been noted in patients with CMC (Thorpe and Handley 1929; Sutphin et al. 1943; Whitaker et al. 1956). To date, the only gene associated with CMC, AIRE (autoimmune regulator), is the disease gene for the autosomal recessive syndrome autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, or APECED (MIM 240300) (Ahonen 1985; Nagamine et al. 1997; The Finnish-German APECED Consortium 1997). Although patients with APECED may exhibit a wide range of endocrinopathies, they typically develop hypoparathyroidism and/or adrenal failure. Patients with hyper-IgE syndrome, which can be inherited in autosomal dominant fashion, have also been shown to exhibit fungal infections of the oropharynx and nails, suggesting that this form of immune deficiency is also associated with CMC (Van Scoy et al. 1975; Grimbacher et al. 1999a, 1999b). However, endocrinopathies are not a common feature of hyper-IgE syndrome.
The present article describes a family in which CMC is associated with thyroid disease. The family includes (a) four members with both candidiasis and thyroid disease, including one with the uncommon condition Riedel thyroiditis; (b) five members, including a pair of phenotype-concordant MZ twins, with candidiasis only; and (c) three members with thyroid disease only. Under a model of autosomal dominant inheritance, there are also two nonpenetrant obligate carriers in the family, as well as young children at risk for development of disease, for whom genetic linkage–based diagnosis would confer potential benefit. We established linkage of CMC and/or thyroid disease in this family to a 15-cM interval on chromosome 2p.
Subjects and Methods
Subjects
During the past 3 decades, members of a large nonconsanguineous white family have received, at the University of Alabama Medical Center, medical management for candidiasis and thyroid disease. A partial pedigree is shown in figure 1. Members of the family are not part of a subpopulation recognized to have increased incidence of immune disorders or thyroid disease. The original probands—the affected father (III-8) and grandmother (II-4)—were first reported by Montes et al. (1971). Subject III-8 has severe CMC and was reported as “patient 2” in a trial of oral amphotericin B administration (Montes et al. 1971). His mother (II-4), deceased after developing two separate squamous cell carcinomas of the face and polycythemia vera, had had lifelong, severe CMC and required thyroidectomy at age 30 for euthyroid goiter with no anti-thyroid antibodies. For the present study, 36 members of the extended family enrolled with informed consent in a study approved by the Institutional Review Board of the National Institutes of Health. Histories, focused physical examinations, and blood samples for DNA analysis were obtained for all enrollees. Subjects were classified as having CMC either by direct examination or by report of family members. The skin and nail involvement with candida are illustrated in figure 2. Other than two subjects with obvious thyroid enlargement as well as elevated thyroid-stimulating hormone (TSH) levels, the diagnosis of thyroid disease was based on a history of diagnosis by a physician and/or documented laboratory values.
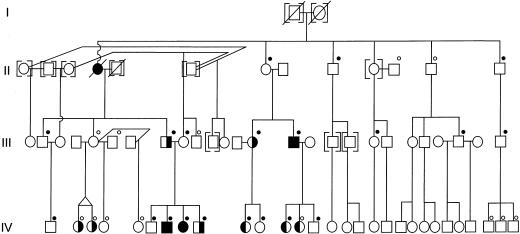
Figure 1.
Partial pedigree segregating CMC (symbols with right half blackened) and thyroid disease (symbols with left half blackened), showing subjects contributing DNA for both phases of analysis (black dots above symbols) or for fine mapping only (white dots above symbols). Individuals for whom CMC was unknown are denoted by symbols enclosed within square brackets. Generation V (not shown) contained four children who were too young for phenotype assignment but whose DNA was genotyped. There was anecdotal evidence that one or more spouses of III-5 were related to individuals in generation II, but we were unable to confirm the relationship(s). Therefore, the father of twins IV-2 and IV-3, the father of IV-4, and the father of IV-5 were designated as unaffected, ungenotyped men with a low likelihood of carrying the disease allele.
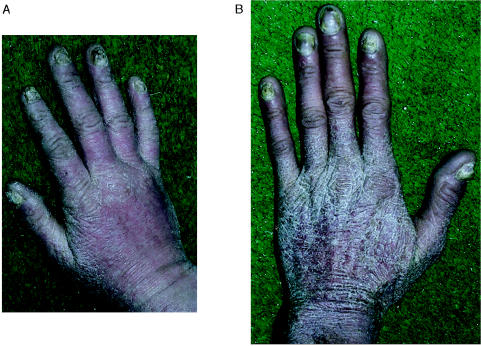
Figure 2.
Candida infection of the skin and nails in subjects IV-7 (left) and III-8 (right)
Additional studies of thyroid and immunologic status were obtained from members of one branch of the family. The four siblings—IV-6, IV-7, IV-8, and IV-9—have been followed, since early childhood, at Children’s Hospital of Alabama. Three of these children have had severe candida infections of the skin, nails, and mucous membranes, with variable responses to chronic administration of oral and topical antifungal antibiotics. Cultures of their lesions have yielded isolates of C. albicans and, in one instance, Trichophyton species. After sustaining a burn injury, subject IV-9 developed respiratory failure and agranulocytosis. Although he recovered and underwent successful skin grafts after intravenous antibiotic and antifungal treatment, his course may have been complicated by candida superinfection. No other instance of invasive candidal disease in the family was documented. Subjects IV-7 and IV-8 had elevated levels of thyroid-stimulating hormone (TSH)—36 and 10.3 μIU/ml, respectively (normal values 0.36–5.8 μIU/ml). The TSH value in IV-7 normalized after treatment with levothyroxine. This child was negative for thyroid peroxidase and anti-thyroglobulin antibodies, but an ultrasound of the thyroid revealed multiple cysts, the largest measuring 0.5 cm in diameter. Subject IV-7 has also had numerous warts on the extremities.
Others in the family were diagnosed with hypothyroidism, including subject III-14, a man who, in addition to fungal infections of the skin, mouth, and nails was diagnosed with Riedel thyroiditis, a fibrosing, invasive thyroid disease usually seen in older women (Zimmermann-Belsing and Feldt-Rasmussen 1994). No family member has been diagnosed with hypoparathyroidism, adrenal failure, or other endocrinopathy.
Immunologic Tests
Skin-test reactivity was assessed by intradermal injection of 0.1 ml of standard antigens. Serum immunoglobulin levels, isohemagglutinins, and cell-surface phenotyping by flow cytometry were performed at the Children’s Hospital of Alabama. Mononuclear cells were isolated, by standard methods, over Ficoll-Hypaque gradients and were cultured either with medium alone, with mitogens phytohemagglutinin (PHA) (Life Sciences), concanavalin A (ConA) (Sigma) or pokeweed (PWM) (Life Sciences, Inc), or with either soluble antigen extract from candida (Greer Laboratories) or preservative-free tetanus toxoid (Wyeth-Ayerst) (James 1994). For proliferation studies, cells were cultured in RPMI 1640 medium, either with 10% human AB serum alone or with PHA or ConA, for 3 d; cultures with medium, PWM, tetanus, and candida were held for 6 d. For the last 6 h of culture, 3H-thymidine was added; cells then were harvested, and incorporated 3H was measured (James 1994). After 3 d, interferon (IFN)-γ secretion into culture supernatants in response to PHA was analyzed with a flow-cytometry bead–assay kit (BioErgonomics). IFN-γ and tumor-necrosis factor (TNF)-α production were also measured 6 d after plating of 106 cells in 24-well flat-bottomed tissue plates in 1 ml RPMI 1640 with 10% fetal bovine serum and antibiotics with or without either tetanus toxoid (10 μg/ml) or candida antigen (320 μg/ml). After incubation, the supernatants were removed, filtered, and analyzed for concentrations of cytokines, by commercial enzyme-linked immunosorbent–assay kits (Endogen and R&D Systems, for INF-γ and TNF-α, respectively).
Marker Selection and Genotyping
Both the genotyping and DNA-sample ascertainment were done in two stages. In the first stage, 20 family members were genotyped at 392 autosomal markers and at X and Y markers (Center for Inherited Disease Research; K. Doheny, personal communication). Customized fluorescent primers and PCR conditions were used, and data were collected by an ABI 377 sequencer using GENOTYPER software (PE Applied Biosystems) (K. Doheny, personal communication). In the second stage, performed in the laboratories of B.G. and J.M.P., 16 additional family members were included. After enrollment of the second set of participants, one individual was dropped from analysis because of uncertainty about his biological relationship to the family, and the affected identical twins were counted as a single meiosis. Thus, genotypes of 34 individuals were analyzed. The markers with high heterozygosity and reliability for fine mapping that were selected included some that had been genotyped in previous linkage studies for unrelated phenotypes mapped to chromosome 2p (Sarfarazi et al. 1995; Bejjani et al. 1998; Patel et al. 1998; Xiao et al. 2000). Fluorescent primers purchased from Life Sciences were used according to Research Genetics' Genome Services Protocol. Genotypes were obtained for all study participants, both at seven original markers spanning a candidate region on chromosome 2p and at additional 2p markers. In this second stage, genotypes at 19 of 21 selected marker loci were determined, including 7 from the genome scan and 12 additional loci. The chromosome 2p markers analyzed, as well as their positions on the Marshfield genetic map (Center for Medical Genetics, Marshfield Medical Research Foundation; also see Broman et al. 1998), are detailed in table 1. The PCR-product lengths described as alleles (below) are those produced by these primer pairs.
Table 1.
Chromosome 2p Markers Used in Linkage Analysis.[Note]
|
Primer Sequence(5′→3′) |
|||
| Marker | Sex-Averaged Distance from 2p Telomere(cM) | Forward | Reverse |
| D2S2207 | 20.57 | CAAGACACCTCTGGTCAGATGCC | TGCACCACTGAGTTCCAGCC |
| D2S1400a | 27.6 | CAAGACACCTCTGGTCAGATGCC | TGCACCACTGAGTTCCAGCC |
| D2S131 | 31.2 | GTATAGGAGCCACACCCCTG | TTTACTGCTGAGACAACCCA |
| D2S1360a | 38.33 | TAACCTTGTGAGCCAATTCC | CAAAACAGAAACAGAACTAATAAGA |
| D2S320 | 38.33 | CAGCCCAGCCATTGATATTT | AACCAACAACTATGCTAGAGATTCC |
| D2S405a | 47.97 | GAACAGGATGGGGAAAACTT | CAGAAGCGTGGCAGTTTATT |
| D2S352 | 50.65 | CTACAGGGCTTCAGCATCC | GCAAAGTCGTTCTCAGGTG |
| D2S367 | 54.96 | AGCTTCTTGTTCACAGGTGT | TTCTTTGGTCTAAGGGTCAC |
| D2S1325 | 54.96 | TATGCATGTATGTGTGTATGTATGC | TTGCTGCAGACCTGTATGAA |
| D2S1788a | 55.51 | AATGGATGGACAAATGGATG | CCCTCCATAATTAGATGAGCC |
| D2S2328 | 61 | CTTTGGCAAACGAGCG | CCGAGCAATTTCACTCTGG |
| D2S1356a | 64.29 | TTCTTGGGCTCCAGGATG | AGGGCAAAACCGCATCTCTA |
| D2S414 | 64.84 | TACTGACTTCTCTGCTCAGAACA | AGCAAAATTAAACTTGGGATATT |
| D2S119 | 65.39 | GAGAATCCCTCAATTTCTTTGGA | CTTGGGGAACAGAGGTCATT |
| D2S2174 | 67.58 | GACGGGTTAGACTCCTGC | TCTTAGAGATGCCCCCA |
| D2S2240 | 69.77 | GGTGTTACTAAACATCCACAGAG | GCGTTGCCCAAAAGAG |
| D2S2739a | 73.61 | CTGGTGTTTAGAATATCTGTCTAGG | ATCTGTTATTCACTTTAACTGAGGC |
| D2S337 | 80.69 | TGCTCCTGGCTTATTTCA | GTCTGCATTCCCACGA |
| D2S441a | ∼88 | AAAAGGCTGTAACAAGGGCT | ATTGGAGCTAAGTGGCTGTG |
Note.— Not included in this table are D2S2221 (at 44.09 cM), which was dropped because of difficulty with PCR and location outside the ultimate region of interest, and D2S2298 (at 65.94 cM), which was dropped because of “stuttering,” producing difficulty in allele assignment.
Used for initial genome scan.
Linkage Analysis
LOD scores were calculated by the software package FASTLINK, version 4.1P (Lathrop and Lalouel 1984; Cottingham et al. 1993; Schäffer et al. 1994). Some multipoint runs were done in parallel (Gupta et al. 1995), with the aid of the p4 parallel-programming software library (Butler and Lusk 1994) on a shared-memory–multiprocessor computer. Loops in the pedigree were broken by Becker et al.'s (1998) method. Inconsistencies in the genotype data were detected by the PedCheck program (O'Connell and Weeks 1998), and 10 (individual, marker) pairs were zeroed out in the genome-scan marker data, in addition to all the pairs zeroed out in the laboratory.
For every marker in the genome scan, two-point (i.e., marker and disease) LOD scores were computed by the MLINK program. In the preliminary analysis, we used equal allele frequencies for the markers and disease-allele frequencies of .00001, for the dominant models, and .005, for a recessive model. A low disease-allele frequency was chosen to maintain a very low relative likelihood for scenarios in which a disease allele entered the family more times than would be necessary to account for all affected subjects. We analyzed all markers by three dominant models and by the recessive analog of the first dominant model (table 2). Models 2 and 3 were chosen to avoid missing any relevant chromosomal regions. The values of .20 and .80 in the second model imply a high degree of diagnostic uncertainty with regard to unaffected subjects. Model 3 assigns a very low penetrance to unaffected subjects. The use of multiple simple screening models is in the spirit of the proposal presented by Abreu et al. (1999)—that is, to screen with a dominant model with 50% penetrance and with a recessive model with 50% penetrance; however, their two generic models are not well suited to our study, in which there was both a rare (i.e., CMC) and a more common (i.e., thyroid) manifestation of the same disease. Moreover, the presence of six parent-to-child transmissions strongly favored a dominant model over a recessive model (fig. 1). A recessive model was considered in order to formally rule out variants of APECED, which is recessive, and because the seminal article on families with CMC (Wells et al. 1972) has given the impression that the primary mode of inheritance might be recessive.
Table 2.
Models Used in Initial Marker Analysis
|
Proportion of Individuals |
||
| Group | Homozygous for Nondisease Allele | Carrying at Least One Disease Allele |
| Model 1: | ||
| Founders who married nonfounders | 0 | 1.0 |
| Unaffected founder couples and unaffected nonfounders: | ||
| Examined | .01 | .99 |
| Not examined | .03 | .97 |
| Individuals with CMC and/or thyroid disease | .01 | .99 |
| Model 2: | ||
| Founders who married nonfounders | 0 | 1.0 |
| Unaffected founder couples and unaffected nonfounders | .20 | .80 |
| Individuals with CMC | .01 | .99 |
| Individuals with thyroid disease only | .05 | .95 |
| Model 3: | ||
| Founders who married nonfounders | 0 | 1.0 |
| Unaffected founder couples and unaffected nonfounders | 0 | .33 |
| Individuals with CMC | 0 | 1.0 |
| Individuals with thyroid disease only | 0 | .33 |
Only a single region of the genome was consistent with linkage. Varying the parameters for that region showed that the following precautions were all unnecessary for study of this family:
-
1.
distinguishing between documented and merely reported unaffected status;
-
2.
allowing for a phenocopy rate in affected individuals;
-
3.
distinguishing between CMC and thyroid disease in the second model;
-
4.
assigning a high degree of diagnostic uncertainty to unaffected individuals in the second model.
For all regions with a marker showing a two-point LOD score >1.5 or for any two consecutive markers with scores >1.0, we performed three-point analysis, using LINKMAP.
For the fine-map analysis of 2p markers, with 34 genotyped subjects, disease-allele frequency was assigned the value .00001. Marker-allele frequencies were estimated by the ILINK program in FASTLINK, with marker-genotype data only (i.e., without phenotype data). We used an autosomal dominant model of inheritance (table 3), with three penetrance classes.
Table 3.
Model Used in Fine Mapping
|
Proportion of Individuals |
||
| Group | Homozygous for Nondisease Allele | Carrying at Least One Disease Allele |
| Founders who married nonfounders | 0 | 1.0 |
| Unaffected founder couples and unaffected nonfounders | 0 | .8 |
| Individuals with CMC and/or thyroid disease | 0 | 1.0 |
Both the analysis of the partial data in the first phase and the fact that 8 of the 11 affected individuals had the rare, CMC phenotype suggested that inclusion of a phenocopy rate would unnecessarily complicate the basic analysis. Since thyroid disease without CMC is not so rare and may be hard to diagnose, we did consider a modified model in which the three individuals with thyroid disease only (i.e., without CMC) were assigned a phenocopy rate of .02 and in which all the unaffected and non–married-in individuals were assigned a diagnostic uncertainty of .01. The penetrance, .8, was estimated according to established principles summarized by Ott (1999). Of 23 offspring of either affected individuals or obligate nonpenetrant carriers, 11 were affected. We added 0/1 for the founder couple in generation I, to get a fraction of 11/24. The simplest possible penalty for ascertainment biased toward affected individuals is subtraction of 1 from both numerator and denominator, but, in this situation, since the cases of an affected mother and son had already been published (Montes et al. 1971), we subtracted 2. Then we multiplied the numerator by 2 (because half the time the nondisease allele is transmitted), to get a penetrance estimate of 18/22, or .8.
Since ascertainment may have been biased toward branches of the family with affected individuals, we assessed the robustness of the .8 estimate by varying it in the two-point LOD-score calculations (see the “Genetic Linkage” subsection, in Results). For each pair of markers consistent with linkage at a recombination fraction (θ) of 0, a three-point analysis with the LINKMAP program of FASTLINK 4.1P was performed to verify the extent of the linked chromosomal interval. Most-likely haplotypes were calculated by SIMWALK, version 2.82 (Sobel and Lange 1996).
Results
Immunologic Evaluations
Diverse patterns of abnormalities have been noted among subjects with CMC (Kirkpatrick 2001). Immunologic data for the family that we studied are summarized in table 4. In subjects II-4 and III-8, cell-mediated immunity had been assessed by skin testing many years ago, revealing anergy to candida and all other antigens tested (Montes et al. 1971; T. P. Atkinson, unpublished data). In contrast, skin reactivity to candida was more recently positive (∼20 mm induration) in affected siblings IV-7, IV-8, and IV-9 (data not shown). However, these children had no reactions to intradermal challenge by tetanus, mumps, or trichophyton, although all three had received the usual tetanus and mumps vaccinations and one had been culture positive for Trichophyton species.
Table 4.
Immunologic Data
|
Statusa |
Immunoglobulin |
In Vitro Responses of Proliferation/INF-γ/TNF-α in Response toa,b |
|||||||
| Subject (Age [years]) | CMC | Thyroid Disease | Candida Skin Test | IgMc(mg/dl) | IgG(mg/dl) | IgA(mg/dl) | Mitogensd | Tetanus | Candida |
| II-4 (55) | + | + | − | 54 | 1,675 | 390 | ND | ND | ND |
| III-8 (22) | + | − | − | 51 | 1,405 | 192 | ND | ND | ND |
| III-9 (33) | − | − | ND | 80 | 954 | 104 | +/+/ND | +/+/+ | +/+/+ |
| IV-6 (12) | − | − | + | 37 | 851 | 47 | +/−/ND | +/+/+ | −/+/+ |
| IV-7 (11) | + | + | + | 29 | 938 | 41 | +/−/ND | −/−/+ | −/+/+ |
| IV-8 (10) | + | + | + | 37 | 1,003 | 71 | +/−/ND | −/−/+ | −/+/+ |
| IV-9 (8) | + | − | + | 34 | 1,039 | 118 | +/−/ND | −/−/+ | −/+/+ |
+ = Present; − = absent.
For INF-γ and TNF-α, data are for production in culture supernatants. ND = not done.
Mean ± SD normal IgM levels are as follows: 3–5 years, 56 ± 18; 6–8 years, 65 ± 25; 9–11 years, 79 ± 33; 12–16 years, 89 ± 20; adult, 99 ± 27 (Stiehm and Fudenberg 1966). Values >1 SD below the mean for age are underlined.
PHA, ConA, and PWM.
Flow-cytometric studies of lymphocyte subpopulations in peripheral blood samples from subject III-8 that were obtained years apart revealed a decreased percentage of CD8+ T cells, producing a CD4:CD8 ratio of 5–6 (normal ratio 2 ± 0.5). Reduced numbers of peripheral blood T cells, 889/mm3, along with an elevated CD4:CD8 ratio, were also observed in his mother, II-4. However, the affected children of III-8 had normal numbers and percentages of CD4+ and CD8+ T cells and B cells (not shown).
All the children of III-8, including the unaffected child, IV-6, had serum IgM levels that were ⩾1 SD below the mean for age; in those sampled more than once, the values appeared to be decreasing (table 4). Total IgG levels of those affected with CMC were normal to mildly elevated, and IV-7 and IV-8 had mild elevations of IgE (normal <50 IU/ml). Isohemagglutinins were present despite the reduced levels of IgM (not shown). Previous evaluations had documented positive antibody titers to C. albicans in III-8 but not in his mother II-4. Both had positive antibody responses to immunization with Salmonella typhi polysaccharide vaccine.
Proliferative responses to mitogens and antigens were studied in affected siblings IV-7, IV-8, and IV-9, in healthy subject IV-6, and in their mother, who had married into the family. Mitogen responses were all normal, but the responses to tetanus challenge in the three affected children were undetectable, despite prior immunization (table 4). Proliferative responses to candida are positive in only approximately half of our laboratory controls. In this family, positive proliferation, indicating specific immunity to candida, occurred only in affected subject IV-7 and in his mother.
PHA-induced IFN-γ production was undetectable in the healthy sibling (IV-6) and in the affected siblings (IV-7, IV-8, and IV-9), although their mother and laboratory controls had positive responses. Antigen-induced IFN-γ and TNF-α production by the affected children’s peripheral blood mononuclear cells in response to high dose C. albicans antigen was robust, but they produced no detectable IFN-γ and relatively normal levels of TNF-α in response to tetanus toxoid. Thus, both the patients’ proliferative responses and their cytokine responses suggested some ability to respond to candida but inconsistent or low responsiveness to some of the other indicators of intact cellular immunity. The in vitro responses of subjects IV-6, IV-7, IV-8, and IV-9 were correlated with their responses to candida and tetanus skin tests, in contrast to the anergy seen in skin testing of their father and paternal grandmother some years earlier.
Genetic Linkage
In the genome-scan two-point analysis, we found regions worthy of follow-up on chromosomes 2, 4, and 17 in at least one dominant model and on chromosome 1 and 11 under a recessive model. The region on chromosome 2 had five consecutive markers—D2S1360-D2S405-D2S1788-D2S1356-D2S2739—spanning >30 cM, with positive scores in the first dominant model and scores >1.0 in the second and third dominant models (table 2). In models 2 and 3, the outside markers D2S1360 and D2S2739 had scores >2.0. Three-point analysis using all pairs of the five markers increased the peak LOD score in the region. In contrast, the other four regions considered had three or fewer adjacent markers with positive scores, no markers with scores >2.0, and three-point LOD scores that were lower than two-point LOD scores. Therefore, we proceeded with fine mapping of only the region on chromosome 2, using additional family members and more markers to extend the analysis.
In the family that we studied, linkage of CMC to AIRE on chromosome 21 was investigated but not confirmed. In the genome scan, the two markers flanking AIRE had two-point LOD scores <−2.0 for θ⩽.07 (complete data not shown). This was not surprising, since the inheritance pattern for AIRE defects in APECED is recessive, whereas the inheritance pattern in the family that we studied appeared to be dominant. Furthermore, the endocrinopathies in the family that we studied were distinct from those in APECED.
Table 5 shows the two-point LOD scores for 19 markers on chromosome 2 when the parameters described for the second phase are used. The markers are listed in map order along the chromosome, except that the order of D2S1360 and D2S320 is uncertain. Although the Marshfield map (Center for Medical Genetics, Marshfield Medical Research Foundation) is also uncertain about the relative order of D2S367 and D2S1325, our data suggest that D2S367 is closer to 2p-ter. All genotyped, affected individuals had the same allele at D2S1325, but, at D2S367, the affected MZ twins did not share an allele with the other affected individuals. The two-point LOD scores at the seven markers defining the linked haplotype varied, primarily because of differences in marker informativeness. Markers D2S1788, D2S2328, and D2S1356 had 9–10 distinct alleles in this family, whereas the other four markers with positive scores at θ=0 had only five or six alleles. Because thyroid disease is not rare in the general population, we explored a modified model in which the three individuals with only thyroid disease were assigned a phenocopy rate of .02 and in which all the unaffected and non–married-in individuals had a diagnostic uncertainty of .01. Under this model, the positive two-point LOD scores shown in table 5 decreased by ⩽0.05: for D2S1788, the LOD score at θ=0 decreased from 3.31 to 3.27; for D2S2328, the LOD score at θ=0 decreased from 3.70 to 3.66; for D2S1566, the LOD score at θ=0 decreased from 3.23 to 3.20.
Table 5.
Two-Point LOD Scores for Markers on Chromosome 2
|
LOD Score When θ between Disease and Marker = |
|||||||
| Marker | 0 | .01 | .03 | .05 | .07 | .10 | .15 |
| D2S2207 | −17.94 | −2.92 | −1.87 | −1.37 | −1.03 | −.69 | −.33 |
| D2S1400 | −6.11 | −2.31 | −1.35 | −.91 | −.62 | −.34 | −.05 |
| D2S131 | −16.34 | −5.18 | −3.73 | −2.96 | −2.43 | −1.86 | −1.22 |
| D2S1360 | −5.05 | −1.29 | −.70 | −.39 | −.18 | .05 | .27 |
| D2S320 | −.12 | −.05 | .06 | .15 | .22 | .30 | .37 |
| D2S405 | −1.09 | −.99 | −.84 | −.71 | −.60 | −.48 | −.32 |
| D2S352 | −4.01 | −1.51 | −.95 | −.66 | −.46 | −.25 | −.03 |
| D2S367 | −1.13 | 1.81 | 2.15 | 2.24 | 2.25 | 2.20 | 2.01 |
| D2S1325 | 1.14 | 1.14 | 1.13 | 1.11 | 1.09 | 1.04 | .94 |
| D2S1788 | 3.31 | 3.26 | 3.17 | 3.07 | 2.97 | 2.80 | 2.50 |
| D2S2328 | 3.70 | 3.65 | 3.54 | 3.42 | 3.30 | 3.11 | 2.77 |
| D2S1356 | 3.23 | 3.19 | 3.09 | 2.99 | 2.88 | 2.71 | 2.40 |
| D2S414 | 1.83 | 1.81 | 1.76 | 1.72 | 1.66 | 1.57 | 1.39 |
| D2S119 | 1.65 | 1.64 | 1.61 | 1.58 | 1.54 | 1.48 | 1.35 |
| D2S2174 | 2.58 | 2.56 | 2.52 | 2.47 | 2.41 | 2.29 | 2.07 |
| D2S2240 | −3.14 | −1.52 | −1.03 | −.81 | −1.67 | −.54 | −.43 |
| D2S2739 | −1.29 | .32 | .77 | .97 | 1.08 | 1.17 | 1.20 |
| D2S337 | −9.05 | −.27 | .20 | .41 | .53 | .64 | .71 |
| D2S441 | −∞ | −4.35 | −2.89 | −2.21 | −1.76 | −1.29 | −.79 |
In the interval from D2S1325 through D2S2174, all affected individuals shared an allele, producing positive two-point LOD scores at θ=0 (table 5). We computed most-likely haplotypes by using marker data only. With different marker sets and random seeds, SIMWALK consistently predicted that the six-marker haplotype D2S1788 (190 bp)–D2S2328 (148 bp)–D2S1356 (241 bp)–D2S414 (215 bp)–D2S119 (225 bp)–D2S2174 (280 bp) segregated in all affected individuals. III-5, IV-2, and IV-3, the individuals whose genotypes defined the boundaries of the linked interval, were ascertained in the second, fine-mapping phase, explaining why the linked interval appeared to be larger when only the initial 20 DNA samples were used in the genome scan. The haplotype shared among affected subjects was used to make a genetic diagnosis for three infant offspring of family members who have either CMC or thyroid disease (not shown).
A penetrance of .8 for the disease allele was estimated on the basis of data on the family (see the “Linkage Analysis” subsection of Subjects and Methods) but may be inaccurate. To determine the effect that penetrance had on our linkage analysis, we recalculated the two-point LOD scores at θ=0, varying the penetrance from .5 to .9 (table 6). As shown, the penetrance value giving the highest LOD score was not uniformly .8 but, instead, varied from marker to marker. For each of the penetrance values used, a least two and as many as four markers had LOD scores >3.0.
Table 6.
Two-Point LOD Scores at Varying Penetrance Values
|
LOD Score at Penetrance = |
|||||
| Marker (Location [cM]) | .5 | .6 | .7 | .8 | .9 |
| D2S1325 (54.96) | 1.79 | 1.66 | 1.47 | 1.14 | .45 |
| D2S1788 (55.51) | 3.33 | 3.37 | 3.36 | 3.31 | 3.12 |
| D2S2328 (61.00) | 3.70 | 3.75 | 3.75 | 3.70 | 3.52 |
| D2S1356 (64.29) | 3.37 | 3.40 | 3.36 | 3.23 | 2.84 |
| D2S414 (64.84) | 2.20 | 2.15 | 2.04 | 1.83 | 1.35 |
| D2S119 (65.39) | 2.23 | 2.10 | 1.93 | 1.65 | 1.16 |
| D2S2174 (67.58) | 3.30 | 3.20 | 2.99 | 2.58 | 1.67 |
The sensitivity-testing method of Hodge and Greenberg (1992) was used to evaluate the effect of possible misclassification on the LOD score. Using a penetrance estimate of 75% (to approximate what the estimate would have been with one fewer affected individuals), we changed the status of each of the 11 affected individuals (the twins counting as one) to unaffected and repeated the two-point analysis for the highest-scoring marker, D2S2328. When the disease status of none of the affected individuals was changed, the LOD score was 3.73. Changing one individual at a time gave the following LOD scores: 3.73 (II-4), 3.73 (III-8), 3.73 (III-13), 3.73 (III-14), 3.24 (IV-2), 3.03 (IV-6, IV-7, or IV-8), 3.07 (IV-10), 2.90 (IV-13), and 3.07 (IV-14). The effect of misdiagnosing any of the four affected individuals in generations II and III was negligible because, having had affected descendants, they were expected to carry the disease-associated allele. The effect of misdiagnosing the twin (IV-2) was less severe than the effect of misdiagnosing other individuals in generation IV, because, for purposes of the linkage analysis, IV-3 was ignored, and thus IV-2 had no other affected individuals in her nuclear family. The effect of misdiagnosing IV-13 was the most severe, because IV-13 has a child carrying the disease-associated allele whose disease status in the analysis was classified as unknown.
Three-point linkage analysis showed positive LOD scores when all 21 pairs of markers among D2S1325, D2S1788, D2S2328, D2S1356, D2S414, D2S119, and D2S2174 were used. Scores for the more informative marker pairs are shown in table 7. The highest three-point score, 3.82, was achieved, not surprisingly, with the two highly informative markers that were farthest apart. These three-point LOD scores suggest that no subinterval of the linked marker interval should be favored as likely to contain the locus conferring susceptibility to CMC and/or thyroid disease.
Table 7.
Three-Point Linkage Analysis for Informative Markers
|
LOD Score |
||||
| UpperMarker | LowerMarker | At Upper Marker | At Lower Marker | Maximum |
| D2S1325 | D2S2328 | 2.90 | 3.16 | 3.16 |
| D2S1325 | D2S414 | 2.68 | 3.05 | 3.05 |
| D2S1325 | D2S2174 | 2.41 | 3.03 | 3.03 |
| D2S1788 | D2S2328 | 3.21 | 3.57 | 3.57 |
| D2S1788 | D2S1356 | 3.82 | 3.77 | 3.82 |
| D2S1788 | D2S119 | 3.54 | 3.19 | 3.54 |
| D2S1788 | D2S2174 | 3.21 | 2.96 | 3.21 |
| D2S2328 | D2S1356 | 3.27 | 3.32 | 3.32 |
| D2S2328 | D2S414 | 3.15 | 3.02 | 3.15 |
| D2S2328 | D2S119 | 3.33 | 3.09 | 3.33 |
| D2S2328 | D2S2174 | 3.18 | 3.07 | 3.18 |
| D2S1356 | D2S414 | 3.06 | 2.75 | 3.06 |
| D2S1356 | D2S119 | 3.15 | 3.06 | 3.15 |
| D2S1356 | D2S2174 | 3.09 | 2.95 | 3.09 |
| D2S414 | D2S2174 | 3.02 | 3.08 | 3.08 |
Discussion
We have presented a large family with an autosomal dominant form of CMC associated with thyroid disease, and we have localized the underlying gene defect to chromosome 2p. This is the first linkage assignment for autosomal dominant CMC. The characteristics of the disorder in the family that we studied are different, in several respects, from those of APECED, the only CMC-associated disorder for which a gene has been identified. Although both conditions have the common findings of CMC and endocrinopathy, APECED is well established as an autosomal recessive disorder, and no variants with autosomal dominant transmission are known. In addition, patients with APECED typically present with adrenal failure and hypoparathyroidism, endocrine disorders not seen in the family that we studied. Finally, APECED is due to mutations in AIRE, located on chromosome 21q22.3, a region that, in the family that we studied, did not show segregation with disease. In this family, we established genetic linkage to a 15-cM region on chromosome 2p, bounded by markers D2S367 and D2S2240. Data on more families segregating CMC/thyroid disease could make possible the identification of critical recombination events to narrow the linkage interval.
Published studies suggest that autosomal dominant CMC (MIM 114580) may be common among patients who do not have APECED. A family reported by Sams et al. (1979) and Jorizzo et al. (1980) demonstrated autosomal transmission of CMC, with no recognized endocrinopathy, in three children and four grandchildren of an affected subject. In another family, an 11-year-old boy, his sister, and her daughter had CMC without reported endocrine abnormalities (Canales et al. 1969). Loeys et al. (1999) reported a 5-year-old child with CMC and invasive fungal disease (a mycotic aneurysm) who had an affected father and an affected paternal grandmother. The syndrome of the family that we studied, in which thyroid disease is frequent, appears to be distinct from autosomal dominant CMC assigned to MIM 114580, which is characterized by a lack of associated endocrinopathy.
An association between thyroid disease and CMC has been recognized in familial CMC. Kroll et al. (1973) described a mother with CMC whose son had CMC and previously undiagnosed hypothyroidism. In a review of 43 patients with CMC who were from eight centers, Herrod (1990) noted three instances of apparent dominant transmission: an affected grandmother, mother, and son (the latter of whom had hypothyroidism); an affected father-daughter pair; and an affected father and three siblings. Herrod stated that, in the cases with dominant inheritance, endocrinopathy appeared to be uncommon, although this may reflect the high incidence of endocrinopathy in families with recessive APECED that were reported in his review. In a recent case report, Steensma et al. (2000) documented autosomal dominant CMC in which the proband, a 27 year-old man, was also found to have subclinical hypoparathyroidism and hypogonadism.
Other series and case reports document the occurrence of CMC and hypothyroidism without adrenal or parathyroid dysfunction (Papazian and Koch 1960; Montes et al. 1971; Kirkpatrick and Smith 1974). Hypothyroidism constitutes only ∼6% of endocrine abnormalities seen in APECED, and almost all patients with hypothyroidism and APECED have hypoparathyroidism and/or adrenal failure as well (Ahonen et al. 1990). An isolated patient with CMC and hypothyroidism, especially one from a family with multiple affected individuals other than siblings, is unlikely to have a mutation in the AIRE gene. Thus, although less well recognized than APECED, dominant CMC, sometimes associated with thyroid disease, is a distinct clinical entity.
Immunologic evaluation did not reveal a uniform pattern of abnormalities in the family that we studied, although in aggregate the studies suggested the possibility of mild T-cell and B-cell dysfunction in those affected with CMC. The most consistent feature was depressed serum concentration of IgM. Although Gill and Portnoy (1989) reported the development of panhypogammaglobulinemia in a patient with CMC and hypothyroidism, low serum IgM has not previously been a notable abnormality in immunologic studies of CMC (Herrod 1990). Low IgG, low IgA, and selective IgA deficiency have been separately identified in patients with CMC (Herrod 1990; Lilic et al. 1996) but were not documented in the family that we studied. One reported patient with CMC and hypothyroidism had severe IgG-subclass deficiencies and deficient anti-polysaccharide antibody responses, resulting in the development of bronchiectasis (Brägger et al. 1989). It is not clear whether antibody deficiencies in CMC are part of a primary immunologic disorder or perhaps a consequence of the long-term immunosuppressive effects of chronic fungal infections.
As has been demonstrated with most other patients with CMC, those in the family that we studied exhibited aberrant T-cell responses. The majority of patients with CMC manifest cutaneous anergy to candida antigen and, in many cases, to other antigens as well (Herrod 1990). Although, by skin testing, the patients in generations II and III whom we tested were anergic to other delayed-type antigens (in parallel to their poor responses to tetanus in vitro), there was a curious disparity between the children in generation IV and their father and paternal grandmother. The younger patients manifested robust delayed-type–hypersensitivity responses to candida antigen, which mirrored their positive cytokine production in vitro, whereas the adults were anergic to all intradermally administered antigens tested. These differences may be a explained by a progressive deterioration of T-cell responses with increasing age in patients with CMC, as has been suggested by others (Sams et al. 1979). Alternatively, the superior responses seen in the children could be the result of better recent management of their infections, by means of azole antifungal therapy. Candida-derived polysaccharide antigens have been shown to be immunosuppressive (Fischer et al. 1978, 1982; Durandy et al. 1987), and improved immune responses are recognized to follow resolution of fungal infections (Paterson et al. 1971; Kirkpatrick and Smith 1974; Durandy et al. 1987; Kirkpatrick 2001). Because early, effective treatment of fungal infections may confer significant benefit, genetic counseling was offered, and inheritance of the affected or unaffected haplotype (as determined in a CLIA-certified laboratory) was shared with parents of infants who were too young for ascertainment of disease status.
Our account has focused on the CMC phenotype, but, in some individuals in the family, only thyroid dysfunction was documented. Previous linkage studies have indicated that Graves disease (MIM 275000) and Hashimoto thyroiditis (MIM 140300) are associated with several genes of the immune system, such as the CTLA4 gene on chromosome 2q33 (Donner at al. 1997; Heward et al. 1999; Vaidya et al. 1999), as well as with additional loci, on 18q21 (Vaidya et al. 2000) and 20q1 (Tomer et al. 1999b, 1999; Pearce et al. 1999). Hashimoto thyroiditis and other types of thyroiditis or hypothyroidism have been associated with 6p (Heward et al. 1998), 5q31-q33 and 8q23-q24 (Sakai et al. 2001), 14q31 (Tomer et al. 1998a), Xq21 (Barbesino et al. 1998), and chromosomes 12 and 13 (Tomer et al. 1999). However, none of these studies found evidence for genetic linkage of thyroid disease alone to chromosome 2p.
According to the publicly available Draft Human Genome sequence (International Human Genome Sequencing Consortium 2001), the 15-cM interval between D2S367 and D2S2240 spans ∼12 Mb and contains >20 actual and predicted genes, as well as dozens of mRNAs and expressed-sequence tags. Some of these genes—such as CYP1B1 (MIM 601771), SLC3A1/rBAT (MIM 104614), and SIX3 (MIM 603714)—are unlikely candidates for candidiasis/thyroid disease because they are known to be mutated in patients with completely different phenotypes (Calonge et al. 1994; Stoilov et al. 1997; Wallis et al. 1999). However, the nature of the gene that has become mutated in the family that we studied is obscure.
The mechanism by which AIRE deficiency leads to failure of selected endocrine tissues remains poorly understood. The gene product is expressed at highest levels in thymus and glandular tissues. Histopathology shows glandular atrophy and lymphocytic infiltrates with occasional lymphoid follicles and fibrosis (Whitaker et al. 1956; Hermans et al. 1969), changes consistent with either a primary autoimmune reaction against glandular tissue or a reactive process developing as the gland is damaged by another mechanism. Autoantibodies are not uniformly found in patients with APECED, although appearance of anti-adrenal or anti-steroidal–cell antibodies presages the development of adrenal failure or ovarian failure in APECED (Ahonen et al. 1987; Björses et al. 1998). One of our affected pediatric patients who had both markedly elevated TSH and cystic changes in the thyroid nevertheless had no detectable anti-thyroid antibodies. In addition, another family member was diagnosed with Riedel thyroiditis, a rare fibrosing thyroid condition that may not have an autoimmune pathogenesis (Zimmermann-Belsing and Feldt-Rasmussen 1994). Thus, an inflammatory or degenerative process that, for reasons other than autoimmunity, arises in the thyroid could result in hypothyroidism with or without autoantibodies or lymphocyte infiltration. The genetic approach to identification of the molecular defect in autosomal dominant CMC/thyroid disease may provide clues to the pathogenesis not only of the disorder in the family that we studied but of other CMC syndromes as well.
Acknowledgments
The authors thank the members of the family for their participation. Genotyping services were provided by the Center for Inherited Disease Research, which is fully funded through National Institutes of Health federal contract N01-HG-65403 to The Johns Hopkins University. We particularly thank Kim Doheny and Elizabeth Pugh for oversight and coordination of the genome scan. Ms. Sara Tartt assisted with flow cytometric analysis on multiple occasions, and Dr. Gulbu Uzel performed proliferative assays. B.G. was supported by Deutsche Forschungsgemeinschaft grant GR1617/2.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Center for Inherited Disease Research, http://www.cidr.jhmi.edu/
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://research.marshfieldclinic.org/genetics/
- FASTLINK, ftp://fastlink.nih.gov/pub/fastlink
- Draft Human Genome, http://www.ncbi.nlm.nih.gov/ (originally available at http://www.genome.ucsc.edu)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for APECED [MIM 240300], autosomal dominant [MIM 114580] and autosomal recessive [MIM 212050] CRC, Graves disease [MIM 275000], Hashimoto thyroiditis [MIM 140300], CYP1B1 [MIM 601771], SLC3A1/rBAT [MIM 104614], and SIX3 MIM 603714])
- PedCheck web site, ftp://watson.hgen.pitt.edu/pub/pedcheck
- Research Genetics' Genome Services Protocol, ftp://ftp.resgen.com/pub/mappairs/humanset/mappairs_protocol.txt
References
- Abreu PC, Greenberg DA, Hodge SE (1999) Direct power comparisons between simple LOD scores and NPL scores for linkage analysis in complex diseases. Am J Hum Genet 65:847–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen P (1985) Autoimmune polyendocrinopathy–candidosis–ectodermal dystrophy (APECED): autosomal recessive inheritance. Clin Genet 27:535–542 [DOI] [PubMed] [Google Scholar]
- Ahonen P, Miettinen A, Perheentupa J (1987) Adrenal and steroidal cell antibodies in patients with autoimmune polyglandular disease type I and risk of adrenocortical and ovarian failure. J Clin Endocrinol Metab 64:494–500 [DOI] [PubMed] [Google Scholar]
- Ahonen P, Myllarniemi S, Sipila I, Perheentupa J (1990) Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med 322:1829–1836 [DOI] [PubMed] [Google Scholar]
- Barbesino G, Tomer Y, Concepcion ES, Davies TF, Greenberg D (1998) Linkage analysis of candidate genes in autoimmune thyroid diseases. II. Selected gender-related genes and the X chromosome. J Clin Endocrinol Metab 83:3290–3295 [DOI] [PubMed] [Google Scholar]
- Becker A, Geiger D, Schäffer AA (1998) Automatic selection of loop breakers for genetic linkage analysis. Hum Hered 48:49–60 [DOI] [PubMed] [Google Scholar]
- Bejjani BA, Lewis RA, Tomey KF, Anderson KL, Dueker DK, Jabak M, Astle WF, Otterud B, Leppert M, Lupski JR (1998) Mutations in CYP1B1, the gene for cytochrome P4501B1, are the predominant cause of primary congenital glaucoma in Saudi Arabia. Am J Hum Genet 62:325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björses P, Aaltonen J, Horelli-Kuitunen N, Yaspo M-L, Peltonen L (1998) Gene defect behind APECED: a new clue to autoimmunity. Hum Mol Genet 7:1547–1553 [DOI] [PubMed] [Google Scholar]
- Brägger C, Seger RA, Aeppli R, Hallè F, Hitzig WH (1989) IgG2/IgG4 subclass deficiency in a patient with chronic mucocutaneous candidiasis and bronchiectases. Eur J Pediatr 149:168–169 [DOI] [PubMed] [Google Scholar]
- Broman KW, Murray JC, Sheffield VC, White RL, Weber JL (1998) Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am J Hum Genet 63:861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R, Lusk E (1994) Monitors, messages, and clusters: the p4 parallel programming system. Parallel Comput 20:547–564 [Google Scholar]
- Calonge MJ, Gasparini P, Chillarón J, Chillón M, Gallucci M, Rousaud F, Zelante L, Testar X, Dallapiccola B, Di Silverio F, Barceló P, Estivill X, Zorzano A, Nunes V, Palacín M (1994) Cystinuria caused by mutations in rBAT, a gene involved in the transport of cystine. Nat Genet 6: 420–425 [DOI] [PubMed] [Google Scholar]
- Canales L, Middlemas RO III, Louro JM, South MA (1969) Immunological observations in chronic mucocutaneous candidiasis. Lancet 2:567–571 [DOI] [PubMed] [Google Scholar]
- Cottingham RW Jr, Idury RM, Schäffer AA (1993) Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263 [PMC free article] [PubMed] [Google Scholar]
- Donner H, Braun J, Seidl C, Rau H, Finke R, Ventz M, Walfish PG,Usadel KH, Badenhoop K (1997) Codon 17 polymorphism of the cytotoxic T lymphocyte antigen 4 gene in Hashimoto's thyroiditis and Addison's disease. J Clin Endocrinol Metab 82:4130–4132 [DOI] [PubMed] [Google Scholar]
- Durandy A, Fischer A, Le Deist F, Drouhet E, Griscelli C (1987) Mannan-specific and mannan-induced T-cell suppressive activity in patients with chronic mucocutaneous candidiasis. J Clin Immunol 7:400–409 [DOI] [PubMed] [Google Scholar]
- Finnish-German APECED Consortium, The (1997) An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet 17:399–403 [DOI] [PubMed] [Google Scholar]
- Fischer A, Ballet J-J, Griscelli C (1978) Specific inhibition of in vitro Candida-induced lymphocyte proliferation by polysaccharidic antigens present in the serum of patients with chronic mucocutaneous candidiasis. J Clin Invest 62:1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Pichat L, Audinot M, Griscelli C (1982) Defective handling of mannan by monocytes in patients with chronic mucocutaneous candidiasis resulting in a specific cellular unresponsiveness. Clin Exp Immunol 47:653–660 [PMC free article] [PubMed] [Google Scholar]
- Gill FF, Portnoy JM (1989) An unusual combination of immunologic abnormalities in a patient with chronic mucocutaneous candidiasis. Ann Allergy 63:98–100 [PubMed] [Google Scholar]
- Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, Miller JA, O'Connell AC, Puck JM (1999a) Hyper-IgE syndrome with recurrent infections, an autosomal dominant multisystem disorder. N Engl J Med 340:692–702 [DOI] [PubMed] [Google Scholar]
- Grimbacher B, Schäffer AA, Holland SM, Davis J, Gallin JI, Malech HL, Atkinson TP, Belohradsky B, Buckley RH, Cossu F, Español T, Garty B-Z, Matamoros N, Myers LA, Nelson RP, Ochs HD, Renner ED, Wellinghausen N, Puck JM (1999b) Genetic linkage of hyper-IgE syndrome to chromosome 4. Am J Hum Genet 65:735–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Schäffer AA, Cox AL, Dwarkadas S, Zwaenepoel W (1995) Integrating parallelization strategies for linkage analysis. Comput Biomed Res 28:116–139 [DOI] [PubMed] [Google Scholar]
- Hermans PE, Ulrich JA, Markowitz H (1969) Chronic mucocutaneous candidiasis as a surface expression of deep-seated abnormalities: report of a syndrome of superficial candidiasis, absence of delayed hypersensitivity and aminoaciduria. Am J Med 47:503–519 [DOI] [PubMed] [Google Scholar]
- Herrod HG (1990) Chronic mucocutaneous candidiasis in childhood and complications of non-candida infection: a report of the Pediatric Immunodeficiency Collaborative Study Group. J Pediatr 116:377–382 [DOI] [PubMed] [Google Scholar]
- Heward JM, Allahabadia A, Armitage M, Hattersley A, Dodson PM, Macleod K, Carr-Smith J, Daykin J, Daly A, Sheppard, MC, Holder RL, Barnett AH, Franklyn JA, Gough SCL (1999) The development of Graves' disease and the CTLA-4 gene on chromosome 2q33. J Clin Endocrinol Metab 84:2398–2401 [DOI] [PubMed] [Google Scholar]
- Heward JM, Allahabadia A, Daykin J, Carr-Smith J, Daly A, Armitage M, Dodson PM, Sheppard MC, Barnett AH, Franklyn JA, Gough SCL (1998) Linkage disequilibrium between the human leukocyte antigen class II region of the major histocompatibility complex and Graves' disease: replication using a population case control and family-based study. J Clin Endocrinol Metab 83:3394–3397 [DOI] [PubMed] [Google Scholar]
- Hodge SE, Greenberg DA (1992) Sensitivity of LOD scores to changes in diagnostic status. Am J Hum Genet 50:1053–1066 [PMC free article] [PubMed] [Google Scholar]
- International Human Genome Sequencing Consortium (2001) Initial sequencing and analysis of the human genome. Nature 409:860–921 [DOI] [PubMed] [Google Scholar]
- James SP (1994) Measurement of proliferative responses in human lymphocytes. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W (eds) Current protocols in immunology. Wiley & Sons, New York, sec 7.10 [Google Scholar]
- Jorizzo JL, Sams WM Jr, Jegasothy BV, Olansky AJ (1980) Cimetidine as an immunomodulator: chronic mucocutaneous candidiasis as a model. Ann Intern Med 92:192–195 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CH (2001) Chronic mucocutaneous candidiasis. Pediatr Infect Dis J 20:197–206 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CH, Smith TK (1974) Chronic mucocutaneous candidiasis: immunologic and antibiotic therapy. Ann Intern Med 80:310–320 [DOI] [PubMed] [Google Scholar]
- Kroll JJ, Einbinder JM, Merz WG (1973) Mucocutaneous candidiasis in a mother and son. Arch Dermatol 108:259–262 [PubMed] [Google Scholar]
- Lathrop GM, Lalouel J-M (1984) Easy calculations of LOD scores and genetic risks on small computers. Am J Hum Genet 36:460–465 [PMC free article] [PubMed] [Google Scholar]
- Lilic D, Calvert JE, Cant AJ, Abinun M, Spickett GP (1996) Chronic mucocutaneous candidiasis. II. Class and subclass of specific antibody responses in vivo and in vitro. Clin Exp Immunol 105:213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeys BL, Van Coster RN, Defreyne LR, Leroy JG (1999) Fungal intracranial aneurysm in a child with familial chronic mucocutaneous candidiasis. Eur J Pediatr 158:650–652 [DOI] [PubMed] [Google Scholar]
- Montes LF, Bradford LG, Lauderdale RO, Taylor CD (1971) Prolonged oral treatment of chronic mucocutaneous candidiasis with amphotericin B. Arch Dermatol 104:45–56 [PubMed] [Google Scholar]
- Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJE, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N (1997) Positional cloning of the APECED gene. Nat Genet 17:393–398 [DOI] [PubMed] [Google Scholar]
- O'Connell JR, Weeks DE (1998) PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 63:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J (1999) Analysis of human genetic linkage, 3d ed. Johns Hopkins University Press, Baltimore [Google Scholar]
- Papazian CE, Koch R (1960) Monilial granuloma with hypothyroidism. N Engl J Med 262:16–18 [DOI] [PubMed] [Google Scholar]
- Patel SB, Salen G, Hidaka H, Kwiterovich PO, Stalenhoef AF, Miettinen TA, Grundy SM, Lee MH, Rubenstein JS, Polymeropoulos MH, Brownstein MJ (1998) Mapping a gene involved in regulating dietary cholesterol absorption: the sitosterolemia locus is found at chromosome 2p21. J Clin Invest 102:1041–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson PY, Semo R, Blumenschein G, Swelstad J (1971) Mucocutaneous candidiasis, anergy and a plasma inhibitor of cellular immunity: reversal after amphotericin B therapy. Clin Exp Immunol 9:595–602 [PMC free article] [PubMed] [Google Scholar]
- Pearce SHS, Vaidya B, Imrie H, Perros P, Kelly WF, Toft AD, McCarthy MI, Young ET, Kendall-Taylor P (1999) Further evidence for a susceptibility locus on chromosome 20q13.11 in families with dominant transmission of Graves disease. Am J Hum Genet 65:1462–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Shirasawa S, Ishikawa N, Ito K, Tamai H, Kuma K, Akamizu T, Tanimura M, Furugaki K, Yamamoto K, Sasazuki T (2001) Identification of susceptibility loci for autoimmune thyroid disease to 5q31-q33 and Hashimoto's thyroiditis to 8q23-q24 by multipoint affected sib-pair linkage analysis in Japanese. Hum Mol Genet 10:1379–1386 [DOI] [PubMed] [Google Scholar]
- Sams WM Jr, Jorizzo JL, Snyderman R, Jegasothy BV, Ward FE, Weiner M, Wilson JG, Yount WJ, Dillard SB (1979) Chronic mucocutaneous candidiasis: immunologic studies of three generations of a single family. Am J Med 67:948–959 [DOI] [PubMed] [Google Scholar]
- Sarfarazi M, Akarsu AN, Hossain A, Turacli ME, Aktan SG, Barsoum-Homsy M, Chevrette L, Sayli BS (1995) Assignment of a locus (GLC3A) for primary congenital glaucoma (buphthalmos) to 2p21 and evidence for genetic heterogeneity. Genomics 30:171–177 [DOI] [PubMed] [Google Scholar]
- Schäffer AA, Gupta SK, Shriram K, Cottingham RW Jr (1994) Avoiding recomputation in linkage analysis. Hum Hered 44:225–237 [DOI] [PubMed] [Google Scholar]
- Sobel E, Lange K (1996) Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker sharing statistics. Am J Hum Genet 58:1323–1337 [PMC free article] [PubMed] [Google Scholar]
- Steensma DP, Tefferi A, Weiler CR (2000) Autoimmune hemolytic anemia in a patient with autosomal dominant chronic mucocutaneous candidiasis. Mayo Clin Proc 75:853–855 [DOI] [PubMed] [Google Scholar]
- Stiehm ER, Fudenberg HH (1966) Serum levels of immune globulins in health and disease: a survey. Pediatrics 37:715–727 [PubMed] [Google Scholar]
- Stoilov I, Akarsu AN, Sarfarazi M (1997) Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum Mol Genet 6:641–647 [DOI] [PubMed] [Google Scholar]
- Sutphin A, Albright F, McCune DJ (1943) Five cases (three in siblings) of idiopathic hypoparathyroidism associated with moniliasis. J Clin Endocrinol 3:625–634 [Google Scholar]
- Thorpe ES, Handley HE (1929) Chronic tetany and chronic mycelial stomatitis in a child aged four and one-half years. Am J Dis Child 38:228–238 [Google Scholar]
- Tomer Y, Barbesino G, Greenberg DA, Concepcion E, Davies TF (1998a) Linkage analysis of candidate genes in autoimmune thyroid disease. III. Detailed analysis of chromosome 14 localizes Graves' disease-1 (GD-1) close to multinodular goiter-1 (MNG-1). J Clin Endocrinol Metab 83:4321–4327 [DOI] [PubMed] [Google Scholar]
- ——— (1999) Mapping the major susceptibility loci for familial Graves' and Hashimoto's diseases: Evidence for genetic heterogeneity and gene interactions. J Clin Endocrinol Metab 84:4656–4664 [DOI] [PubMed] [Google Scholar]
- Tomer Y, Barbesino G, Greenberg DA, Concepcion E, Davies TF, International Consortium for the Genetics of Autoimmune Thyroid Disease (1998b) A new Graves disease–susceptibility locus maps to chromosome 20q11.2. Am J Hum Genet 63:1749–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya B, Imrie H, Perros P, Young ET, Kelly WF, Carr D, Large DM, Toft AD, Kendall-Taylor P, Pearce SHS (2000) Evidence for a new Graves disease susceptibility locus at chromosome 18q21. Am J Hum Genet 66:1710–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya B, Imrie H, Perros P, Young ET, Kelly WF, Carr D, Large DM, Toft AD, McCarthy MI, Kendall-Taylor P, Pearce SHS (1999) The cytotoxic T lymphocyte antigen-4 is a major Graves' disease locus. Hum Mol Genet 8:1195–1199 [DOI] [PubMed] [Google Scholar]
- Van Scoy RE, Hill HR, Ritts RE, Quie PG (1975) Familial neutrophil chemotaxis defect, recurrent bacterial infections, mucocutaneous candidiasis, and hyperimmunoglobulinemia E. Ann Intern Med 82:766–771 [DOI] [PubMed] [Google Scholar]
- Wallis DE, Roessler E, Hehr U, Nanni L, Wiltshire T, Richieri-Costa A, Gillessen-Kaesbach G, Zackai EH, Rommens J, Muenke M (1999) Mutations in the homeodomain of the human SIX3 gene cause holoprosencephaly. Nat Genet 22:196–198 [DOI] [PubMed] [Google Scholar]
- Wells RS, Higgs JM, MacDonald A, Valdimarsson H, Holt PJL (1972) Familial chronic mucocutaneous candidiasis. J Med Genet 9:302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker J, Landing BH, Esselborn VM, Williams RR (1956) The syndrome of familial juvenile hypoadrenocorticism, hypoparathyroidism, and superficial moniliasis. J Clin Endocrinol Metab 16:1374–1387 [DOI] [PubMed] [Google Scholar]
- Xiao S, Wang X, Qu B, Yang M, Liu G, Bu L, Wang Y, Zhu L, Lei H, Hu L, Zhang X, Liu J, Zhao G, Kong X (2000) Refinement of the locus for autosomal dominant hereditary gingival fibromatosis (GINGF) to a 3.8-cM region on 2p21. Genomics 68:247–252 [DOI] [PubMed] [Google Scholar]
- Zimmermann-Belsing T, Feldt-Rasmussen U (1994) Riedel’s thyroiditis: an autoimmune or primary fibrotic disease? J Intern Med 235:271–274 [DOI] [PubMed] [Google Scholar]