Abstract
Rupture of intracranial aneurysms (IAs) causes subarachnoid hemorrhage, a devastating condition with high morbidity and mortality. Angiographic and autopsy studies show that IA is a common disorder, with a prevalence of 3%–6%. Although IA has a substantial genetic component, little attention has been given to the genetic determinants. We report here a genomewide linkage study of IA in 104 Japanese affected sib pairs in which positive evidence of linkage on chromosomes 5q22-31 (maximum LOD score [MLS] 2.24), 7q11 (MLS 3.22), and 14q22 (MLS 2.31) were found. The best evidence of linkage is detected at D7S2472, in the vicinity of the elastin gene (ELN), a candidate gene for IA. Fourteen distinct single-nucleotide polymorphisms (SNPs) were identified in ELN, and no obvious allelic association between IA and each SNP was observed. The haplotype between the intron-20/intron-23 polymorphism of ELN is strongly associated with IA (P=3.81×10-6), and homozygous patients are at high risk (P=.002), with an odds ratio of 4.39. These findings suggest that a genetic locus for IA lies within or close to the ELN locus on chromosome 7.
Introduction
Large autopsy studies reveal that intracranial aneurysms (IAs [MIM 105800]) have a prevalence of 4.6% (Iwamoto et al. 1999), and angiographic studies indicate the prevalence of unruptured incidental IA among adults to be 2.7%–6.5% (Ujiie et al. 1993; Nakagawa and Hashi 1994). Rupture of an IA causes sudden subarachnoid hemorrhage (SAH), with high morbidity and mortality. For all ages, the annual incidence of SAH due to aneurysmal rupture is 18–23/100,000 (Inagawa et al. 1988, 1995); for individuals ⩾40 years old, it is 96/100,000 (Kiyohara et al. 1989). For patients with SAH, 8%–12% die before receiving medical attention (Phillips et al. 1980; Inagawa et al. 1995; Schievink et al. 1995b), 40%–60% die ⩽1 mo after onset of the disease (Sacco et al. 1984; Kiyohara et al. 1989; Inagawa et al. 1995), and more than a third of those who survive show major neurological deficits (Longstreth et al. 1993; Inagawa et al. 1995). Despite the improvements in medical and surgical care and in diagnostic methods during the past decades, aneurysmal SAH is still a major public health problem.
Although genetic and environmental factors play equally important roles in the etiology of IA, recent progress in molecular genetics enables us to approach the genetic determinants directly. The risk of ruptured IA in first-degree relatives of patients with aneurysmal SAH is four times higher, and the relative risk in siblings is six times higher, than that in the general population (Schievink et al. 1995a; Ronkainen et al. 1997). A small fraction of IA is associated with heritable connective-tissue diseases such as polycystic kidney disease, Ehlers-Danlos syndrome type IV, and Marfan syndrome (Schievink 1997). Segregation analysis has been unable to define the inheritance pattern of IA (Schievink et al. 1994), possibly because of the complex etiology of the disease. We performed a genetic linkage study with Japanese nuclear families, to identify susceptible loci underlying IA, especially ruptured IA. Because IA has late onset and low penetrance, an occult phenotype may exist in a family, and complicated etiologies frequently are involved, so only affected sib pairs (ASPs) were used for the nonparametric linkage study. Difficulties in collection of ASPs were expected, because of the high mortality in ruptured IA. At all 1,100 hospitals in Japan that have been certified as training hospitals by the Japan Neurosurgical Society, we inquired regarding ASPs with IA. We were able to enroll 104 ASPs, comprising mainly patients with ruptured IA, at 94 of these hospitals. The subjects were examined in a genomewide linkage study. The best evidence of linkage was obtained on chromosome 7, near marker D7S2472, and the elastin gene (ELN), encoding a major component of the blood-vessel wall, was found to lie very close to the marker. Since ELN is both a positional and functional candidate gene for IA, it was analyzed for allelic association, haplotype association, and linkage disequilibrium (LD).
Subjects and Methods
Subjects
Current samples comprise 85 nuclear families collected through neurosurgical services certified by the Japan Neurosurgical Society, and the number of possible ASPs was 104. The Ethical Committee of the Tokyo Women's Medical University approved the study, and all the participants (or their family members) gave written, informed consent. The families included at least two affected siblings, each of whom had an IA >5 mm, as ascertained by conventional angiography, three-dimensional computed tomography (CT) angiography, magnetic resonance (MR) angiography, or surgical findings. This collected sample comprised 179 individuals—51 males and 128 females. ASPs comprised 77 pairs, 7 trios, and 1 quartet of siblings with IA. In the 77 pairs, SAH (i.e., ruptured IA) was present in both siblings in 41 pairs, in one sibling in 27 pairs, and in neither sibling in 9 pairs; in the 7 trios, SAH was present in all three siblings in 3 trios, one sibling in 1 trio, and in none of the siblings in 3 trios; in the quartet, SAH was present in only one individual. Of the 85 families, 73 had at least one member with SAH (table 1). The detailed clinical features of these families have been reported elsewhere (Kasuya et al. 2000).
Table 1.
Samples Used for Affected Sib Pair Linkage Analysis
|
No. of |
|||||
| Families |
|||||
| FamilyStructure | SAHPositive | SAHNegative | Total | Individuals | Sib Pairs |
| Pairs | 68 | 9 | 77 | 154 | 77 |
| Trios | 4 | 3 | 7 | 21 | 21 |
| Quartets | 1 | 0 | 1 | 4 | 6 |
| Total | 73 | 12 | 85 | 179 | 104 |
For the allelic-ssociation study, 172 patients with IA (70 men and 102 women; mean [SD] age 59.8 [10.5] years) and 192 controls (91 men and 101 women; mean [SD] age 59.0 [16.5] years) were enrolled. All subjects were of Japanese ethnicity. The 172 patients with IA include 78 probands in nuclear families, 9 patients with first-degree relatives with IA, and 85 patients without known family history of IA. The 192 controls were outpatients of Tokyo Women's Medical University Hospital who presented with headache and other neurological complaints. Selected controls had no history of SAH and were of ages similar to those of the patients with IA and, on conventional CT examination, showed no evidence of IA.
Genotyping
PCR amplifications were performed on the basis of standard protocols. Genotyping was performed, by a fluorescence-based semiautomated technique, on a DNA Sequencer model 377 (Applied Biosystems), with Linkage Mapping Set version 2 (Applied Biosystems). The marker alleles were assigned by GENESCAN and GENOTYPER software (Applied Biosystems). Heterozygosity of each microsatellite marker was determined on the basis of 64 unrelated healthy Japanese from various regions in Japan. Of the 400 markers in the set, 43 were not informative; the other 357, which, in 64 unrelated Japanese healthy subjects, had heterozygosities >60%, were analyzed. A set of 47 markers obtained from online information was added to the original set, to fill in gaps >20 cM (primer sequences of these additional markers are available on request). The average heterozygosity of the total of 404 markers was .756 in Japanese. The average interval between markers was 8.7 cM, and two gaps were >20 cM (maximum 26.8 cM).
Linkage Analysis
Because the mode of inheritance of IA is not known, we applied two different nonparametric linkage methods—the SIBPAL program from the S.A.G.E. package (version 3.1) (Elston et al. 1997) and the GENEHUNTER program (version 1.2) (Kruglyak et al. 1996). The SIBPAL program estimated the mean ratio (π) of alleles shared identical by descent (IBD) among ASPs, at each microsatellite marker. The π obtained was tested against the null hypothesis of no linkage (π=.5). The test statistic has a standard normal distribution under the null hypothesis, and, because the alternative hypothesis of linkage is given when IBD sharing is >50%, the test is one sided. Accordingly, accurate π values can be obtained by use of a one sided π-test as implemented in the SIBPAL program. Multipoint linkage analysis was performed by a maximum-likelihood method implemented in the GENEHUNTER program. Maximum LOD score (MLS) was calculated by the method of possible triangle constraints (Kruglyak et al. 1996). All sib pairs from sibships containing more than two affected individuals were counted, and the unweighted option was used.
Physical Map of the ELN Locus
A physical map of the ELN locus was constructed on the basis of both the GenBank database (accession numbers AC005089, AC005056, U93037, U63721, U62292, U62293, AC005057, AF045555, AC005081, and AC005015.2) and the physical map of the microdeletion in Williams-Beuren syndrome (WBS) (Peoples et al. 2000). Eight polymorphic dinucleotide or tetranucleotide repeats in the vicinity were discovered, and linkage and allelic association studies were performed.
Single-Nucleotide Polymorphisms (SNPs) of ELN
A total of 16 IA probands and 8 controls were screened to identify SNPs of ELN. Direct sequencing was performed on PCR-amplified segments spanning all 34 exons, acceptor, donor, and branch-point sequences in the introns, 1.0 kb of putative promoter sequence, and 1.2 kb of the 3′UTR sequence. A total of 42 primer sets were designed on the basis of the human ELN cDNA and genomic sequences (accession numbers M36860 and AC005056, respectively), obtained from the GenBank database. Primer sequences and PCR conditions are available from the authors on request.
Allelic Association, Haplotype Analysis, and LD
Allelic association with IA was evaluated by χ2 test statistic, for each SNP; the odds ratio and 95% confidence interval (95%CI) also were calculated for each SNP. Because the gametic phase was unknown, the haplotype frequencies were calculated from two-locus genotype data by the maximum-likelihood estimates, by use of the ARLEQUIN program. The haplotype frequencies of ELN were compared in patients with IA versus controls and were evaluated by the contingency table of χ2 test statistics. The extent of pairwise LD (D) was evaluated as D=p11p22-p12p21, where p11, p22, p12, and p21 are the frequencies of haplotypes of A1B1, A2B2, A1B2, and A2B1, respectively, at loci A and B. p1, p2, q1, and q2 are the frequencies of alleles A1, A2, B1, and B2, respectively and two LD measures are applied: first, coefficient D′ is given by D/Dmax, where either Dmax is the smaller of p1q2 and p2q1 when D>0 or Dmax is the smaller of p1q1 and p2q2 when D<0 (Lewontin 1964); second, r2 is given by D2/(p1p2q1q2) (Hill and Robertson 1968).
Results
Radiographic Examinations of IA
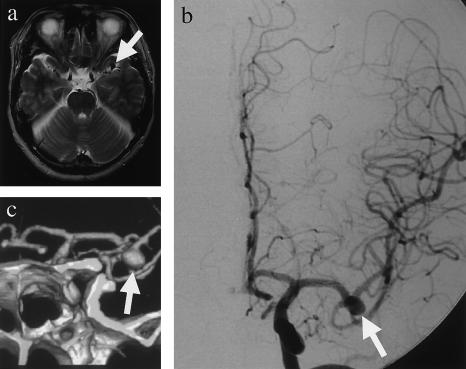
Modern diagnostic techniques allow the detection of many potentially dangerous conditions before symptoms occur. Most patients with IA are asymptomatic, however, until sudden rupture and life-threatening SAH. IA could be diagnosed by various radiographic methods, such as cerebral angiography, three-dimensional CT angiography, and MR angiography. Figure 1 shows MR imaging (fig. 1a), cerebral angiography (fig. 1b), and three-dimensional CT angiography (fig. 1c) of a typical patient with IA who has a saccular aneurysm in the left middle cerebral artery.
Figure 1.
Radiographic examinations for IA. A 51-year-old male patient with a complaint of vertigo received MR imaging, which showed a flow void, an indication of aneurysm, in the left middle cerebral artery (a, arrow). Cerebral angiography (b, arrow) and three-dimensional CT angiography (c, arrow) further confirmed IA.
Genomewide Linkage Studies
The sample for linkage study comprised 85 Japanese nuclear families, and the maximum number of ASPs with IA was 104 (table 1). Because the quantity of DNA available from 25 participants was insufficient for genotyping of the 404 microsatellite markers for genomewide scan, 154 of the 179 individuals comprised by the 83 ASPs were genotyped. The statistical probability of linkage between each marker and IA was tested by SIBPAL (table 2). Regions of the genome were considered to have suggestive evidence of linkage in the first data set either if an individual marker attained statistical significance P<.01 or if two or more adjacent markers each attained statistical significance at P<.05. By these criteria, suggestive evidence of linkage to IA obtains for markers within three distinct chromosomes: chromosome 5 (markers D5S428 and D5S644), chromosome 7 (markers D7S669 and D7S630), and chromosome 14 (markers D14S258 and D14S74) (table 2). These chromosomes were further tested by multipoint linkage analysis using GENEHUNTER. Multipoint analyses of the three regions showed at least nominal evidence for linkage (defined by MLS >1.0; data not shown). Linkage analysis of chromosome X by GENEHUNTER indicated no evidence of linkage (data not shown).
Table 2.
Single-Point Linkage Analysis by SIBPAL
| Chromosome and Marker | Distance(cM) | Heterozygosity | No. of Pairs | IBD | t | P |
| 1: | ||||||
| D1S468 | 6.2 | .71 | 83 | .52 | .73 | .234 |
| D1S214 | 16.4 | .72 | 83 | .51 | .47 | .320 |
| D1S450 | 22.9 | .85 | 81 | .49 | −.41 | .659 |
| D1S2667 | 26.9 | .82 | 82 | .47 | −.9 | .815 |
| D1S507 | 36.2 | .83 | 80 | .44 | −2.07 | .979 |
| D1S199 | 47.7 | .77 | 83 | .48 | −.6 | .726 |
| D1S234 | 56.6 | .81 | 81 | .49 | −.35 | .638 |
| D1S496 | 65.6 | .87 | 70 | .48 | −.54 | .706 |
| D1S2797 | 77.6 | .78 | 83 | .55 | 1.81 | .037* |
| D1S2700 | 89.3 | .83 | 82 | .52 | .51 | .304 |
| D1S230 | 97.4 | .61 | 83 | .49 | −.3 | .619 |
| D1S2841 | 108.8 | .83 | 83 | .50 | .11 | .456 |
| D1S207 | 117.6 | .83 | 81 | .49 | −.31 | .622 |
| D1S2868 | 129.9 | .61 | 80 | .55 | 2.05 | .022* |
| D1S206 | 137.6 | .78 | 83 | .45 | −1.88 | .968 |
| D1S2726 | 149 | .72 | 83 | .49 | −.28 | .612 |
| D1S252 | 155.1 | .80 | 82 | .50 | .01 | .496 |
| D1S498 | 160.7 | .73 | 82 | .51 | .21 | .418 |
| D1S484 | 173.9 | .68 | 82 | .47 | −1.08 | .859 |
| D1S2878 | 181.7 | .84 | 83 | .47 | −.84 | .798 |
| D1S196 | 186.4 | .72 | 83 | .53 | 1.22 | .114 |
| D1S218 | 196.5 | .82 | 83 | .50 | −.13 | .553 |
| D1S238 | 206.7 | .81 | 82 | .52 | .6 | .274 |
| D1S413 | 216.5 | .63 | 82 | .50 | .12 | .453 |
| D1S249 | 225.1 | .67 | 83 | .52 | .54 | .296 |
| D1S425 | 235.3 | .61 | 82 | .51 | .49 | .314 |
| D1S213 | 246.2 | .85 | 81 | .50 | .04 | .483 |
| D1S2800 | 256.1 | .71 | 83 | .50 | .12 | .452 |
| D1S2785 | 269.7 | .85 | 82 | .50 | −.11 | .542 |
| D1S2842 | 277.3 | .72 | 81 | .47 | −.93 | .822 |
| D1S2836 | 290.1 | .68 | 83 | .44 | −1.98 | .975 |
| 2: | ||||||
| D2S319 | 6 | .72 | 82 | .47 | −1.00 | .839 |
| D2S2211 | 14 | .64 | 83 | .51 | .33 | .371 |
| D2S162 | 21.3 | .79 | 83 | .48 | −.69 | .755 |
| D2S168 | 28.6 | .80 | 82 | .49 | −.43 | .664 |
| D2S305 | 40.7 | .77 | 81 | .51 | .37 | .358 |
| D2S165 | 50.7 | .86 | 81 | .46 | −1.02 | .845 |
| D2S367 | 58.3 | .87 | 83 | .50 | −.15 | .558 |
| D2S2259 | 67.4 | .62 | 83 | .49 | −.25 | .598 |
| D2S391 | 73.8 | .71 | 82 | .48 | −.59 | .720 |
| D2S337 | 84.1 | .84 | 82 | .43 | −2.24 | .986 |
| D2S2368 | 89.2 | .83 | 83 | .44 | −2.22 | .986 |
| D2S286 | 98.4 | .74 | 81 | .47 | −.86 | .803 |
| D2S2333 | 107.7 | .84 | 83 | .52 | .77 | .223 |
| D2S2216 | 115.3 | .72 | 82 | .48 | −.84 | .799 |
| D2S160 | 127.4 | .71 | 83 | .54 | 1.73 | .044* |
| D2S347 | 135.7 | .61 | 81 | .45 | −1.8 | .962 |
| D2S112 | 145.8 | .60 | 83 | .47 | −1.18 | .880 |
| D2S151 | 156.4 | .77 | 82 | .44 | −2.29 | .988 |
| D2S142 | 166.3 | .73 | 82 | .44 | −2.08 | .980 |
| D2S2330 | 175.5 | .84 | 83 | .49 | −.20 | .580 |
| D2S335 | 182.5 | .84 | 83 | .49 | −.30 | .617 |
| D2S364 | 192.9 | .78 | 82 | .45 | −1.66 | .949 |
| D2S117 | 201.4 | .88 | 83 | .47 | −.79 | .784 |
| D2S325 | 210.9 | .79 | 82 | .45 | −1.67 | .951 |
| D2S164 | 222 | .65 | 80 | .45 | −1.85 | .966 |
| D2S126 | 228.8 | .80 | 82 | .48 | −.60 | .725 |
| D2S396 | 240.2 | .85 | 82 | .50 | −.16 | .562 |
| D2S206 | 248.3 | .80 | 82 | .54 | 1.35 | .091 |
| D2S338 | 258.7 | .81 | 83 | .51 | .38 | .353 |
| D2S125 | 269.5 | .81 | 83 | .52 | .74 | .232 |
| 3: | ||||||
| D3S1297 | 2.5 | .76 | 83 | .54 | 1.47 | .073 |
| D3S1304 | 16.5 | .79 | 82 | .50 | −.17 | .569 |
| D3S1263 | 30.4 | .89 | 83 | .47 | −1.08 | .858 |
| D3S2338 | 36.3 | .73 | 82 | .48 | −.63 | .736 |
| D3S1266 | 46.9 | .66 | 83 | .48 | −.85 | .801 |
| D3S1277 | 56.1 | .69 | 80 | .47 | −1.22 | .887 |
| D3S1289 | 69.1 | .81 | 81 | .48 | −.49 | .686 |
| D3S1300 | 79 | .81 | 80 | .55 | 1.42 | .080 |
| D3S1285 | 91 | .76 | 83 | .48 | −.61 | .728 |
| D3S1566 | 97.2 | .84 | 80 | .46 | −1.26 | .895 |
| D3S3681 | 108.8 | .82 | 79 | .52 | .73 | .235 |
| D3S1271 | 117.7 | .60 | 83 | .53 | 1.42 | .080 |
| D3S1278 | 131.8 | .70 | 83 | .51 | .33 | .373 |
| D3S1267 | 141.1 | .65 | 83 | .47 | −1.08 | .859 |
| D3S1292 | 148.7 | .89 | 83 | .51 | .42 | .338 |
| D3S1569 | 162 | .79 | 83 | .45 | −1.84 | .965 |
| D3S1279 | 173 | .62 | 83 | .45 | −1.98 | .975 |
| D3S1614 | 183.1 | .67 | 83 | .54 | 1.63 | .053 |
| D3S1565 | 193 | .80 | 83 | .52 | .66 | .257 |
| D3S1262 | 207.2 | .72 | 83 | .52 | .65 | .259 |
| D3S1580 | 213.7 | .84 | 82 | .53 | .95 | .172 |
| D3S1601 | 220.4 | .79 | 83 | .53 | 1.04 | .150 |
| D3S1311 | 230.7 | .73 | 82 | .55 | 2.22 | .014* |
| 4: | ||||||
| D4S412 | 3.7 | .68 | 83 | .47 | −.94 | .824 |
| D4S2935 | 12.2 | .64 | 82 | .50 | .06 | .476 |
| D4S3036 | 23.1 | .78 | 82 | .50 | −.09 | .534 |
| D4S419 | 32.6 | .69 | 83 | .53 | 1.11 | .134 |
| D4S391 | 43.2 | .78 | 83 | .53 | 1.23 | .112 |
| D4S405 | 56.7 | .75 | 83 | .49 | −.21 | .582 |
| D4S1592 | 68.4 | .78 | 83 | .50 | −.15 | .558 |
| D4S392 | 77.9 | .82 | 82 | .54 | 1.16 | .125 |
| D4S2964 | 87.1 | .70 | 83 | .50 | −.16 | .565 |
| D4S1534 | 93.5 | .80 | 83 | .52 | .56 | .287 |
| D4S414 | 99.2 | .75 | 83 | .52 | .62 | .268 |
| D4S1572 | 106.3 | .83 | 83 | .52 | .72 | .236 |
| D4S406 | 115.8 | .70 | 81 | .51 | .31 | .379 |
| D4S402 | 123.5 | .84 | 81 | .54 | 1.16 | .124 |
| D4S3039 | 131.9 | .82 | 81 | .53 | .89 | .189 |
| D4S424 | 143.8 | .76 | 83 | .52 | .69 | .247 |
| D4S413 | 157.9 | .62 | 83 | .54 | 1.57 | .060 |
| D4S2979 | 170.9 | .65 | 81 | .50 | −.17 | .567 |
| D4S2991 | 179.6 | .81 | 82 | .55 | 1.77 | .040* |
| D4S415 | 185 | .73 | 82 | .52 | .62 | .269 |
| D4S1535 | 198.5 | .76 | 81 | .52 | .85 | .198 |
| D4S426 | 211 | .72 | 83 | .48 | −.62 | .733 |
| 5: | ||||||
| D5S1981 | .6 | .76 | 83 | .51 | .47 | .321 |
| D5S406 | 10.7 | .73 | 82 | .51 | .43 | .333 |
| D5S630 | 18.6 | .90 | 83 | .52 | .46 | .322 |
| D5S416 | 27.9 | .64 | 81 | .50 | .07 | .473 |
| D5S419 | 39.5 | .87 | 80 | .47 | −1.02 | .846 |
| D5S426 | 51.6 | .78 | 82 | .48 | −.75 | .772 |
| D5S418 | 58.1 | .78 | 81 | .54 | 1.19 | .12 |
| D5S407 | 65 | .86 | 83 | .52 | .48 | .316 |
| D5S647 | 74.7 | .82 | 83 | .54 | 1.25 | .108 |
| D5S424 | 82.8 | .68 | 82 | .52 | .86 | .196 |
| D5S641 | 92.3 | .81 | 82 | .51 | .33 | .371 |
| D5S428 | 95.4 | .68 | 81 | .57 | 3.09 | .001** |
| D5S644 | 104.5 | .83 | 81 | .56 | 2.14 | .018* |
| D5S433 | 112.2 | .75 | 83 | .53 | 1.08 | .141 |
| D5S2027 | 118.9 | .59 | 82 | .52 | .79 | .216 |
| D5S471 | 129.6 | .71 | 83 | .53 | 1.40 | .083 |
| D5S2115 | 138.6 | .74 | 82 | .54 | 1.45 | .075 |
| D5S436 | 147.2 | .75 | 83 | .52 | .74 | .231 |
| D5S410 | 156 | .57 | 83 | .50 | .01 | .496 |
| D5S422 | 163.9 | .81 | 82 | .50 | −.11 | .545 |
| D5S400 | 174.3 | .88 | 82 | .50 | .01 | .496 |
| D5S1960 | 179.1 | .73 | 64 | .48 | −.59 | .721 |
| D5S408 | 195.8 | .68 | 81 | .49 | −.24 | .596 |
| 6: | ||||||
| D6S1574 | 8.7 | .73 | 81 | .50 | −.12 | .549 |
| D6S309 | 13.6 | .76 | 80 | .52 | .84 | .200 |
| D6S470 | 17.7 | .72 | 82 | .47 | −1.08 | .859 |
| D6S289 | 29.6 | .81 | 83 | .50 | .11 | .458 |
| D6S422 | 35.7 | .67 | 83 | .52 | .77 | .223 |
| D6S276 | 44.9 | .73 | 83 | .53 | 1.05 | .148 |
| D6S1610 | 53.9 | .78 | 83 | .51 | .42 | .339 |
| D6S1575 | 60.7 | .84 | 83 | .54 | 1.32 | .095 |
| D6S452 | 72.2 | .85 | 80 | .47 | −1.01 | .843 |
| D6S257 | 80 | .88 | 83 | .51 | .30 | .384 |
| D6S460 | 90 | .80 | 82 | .50 | .09 | .462 |
| D6S300 | 103.5 | .71 | 82 | .53 | .96 | .170 |
| D6S434 | 109.2 | .78 | 83 | .53 | 1.00 | .161 |
| D6S287 | 122 | .68 | 81 | .51 | .55 | .291 |
| D6S262 | 129.8 | .77 | 83 | .52 | .48 | .317 |
| D6S292 | 138.2 | .85 | 82 | .49 | −.25 | .599 |
| D6S308 | 145.5 | .65 | 83 | .50 | .14 | .444 |
| D6S441 | 155.3 | .79 | 81 | .50 | .08 | .469 |
| D6S305 | 166.6 | .82 | 80 | .48 | −.47 | .682 |
| D6S1719 | 177.9 | .77 | 79 | .47 | −.89 | .812 |
| D6S281 | 201.1 | .81 | 83 | .46 | −1.18 | .880 |
| 7: | ||||||
| D7S531 | 4.8 | .77 | 81 | .53 | 1.14 | .130 |
| D7S517 | 7.8 | .79 | 83 | .54 | 1.55 | .063 |
| D7S513 | 17.7 | .9 | 83 | .53 | .89 | .189 |
| D7S507 | 29.1 | .82 | 83 | .50 | −.09 | .538 |
| D7S493 | 35 | .73 | 83 | .49 | −.29 | .615 |
| D7S516 | 42.1 | .76 | 83 | .49 | −.24 | .596 |
| D7S484 | 55.6 | .79 | 82 | .51 | .34 | .367 |
| D7S510 | 60.5 | .82 | 83 | .55 | 1.87 | .032* |
| D7S519 | 70.5 | .74 | 83 | .53 | 1.32 | .095 |
| D7S502 | 79.6 | .85 | 82 | .54 | 1.13 | .131 |
| D7S669 | 90.9 | .83 | 83 | .56 | 2.06 | .021* |
| D7S630 | 98.7 | .77 | 82 | .55 | 1.77 | .040* |
| D7S657 | 105.2 | .77 | 83 | .54 | 1.52 | .066 |
| D7S515 | 112.9 | .75 | 83 | .56 | 2.24 | .014* |
| D7S486 | 125.3 | .76 | 83 | .50 | .05 | .478 |
| D7S530 | 136.4 | .72 | 81 | .48 | −.59 | .722 |
| D7S640 | 139.7 | .85 | 83 | .48 | −.59 | .722 |
| D7S684 | 149.6 | .78 | 83 | .45 | −1.68 | .952 |
| D7S661 | 157.5 | .84 | 83 | .47 | −.95 | .828 |
| D7S636 | 165 | .93 | 82 | .47 | −.70 | .757 |
| D7S798 | 171.3 | .75 | 80 | .52 | .62 | .270 |
| D7S2465 | 182.1 | .77 | 81 | .53 | .83 | .204 |
| 8: | ||||||
| D8S264 | .7 | .83 | 83 | .52 | .65 | .260 |
| D8S277 | 8.4 | .81 | 80 | .48 | −.58 | .717 |
| D8S550 | 20.4 | .72 | 83 | .48 | −.62 | .731 |
| D8S1731 | 30.7 | .70 | 82 | .49 | −.20 | .581 |
| D8S258 | 40.3 | .68 | 83 | .51 | .51 | .304 |
| D8S177 1 | 49.6 | .68 | 80 | .45 | −1.70 | .953 |
| D8S505 | 60 | .77 | 83 | .46 | −1.31 | .904 |
| D8S285 | 70.6 | .70 | 83 | .46 | −1.31 | .904 |
| D8S260 | 78.8 | .76 | 83 | .50 | .04 | .482 |
| D8S543 | 86.7 | .73 | 80 | .52 | .93 | .177 |
| D8S1705 | 94.3 | .75 | 83 | .55 | 1.60 | .057 |
| D8S270 | 102.1 | .70 | 82 | .52 | .87 | .194 |
| D8S514 | 128.9 | .77 | 83 | .50 | .18 | .428 |
| D8S284 | 142.7 | .80 | 81 | .51 | .17 | .432 |
| D8S272 | 152.5 | .80 | 83 | .50 | −.06 | .525 |
| 9: | ||||||
| D9S288 | 8.8 | .81 | 83 | .57 | 2.34 | .011* |
| D9S286 | 16.8 | .75 | 81 | .53 | 1.13 | .131 |
| D9S285 | 27.9 | .62 | 83 | .49 | −.39 | .650 |
| D9S157 | 31.8 | .83 | 83 | .52 | .50 | .309 |
| D9S265 | 42 | .63 | 83 | .53 | 1.35 | .090 |
| D9S1678 | 50.3 | .75 | 79 | .51 | .34 | .368 |
| D9S1817 | 57.9 | .86 | 83 | .51 | .47 | .320 |
| D9S166 | 65 | .75 | 82 | .53 | 1.19 | .118 |
| D9S175 | 68.8 | .62 | 82 | .50 | .13 | .447 |
| D9S167 | 82.4 | .84 | 83 | .55 | 1.50 | .069 |
| D9S283 | 93.2 | .73 | 81 | .51 | .42 | .338 |
| D9S287 | 103.3 | .64 | 82 | .52 | .99 | .162 |
| D9S1690 | 106.5 | .78 | 83 | .52 | .58 | .281 |
| D9S1677 | 117.8 | .87 | 82 | .53 | 1.00 | .160 |
| D9S1776 | 124.2 | .76 | 83 | .51 | .22 | .413 |
| D9S1682 | 132.9 | .64 | 78 | .51 | .52 | .301 |
| D9S290 | 141.1 | .66 | 83 | .46 | −1.44 | .923 |
| D9S164 | 148.1 | .79 | 80 | .49 | −.39 | .651 |
| D9S1826 | 160.2 | .82 | 82 | .51 | .35 | .364 |
| D9S158 | 163 | .72 | 82 | .54 | 1.49 | .071 |
| 10: | ||||||
| D10S249 | 0 | .82 | 82 | .49 | −.21 | .583 |
| D10S552 | 13 | .76 | 83 | .55 | 1.50 | .069 |
| D10S189 | 17.3 | .72 | 83 | .55 | 1.62 | .055 |
| D10S570 | 32.1 | .73 | 83 | .49 | −.39 | .651 |
| D10S1653 | 38.8 | .75 | 83 | .48 | −.61 | .730 |
| D10S548 | 43.4 | .59 | 82 | .48 | −.90 | .814 |
| D10S197 | 50.5 | .72 | 83 | .51 | .32 | .376 |
| D10S208 | 60.2 | .79 | 83 | .52 | .57 | .286 |
| D10S196 | 72.5 | .70 | 80 | .44 | −2.39 | .990 |
| D10S1652 | 83.3 | .70 | 79 | .53 | 1.19 | .119 |
| D10S537 | 93.8 | .82 | 82 | .51 | .38 | .352 |
| D10S1686 | 109.2 | .66 | 82 | .45 | −1.75 | .958 |
| D10S185 | 123.3 | .78 | 83 | .52 | .66 | .256 |
| D10S192 | 131.2 | .84 | 82 | .49 | −.29 | .612 |
| D10S1269 | 140.2 | .64 | 81 | .50 | −.04 | .515 |
| D10S1693 | 146.1 | .82 | 77 | .44 | −1.96 | .973 |
| D10S587 | 156.6 | .82 | 83 | .45 | −1.52 | .934 |
| D10S217 | 167.2 | .82 | 81 | .49 | −.25 | .600 |
| D10S1651 | 178.3 | .64 | 80 | .47 | −.99 | .838 |
| D10S1711 | 180.5 | .61 | 80 | .46 | −1.47 | .928 |
| 11: | ||||||
| D11S4046 | 3.9 | .85 | 83 | .51 | .50 | .310 |
| D11S1338 | 14.9 | .62 | 83 | .53 | 1.21 | .114 |
| D11S902 | 24.7 | .84 | 80 | .54 | 1.14 | .129 |
| D11S904 | 37 | .71 | 83 | .50 | .10 | .460 |
| D11S935 | 49.6 | .72 | 82 | .50 | −.14 | .555 |
| D11S905 | 55.7 | .81 | 83 | .47 | −1.01 | .843 |
| D11S4191 | 63.4 | .88 | 83 | .54 | 1.22 | .113 |
| D11S987 | 67.5 | .84 | 83 | .55 | 1.75 | .042* |
| D11S1314 | 77.5 | .79 | 82 | .54 | 1.30 | .099 |
| D11S937 | 84.6 | .76 | 83 | .53 | 1.20 | .118 |
| D11S901 | 89.8 | .68 | 83 | .51 | .30 | .381 |
| D11S4175 | 96.3 | .84 | 82 | .50 | −.05 | .518 |
| D11S1339 | 104.8 | .70 | 82 | .52 | .61 | .272 |
| D11S4111 | 112.9 | .80 | 82 | .49 | −.16 | .564 |
| D11S925 | 123.5 | .81 | 83 | .54 | 1.11 | .136 |
| D11S4151 | 132.9 | .61 | 80 | .54 | 1.58 | .060 |
| D11S910 | 145.6 | .72 | 82 | .55 | 2.02 | .023* |
| D11S4125 | 152.8 | .74 | 81 | .51 | .44 | .332 |
| 12: | ||||||
| D12S352 | 0 | .68 | 83 | .46 | −1.74 | .957 |
| D12S99 | 13.9 | .81 | 82 | .46 | −1.36 | .911 |
| D12S336 | 21 | .74 | 83 | .50 | −.09 | .535 |
| D12S364 | 31.7 | .81 | 82 | .46 | −1.45 | .924 |
| D12S310 | 36.1 | .64 | 80 | .52 | .87 | .193 |
| D12S1617 | 45.1 | .84 | 83 | .48 | −.62 | .731 |
| D12S345 | 54.4 | .84 | 83 | .49 | −.30 | .619 |
| D12S85 | 62.7 | .80 | 82 | .45 | −1.41 | .919 |
| D12S368 | 67.3 | .66 | 82 | .49 | −.56 | .713 |
| D12S83 | 76.5 | .81 | 83 | .51 | .48 | .316 |
| D12S326 | 87.6 | .61 | 80 | .47 | −1.08 | .859 |
| D12S351 | 97.1 | .74 | 83 | .53 | .92 | .181 |
| D12S346 | 106.1 | .73 | 81 | .48 | −.60 | .726 |
| D12S78 | 113.3 | .79 | 81 | .53 | .92 | .180 |
| D12S79 | 126.1 | .80 | 82 | .49 | −.20 | .581 |
| D12S86 | 135.1 | .68 | 83 | .50 | .05 | .480 |
| D12S32 4 | 148.3 | .64 | 83 | .47 | −1.16 | .876 |
| D12S36v7 | 160.9 | .71 | 82 | .49 | −.39 | .649 |
| D12S1723 | 165.7 | .79 | 83 | .50 | −.04 | .515 |
| 13: | ||||||
| D13S175 | 7.4 | .67 | 83 | .49 | −.24 | .594 |
| D13S217 | 19.1 | .67 | 82 | .52 | .75 | .229 |
| D13S171 | 27.3 | .65 | 82 | .51 | .25 | .401 |
| D13S218 | 35.3 | .60 | 82 | .49 | −.28 | .611 |
| D13S263 | 40.4 | .81 | 82 | .52 | .64 | .262 |
| D13S153 | 47.5 | .89 | 83 | .56 | 1.84 | .034* |
| D13S156 | 57.3 | .82 | 83 | .54 | 1.4 | .083 |
| D13S170 | 65.4 | .83 | 82 | .54 | 1.21 | .114 |
| D13S265 | 70.6 | .66 | 83 | .53 | 1.09 | .138 |
| D13S159 | 81.5 | .73 | 82 | .50 | −.06 | .524 |
| D13S158 | 86.9 | .73 | 83 | .51 | .41 | .342 |
| D13S173 | 95.9 | .63 | 83 | .53 | .89 | .188 |
| D13S1265 | 101.7 | .83 | 82 | .51 | .27 | .396 |
| D13S285 | 112.8 | .85 | 78 | .51 | .38 | .353 |
| 14: | ||||||
| D14S261 | 0 | .59 | 82 | .51 | .22 | .413 |
| D14S283 | 7.5 | .8 | 83 | .52 | .83 | .203 |
| D14S275 | 21.9 | .6 | 83 | .51 | .52 | .303 |
| D14S70 | 32.9 | .67 | 83 | .45 | −1.77 | .960 |
| D14S288 | 39.1 | .87 | 83 | .53 | .90 | .187 |
| D14S276 | 47 | .77 | 83 | .54 | 1.35 | .090 |
| D14S63 | 59 | .76 | 83 | .53 | 1.01 | .158 |
| D14S258 | 65.8 | .64 | 83 | .55 | 1.86 | .034* |
| D14S74 | 76.4 | .8 | 83 | .58 | 2.82 | .003** |
| D14S68 | 86.3 | .83 | 83 | .52 | .55 | .292 |
| D14S280 | 95.5 | .68 | 83 | .48 | −.58 | .719 |
| D14S65 | 108.1 | .71 | 83 | .52 | .65 | .258 |
| D14S985 | 117.1 | .72 | 83 | .53 | 1.20 | .117 |
| D14S292 | 124.2 | .71 | 83 | .51 | .33 | .371 |
| 15: | ||||||
| D15S128 | 6.1 | .85 | 82 | .50 | −.14 | .556 |
| D15S1002 | 14.5 | .76 | 83 | .50 | −.07 | .528 |
| D15S1048 | 19.1 | .66 | 81 | .50 | −.11 | .543 |
| D15S1007 | 25.9 | .82 | 83 | .52 | .48 | .317 |
| D15S1042 | 32.3 | .78 | 81 | .49 | −.25 | .599 |
| D15S994 | 40 | .76 | 83 | .49 | −.21 | .582 |
| D15S978 | 45.5 | .74 | 81 | .50 | −.10 | .540 |
| D15S117 | 50.8 | .74 | 82 | .50 | −.01 | .503 |
| D15S153 | 62.1 | .79 | 83 | .51 | .38 | .352 |
| D15S131 | 70.7 | .75 | 82 | .49 | −.37 | .645 |
| D15S205 | 77.4 | .88 | 83 | .53 | .96 | .171 |
| D15S127 | 84.8 | .83 | 81 | .47 | −1.06 | .853 |
| D15S1004 | 95.7 | .62 | 81 | .48 | −.93 | .822 |
| D15S120 | 109.6 | .79 | 83 | .47 | −.97 | .832 |
| 16: | ||||||
| D16S423 | 8.4 | .85 | 75 | .51 | .22 | .413 |
| D16S404 | 16.7 | .69 | 82 | .51 | .30 | .383 |
| D16S3075 | 21.8 | .8 | 81 | .49 | −.17 | .566 |
| D16S3017 | 31.1 | .73 | 79 | .47 | −.94 | .824 |
| D16S3046 | 39.3 | .65 | 83 | .47 | −1.30 | .902 |
| D16S3068 | 46.6 | .73 | 83 | .54 | 1.36 | .089 |
| D16S3136 | 60 | .65 | 82 | .48 | −.70 | .758 |
| D16S415 | 65.6 | .69 | 81 | .48 | −.61 | .727 |
| D16S503 | 81.8 | .66 | 76 | .51 | .37 | .355 |
| D16S515 | 90.2 | .87 | 77 | .48 | −.59 | .723 |
| D16S516 | 98.3 | .72 | 75 | .48 | −.61 | .729 |
| D16S3091 | 109.1 | .83 | 83 | .50 | .02 | .491 |
| D16S520 | 123.3 | .8 | 80 | .52 | .68 | .248 |
| 17: | ||||||
| D17S849 | .6 | .74 | 83 | .48 | −.70 | .758 |
| D17S831 | 6.6 | .85 | 78 | .48 | −.57 | .714 |
| D17S938 | 14.8 | .82 | 81 | .48 | −.76 | .774 |
| D17S1852 | 23.2 | .8 | 83 | .49 | −.25 | .598 |
| D17S947 | 32.8 | .85 | 80 | .46 | −1.23 | .888 |
| D17S921 | 37.3 | .73 | 80 | .53 | 1.11 | .135 |
| D17S925 | 49.5 | .71 | 80 | .55 | 1.96 | .027* |
| D17S1872 | 58.3 | .90 | 79 | .47 | −.89 | .811 |
| D17S1868 | 65.1 | .78 | 76 | .52 | .56 | .290 |
| D17S787 | 75.7 | .83 | 83 | .46 | −1.25 | .892 |
| D17S948 | 84.1 | .70 | 80 | .48 | −.62 | .732 |
| D17S949 | 94.9 | .80 | 82 | .51 | .40 | .344 |
| D17S785 | 104.7 | .70 | 83 | .47 | −.98 | .836 |
| D17S784 | 117.7 | .60 | 83 | .51 | .41 | .340 |
| D17S928 | 128.7 | .83 | 82 | .52 | .46 | .325 |
| 18: | ||||||
| D18S59 | .1 | .80 | 76 | .55 | 1.68 | .049* |
| D18S63 | 7.9 | .71 | 80 | .47 | −1.25 | .892 |
| D18S452 | 17.7 | .81 | 82 | .50 | .04 | .485 |
| D18S1153 | 34.7 | .81 | 83 | .50 | −.14 | .554 |
| D18S53 | 40.4 | .82 | 78 | .48 | −.68 | .751 |
| D18S478 | 52.3 | .65 | 83 | .50 | −.14 | .554 |
| D18S1102 | 61.7 | .68 | 82 | .44 | −2.29 | .988 |
| D18S474 | 71.3 | .72 | 76 | .49 | −.39 | .651 |
| D18S64 | 83 | .82 | 83 | .50 | .06 | .476 |
| D18S68 | 94.4 | .72 | 78 | .50 | −.16 | .563 |
| D18S61 | 102.8 | .82 | 82 | .54 | 1.45 | .076 |
| D18S1161 | 112 | .74 | 82 | .54 | 1.41 | .082 |
| D18S462 | 118 | .72 | 81 | .50 | .02 | .494 |
| D18S70 | 123.8 | .75 | 82 | .50 | −.07 | .528 |
| 19: | ||||||
| D19S209 | 10.8 | .82 | 83 | .48 | −.56 | .712 |
| D19S894 | 15.4 | .81 | 74 | .49 | −.26 | .603 |
| D19S884 | 26 | .84 | 83 | .47 | −1.13 | .868 |
| D19S221 | 35.5 | .81 | 81 | .45 | −1.69 | .952 |
| D19S226 | 41.7 | .86 | 82 | .45 | −1.42 | .920 |
| D19S414 | 53.2 | .60 | 79 | .47 | −1.19 | .882 |
| D19S220 | 61.4 | .87 | 80 | .49 | −.35 | .637 |
| D19S420 | 66 | .79 | 82 | .51 | .21 | .419 |
| D19S902 | 76.2 | .79 | 83 | .51 | .33 | .370 |
| D19S921 | 91.7 | .84 | 75 | .52 | .50 | .309 |
| D19S418 | 97.5 | .65 | 83 | .49 | −.4 | .655 |
| D19S210 | 104.9 | .67 | 83 | .51 | .30 | .382 |
| 20: | ||||||
| D20S117 | 2.9 | .82 | 83 | .52 | .50 | .310 |
| D20S889 | 11 | .78 | 83 | .50 | .13 | .447 |
| D20S192 | 18.5 | .76 | 81 | .46 | −1.24 | .891 |
| D20S186 | 33.2 | .88 | 83 | .48 | −.42 | .661 |
| D20S112 | 39.3 | .73 | 83 | .51 | .26 | .399 |
| D20S195 | 50.2 | .74 | 81 | .52 | .92 | .180 |
| D20S107 | 54.9 | .71 | 82 | .48 | −.69 | .755 |
| D20S119 | 61 | .79 | 83 | .47 | −.97 | .833 |
| D20S178 | 65.5 | .77 | 82 | .48 | −.69 | .755 |
| D20S196 | 74.5 | .81 | 83 | .45 | −1.69 | .953 |
| D20S100 | 83.4 | .71 | 83 | .47 | −1.28 | .899 |
| D20S171 | 94.4 | .71 | 82 | .47 | −.98 | .836 |
| D20S173 | 96.5 | .61 | 83 | .53 | 1.11 | .136 |
| 21: | ||||||
| D21S1256 | 8.6 | .82 | 83 | .48 | −.45 | .671 |
| D21S1914 | 23 | .81 | 83 | .48 | −.50 | .690 |
| D21S263 | 31.4 | .82 | 81 | .54 | 1.14 | .129 |
| D21S1252 | 38.7 | .82 | 83 | .52 | .70 | .243 |
| D21S266 | 49.9 | .82 | 82 | .49 | −.38 | .649 |
| 22: | ||||||
| D22S420 | 0 | .70 | 82 | .47 | −1.19 | .881 |
| D22S446 | 9 | .65 | 82 | .46 | −1.55 | .938 |
| D22S315 | 16.2 | .80 | 83 | .46 | −1.21 | .884 |
| D22S280 | 25.9 | .79 | 83 | .49 | −.42 | .662 |
| D22S283 | 33.4 | .75 | 82 | .51 | .50 | .308 |
| D22S423 | 40.2 | .83 | 82 | .49 | −.20 | .581 |
| D22S274 | 45.5 | .84 | 79 | .51 | .29 | .386 |
P<.05.
P<.01.
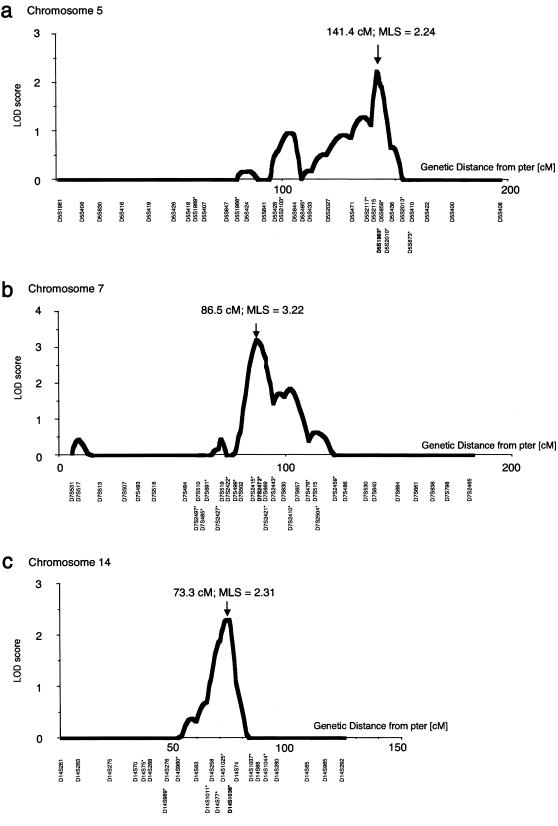
Next, all individuals in the 104 ASPs were genotyped by addition of microsatellite markers covering the candidate regions, on chromosomes 5q, 7q, and 14q, that showed putatively positive evidence of linkage; 10 markers on chromosome 5 (D5S1969, D5S1988, D5S2103, D5S495, D5S2117, D5S1983, D5S658, D5S2010, D5S2013, and D5S673), 14 markers on chromosome 7 (D7S2497, D7S485, D7S691, D7S2427, D7S2422, D7S499, D7S2415, D7S2472, D7S2421, D7S2443, D7S2410, D7S479, D7S2504, and D7S2459), and 9 markers on chromosome 14 (D14S75, D14S989, D14S980, D14S1011, D14S77, D14S1025, D14S1036, D14S1037, and D14S1044) were added, for high-resolution mapping (see The Whitehead Institute for Biomedical Research/MIT Center for Genome Research web site). Multipoint linkage analyses by GENEHUNTER revealed evidence of linkage to loci on chromosomes 5q22-31 (MLS 2.24, P=.00149), 7q11 (MLS 3.22, P=.00046), and 14q22 (MLS 2.31, P=.00120) (fig. 2); the MLSs were near markers D5S1983, D7S2472, and D14S1036, respectively. 1-LOD support intervals lay between D5S471 and D5S2010, between D7S2415 and D7S657, and between D14S258 and D14S74, comprising regions of ∼14, ∼21, and ∼11 cM, respectively.
Figure 2.
Results of multipoint linkage analyses for high-resolution mapping on chromosomes 5 (a), 7 (b), and 14 (c) in 104 Japanese sib pairs with IA. Positions of the MLS are indicated by arrows: MLS = 2.24, 3.22, and 2.31, and distances from the p-terminal end (pter) were 141.4, 86.5, and 73.3 cM, on chromosome 5, 7, and 14, respectively. The borders (defined by LOD >1.0) of positive linkage lie between markers D5S471 and D5S2010, D7S2415 and D7S657, and D14S258 and D14S74. Additional markers used for high-resolution mapping are indicated by asterisks (*).
Physical Map of the ELN Locus
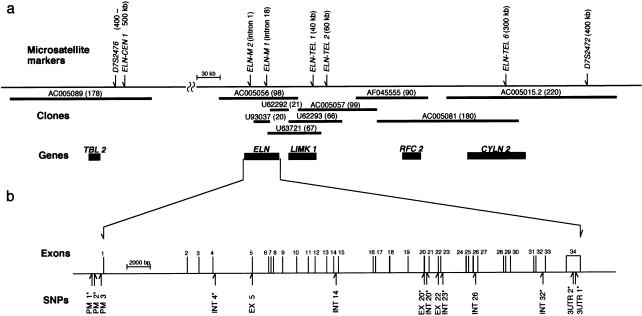
In the search for candidate genes in the linkage regions, the candidate gene ELN was found, 400 kb from marker D7S2472 on chromosome 7. Elastin is the predominant protein of mature elastic fibers in arterial walls. The ELN locus on 7q11.23 has been extensively analyzed in studies of WBS (Peoples et al. 2000) and supravalvular aortic stenosis (Curran et al. 1993; Li et al. 1997; Tassabehji et al. 1997). A physical map of the ELN locus was reconstructed on the basis of the GenBank database and a physical map of microdeletion in WBS (Peoples et al. 2000). A clone (accession number AC005056) contained the full length of ELN, and the contig was extended to marker D7S2472, telomeric to ELN. Another clone (accession number AC005089) was closest to ELN on the centromeric side; the sequences between ELN and the latter clone have not been registered in the GenBank database. Eight polymorphic dinucleotide or tetranucleotide repeats identified in the ELN locus were distributed as follows: D7S2476 and ELN-CEN1, both at 400–500 kb from ELN, ELN-M2 at intron 1 of ELN (Urban et al. 1997), ELN-M1 at intron 18 of ELN (Foster et al. 1993), ELN-TEL1 at 40 kb, ELN-TEL2 at 60 kb, ELN-TEL6 at 300 kb, and D7S2472 at 400 kb, respectively (fig. 3). All eight markers were tested for linkage, by SIBPAL. Although evidence of linkage was not strong, all the markers showed means >.5, for alleles sharing linkage, throughout the region (table 3). The allelic frequencies of the eight markers were compared in patients with IA versus controls. A weak allelic association was detected in the allele-frequency distribution of the marker ELN-M2 (χ2=19.22, df=8, P=.0137) (table 3). Considered together, these findings indicate that ELN is a primary candidate gene for IA.
Figure 3.
Physical map of ELN locus. a, Contigs and microsatellite-marker locations at locus. The thicker lines denote clones, which have been registered in the GenBank database, in the ELN locus; and the numbers in parentheses are the length (in kb) of the clones. The vertical arrows above the thinner lines indicate positions of eight microsatellite markers at the locus; and distances from ELN are in parentheses. Blackened rectangles indicate positions of known genes lying near ELN: TBL2 = transducin β-like 2; LIMK1 = LIM domain kinase 1; RFC2 = replication factor C 2; CYLN2 = cytoplasmic linker 2. b, Expanded view of 43-kb segment of ELN. The exon-intron organization of ELN and the positions of 14 distinct SNPs are indicated. Nine SNPs, indicated by asterisks (*), were used for pairwise haplotype-association study.
Table 3.
Linkage Analysis and Association Study Using Microsatellite Markers at the ELN Locus
|
Linkage Analysis |
Association Study |
|||||||
| Microsatellite Marker | Heterozygosity | Distance from ELN | IBD | t | P | No. of Alleles(CTR/IA)a | χ2 (df) | P |
| D7S2476 | .42 | 400–500 kb (centromere) | .5367 | 2.021 | .0232* | 384/328 | 13.52 (12) | .3327 |
| ELN-CEN1 | .12 | 400–500 kb (centromere) | .5018 | .138 | .4453 | 382/322 | 4.79 (3) | .1881 |
| ELN-M2 | .78 | Intron 1 (ELN) | .5413 | 1.499 | .0687 | 378/320 | 19.22 (8) | .0137* |
| ELN-M1 | .59 | Intron 18 (ELN) | .5051 | .228 | .4100 | 384/328 | 3.43 (5) | .6338 |
| ELN-TEL1 | .87 | 40 kb (telomere) | .5647 | 2.061 | .0212* | 382/322 | 20.78 (14) | .1075 |
| ELN-TEL2 | .76 | 60 kb (telomere) | .5302 | 1.209 | .1150 | 382/326 | 9.20 (10) | .5132 |
| ELN-TEL6 | .77 | 300 kb (telomere) | .5363 | 1.264 | .1049 | 374/320 | 8.39 (9) | .4952 |
| D7S2472 | .74 | 400 kb (telomere) | .6084 | 4.426 | .000029** | 374/328 | 8.80 (10) | .5511 |
CTR = controls; IA = patients with IA.
P<.05.
P<.01.
SNPs in ELN
ELN was extensively screened for SNPs. Systematic direct sequencing was performed on all 34 exons; acceptor, donor, and branch-point sequences of all introns; 1.0 kb of the putative promoter sequence (Kahari et al. 1990); and 1.2 kb of 3′UTR sequence. Fourteen distinct SNPs, including two previously published polymorphisms (Tromp et al. 1991; Urban et al. 1999), were identified (fig. 3 and table 4). Three of the SNPs occur in the coding regions: EX5, a C→T substitution at exon 5 (+16), and EX20, a G→A substitution at exon 20 (+114), resulted in amino acid substitutions A71V and G422S, respectively, whereas EX22, a G→A substitution at exon 22 (+23), was a silent substitution. Allelic frequencies of the 14 SNPs were compared in patients versus controls (table 4). Allelic-frequency differences between cases and controls were found for two SNPs; but they did not reach statistical significance (χ2=3.39, df=1, and P=.067, for INT20; and χ2=2.97, df=1, and P=.085, for 3UTR1) (table 4). All of the SNP frequencies of controls were in Hardy-Weinberg equilibrium.
Table 4.
Polymorphisms in ELN, and Association Study of Patients with IA and of Controls
|
Change |
Allele Frequencyb |
||||||
| SNP Name | Location (Position a) | M→mc | Amino Acid | Controls | Patients with IA | χ2d | P |
| PM 1 | Promoter (−1042) | C→T | .202 (77/382) | .208 (69/332) | .043 | .836 | |
| PM 2 | Promoter (−972) | G→A | .178 (68/382) | .145 (48/332) | 1.459 | .227 | |
| PM 3 | Promoter (−38) | C→T | .021 (4/188) | .040 (7/174) | 1.102 | .294 | |
| INT 4 | Intron 4 (+71) | G→A | .201 (76/378) | .178 (60/338) | .643 | .423 | |
| EX 5 | Exon 5 (+16) | C→T | Ala→Val | .021 (4/188) | .029 (5/174) | .207 | .649 |
| INT14 | Intron 14 (−28) | G→A | .016 (3/186) | .035 (6/170) | 1.324 | .250 | |
| EX 20 | Exon 20 (+114) | G→A | Gly→Ser | .189 (71/376) | .210 (71/338) | .503 | .478 |
| INT 20 | Intron 20 (+17) | T→C | .269 (101/376) | .210 (71/338) | 3.388 | .067 | |
| EX 22 | Exon 22 (+23) | G→A | Leu→Leu | .011 (4/376) | .018 (6/326) | .750 | .387 |
| INT 23 | Intron 23 (+24) | T→C | .294 (113/384) | .308 (106/344) | .166 | .684 | |
| INT 26 | Intron 26 (−20) | C→T | .016 (3/192) | .006 (1/174) | .824 | .364 | |
| INT 32 | Intron 32 (−34) | C→T | .052 (20/384) | .056 (19/340) | .051 | .821 | |
| 3UTR 1 | 3′UTR (+502) | A insertion | .102 (39/382) | .144 (49/340) | 2.968 | .085 | |
| 3UTR 2 | 3′UTR (+659) | G→C | .050 (19/382) | .060 (20/336) | .153 | .696 | |
No. of nucleotides from nearest start of promoter, intron, exon, or 3′UTR.
CTR = controls; IA = patients with IA.
M = major, common allele; m = minor, less common allele.
df = 1.
Pairwise Haplotype Association Study of IA
Thirty-six pairwise haplotype combinations were constructed from nine SNPs (i.e., PM1, PM2, INT4, EX20, INT20, INT23, INT32, 3UTR1, and 3UTR2; table 4) that showed relatively high allelic frequencies (⩾.05). The pairwise haplotype frequencies were calculated by a maximum-likelihood estimation using theARLEQUIN program, and the haplotype frequencies were compared in the 172 patients with IA versus the 192 controls (fig. 4); most often, two SNPs of any combination created four haplotypes, reflecting weak LD throughout the gene. The haplotype frequencies of all pairwise combinations then were compared in a global test with 3 df. The best evidence of haplotype association was observed for INT20/INT23 (χ2=27.90, df=3, P=3.81×10-6). An Mm haplotype (major allele [i.e., M] for INT20 and minor allele [i.e., m] for INT23) was more prevalent in patients with IA than in controls (χ2=11.17, df=1, P=.0008), with an odds ratio of 1.85 (95%CI 1.53–2.65). Subjects who were homozygous for the Mm haplotype and whose haplotype was unambiguously determined appeared more frequently among patients with IA than among controls (10.7% vs. 2.7%; χ2=9.52, df=1, P=.002), with an odds ratio of 4.39 (95%CI 2.62–12.11). Significant associations also were detected in the haplotype-frequency distribution of two combinations: PM2/INT23 (χ2=14.85, df=3, P=1.94×10-3), and INT4/INT23 (χ2=24.10, df=3, P=2.39×10-4) (fig. 4).
Figure 4.
Association study using pairwise haplotype frequencies in patients with IA versus those in controls. P values <.01 are indicated by double asterisks (**).
Pairwise LD of ELN
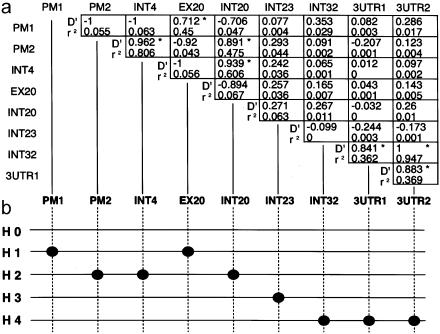
The extent of pairwise LD in the 36 pairs of combinations was investigated in complete detail, by two LD measures—D′ and r2—in 192 controls. LD is generally a measure of distance between SNPs. However, in ELN, the distribution of LD is highly irregular, and generally weak degrees were observed between the SNPs (fig. 5a). Among these, LD was conserved at PM1/EX20, PM2/INT4, PM2/INT20, INT4/INT20, INT32/3UTR1, INT32/3UTR2, and 3UTR1/3UTR2 (D′>.7 and r2>.3). A very weak LD was indicated between INT23 and other SNPs. A similar LD was observed for patients with IA. According to the extent of LD, the five putative ancestral haplotype groups could be classified: H0 = no polymorphism in the allele; H1= polymorphisms at PM1 and EX20; H2 = polymorphisms at PM2, INT4, and INT20; H3 = polymorphism at INT23; and H4 = polymorphisms at INT32, 3UTR1, and 3UTR2 (fig. 5b). Because Mm haplotypes at PM2/INT23, INT4/INT23, and INT20/INT23 were significantly more frequent in patients with IA than in controls (fig. 4), it may well be that the ancestral H3 haplotype puts an individual at risk for IA. A recombinant haplotype of either H2 and H3 or INT20 and INT23 was more common in controls than in patients with IA.
Figure 5.
Pairwise LD between SNPs in ELN. a, Extent of pairwise LD of ELN, measured by two distinct formulas. The upper and lower numerals in each box indicate coefficient D′ and r2, respectively. Formulas for D′ and r2 are described in the text. Pairwise combinations with relatively high LD measures (defined by D′>.7 and r2>.3) in 192 controls are indicated, by boxes containing an asterisk (*). b, Illustration of five ancestral haplotype groups. Black dots represent the SNPs at each site. H0 = no polymorphism in the allele; H1 = polymorphisms at PM1 and EX20; H2 = polymorphisms at PM2, INT4, and INT20; H3 = one polymorphism, at INT23; H4 = polymorphisms at INT32, 3UTR1, and 3UTR2.
Discussion
In the present study, we have identified, by genomewide linkage study and the candidate-gene approach, a molecular determinant of IA, a determinant that suggests both the pathogenesis of the disease and also novel therapeutic targets. Evidence of linkage throughout the genome in 83 Japanese ASPs was established first by SIBPAL analysis, in which excess allele sharing was found. Evidence of linkage was observed at markers on chromosomes 5q, 7q, and 14q. Dense mapping with all 104 ASPs was performed in these regions by multipoint analysis with GENEHUNTER. The best evidence of linkage was observed on chromosome 7q11, near marker D7S2472. In the three chromosomal regions, the candidate genes for either vascular components or vascular formation are those for lysyl oxidase (LOX), fibrillin 2 (FBN2), and fibroblast growth factor 1 (FGF1), all on chromosome 5; ELN and the genes for KREV interaction trapped 1 (KIRT1) and collagen type 1 α2 (COL1A2), all on chromosome 7; and that for latent transforming growth factor β–binding protein 2 (LTBP2), on chromosome 14.
Defects relating to the medial muscle layer at the branch points of intracranial major arteries, favorable sites for IA, are observed frequently, and external elastic lamina is absent in cerebral arteries. Internal elastic lamina is the major structural support in the cerebral vessels (Glynn 1940). It is interesting to note that the several genes associated with elastic-fiber production—that is, LOX, FBN2, and ELN—lie within the linkage regions. Pathology examination of IA often reveals a defect in the internal elastic lamina of IA lesions (Carmichael 1945, 1950; Stebens 1963). Animal models of IA have been established by treatment with either elastase (Anidjar et al. 1990; Miskolczi et al. 1998) or β-aminopropionitrile, which inhibit cross-linking reactions between elastin molecules (Hashimoto et al. 1978). Defects in or degeneration of the internal elastic lamina, therefore, might play an important role in the etiology of IA.
In this study, ELN was extensively screened for the presence of molecular variants, since (a) it gene lies very close to the marker—D7S2472—that showed the best evidence for linkage and (b) elastin constitutes the predominant protein in elastic fibers. We identified 14 SNPs in ELN, and the allelic frequencies in patients with IA were compared with those in controls. Although there appeared to be no allelic association with any SNP, increasing the sample size led to statistical significance, at the intron 20 polymorphism. Pairwise haplotype analysis was performed with combinations of nine SNPs (fig. 4). Haplotype analysis can reveal the degree of predisposition of a specific allele to a disease and is especially useful when causal variants have not been identified. The Mm haplotype at INT20/INT23 was observed more frequently in patients with IA than in controls and indicates risk for IA among Japanese (χ2=27.90, df=3, P=3.81×10-6) (fig. 4). The functional role of the ELN haplotype in pathophysiology is not known. Similarly, a recent report has shown that a specific haplotype combination of calpain-10 is a risk factor for type II diabetes in a Mexican American population (Horikawa et al. 2000). That study suggests that heterozygosity for two different, common haplotypes may be necessary for the development of diabetes. Analyses of all the possible pairwise LD revealed weak LD throughout ELN, and the pairwise LD between INT23 and others in the vicinity was especially weak (fig. 5). ELN is highly rich in Alu repeats, with possibly ⩾30 Alu sequences within the 43-kb region (data not shown). Alu repeats may be associated with genome instability (Calabretta et al. 1982), which could partly explain the low LD observed in ELN. Indeed, loss of the exons in primates may be due to an Alu-mediated recombination event that might confer an evolutionary advantage in elastic tissue (Szabo et al. 1999). It is curious that poor LD was found between INT20 and INT23, whereas a strong association was observed in the haplotype created by the two SNPs. In general, with phase-unknown samples, the haplotype may not be precisely defined at low LD; however, individuals who were homozygous for the Mm haplotype and whose haplotype was unambiguously determined were remarkably more common among the patients with IA patients than among controls (10.7% vs. 2.7%; χ2=9.52, df=1, P=.002), with an odds ratio of 4.39. The Mm haplotype, therefore, is associated with IA, and a disease-causing variant should lie either on the allele within ELN or, possibly, in a nearby gene.
We have mapped three chromosomal loci for IA and have identified a candidate gene, ELN, on the basis of its chromosomal position and function. We have determined that the Mm haplotype at INT20/INT23 indicates risk for the disease in the Japanese. Long-term investigation including replication studies in distinct ethnic groups, as well as functional studies using biochemical and cellular biological techniques, will be necessary to clarify the mechanism of the relationship between the genetic variation and the disease.
Acknowledgments
This work was supported in part by a Research for the Future Program Grant (to I.I.) from The Japan Society for the Promotion of Science and by a grant-in-aid for scientific research (C) from the Japanese Ministry of Education, Science, Sports and Culture (to H.K.). The following investigators participated in diagnosis and recruitment of sib pairs with IA: in Aichi—T. Kawabe; in Akita—N. Yanagida and A. Sugawara; in Aomori—K. Ito; in Chiba—S. Watanabe; in Ehime—T. Shiraishi; in Fukui—K. Kashiwabara and A. Arai; in Fukuoka—H. Egami and T. Soejima; in Fukushima—T. Nagayama, H. Kojima, H. Abiko, and S. Ishikawa; in Gumma—S. Omori, S. Nakajima, M. Kobayashi, and S. Takayama; in Hiroshima—K. Yuki, S. Tsuchimoto, K. Watanabe, S. Sato, and K. Mukada; in Hokkaido—C. Obonai, M. Hashimoto, T. Matsuzaki, T. Sasaki, and H. Kamata; in Hyogo—Y. Kang and H. Kudo; in Ibaraki—S. Tsuruoka; in Ishikawa—S. Someya; in Iwate—T. Yamanome; in Kagawa—Y Baba; in Kagoshima—K. Kimotsuki, T. Masuda, T. Tomosugi, H. Tokimura, and T. Masuda; in Kanagawa—Y. Miyasaka, T. Kuramitsu, and N. Takenaka; in Kochi—Y. Ohara; in Kumamoto—T. Masumitsu; in Kyoto—F. Asakura; in Mie—Y. Domoto; in Miyagi—K. Koshu, Y. Nagamine, and H. Oyama; in Miyazaki—S. Kodama; in Nagano—F. Nakagawa, T. Iwashita, G. Momose, A. Yokoo, K. Kawano, S. Usuda, K. Hongo, and T. Tsuji; in Nara—I. Nakagawa; in Niigata—T. Tsuchida, H. Abe, and I. Ezuka; in Okinawa—H. Shimabukuro; in Osaka—M. Shiguma, S. Yoneda, T. Suzuki, H. Nakagawa, M. Ouchi, Y. Shimamura, and H. Nakajima; in Saitama—S. Okui, H. Wanifuchi, T. Shimizu, K. Okada, S. Iwasa, U. Kaneko, and S. Yato; in Shiga—T. Yamada, M. Uchibori, A. Hino, and M. Ichikawa; in Shizuoka—H. Uchiyama, T. Shimada, E. Kihashi, and K. Mori; in Tochigi—M. Uchida, J. Narita, and M. Yodonawa; in Tokyo—T. Hanada, M. Nakajima, and A. Oikawa; in Toyama—T. Komai; in Wakayama—K. Miki; in Yamagata—E. Kamatsuka, K. Sato, S. Sato, and Y. Ito; in Yamaguchi—H. Adachi and T. Tokumaru; and, in Yamanashi—N. Aoki and J. Kuwazawa.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- ARLEQUIN, http://anthro.unige.ch/arlequin (for software for population genetics data analysis)
- GenBank Overview, http://www.ncbi.nlm.nih.gov/Genbank/GenbankOverview.html (for ELN locus [accession numbers AC005089, AC005056, U93037, U63721, U62292, U62293, AC005057, AF045555, AC005081, AC005015.2, and M36860])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for IA [MIM 105800])
- Whitehead Institute for Biomedical Research/MIT Center for Genome Research, The, http://www-genome.wi.mit.edu/ (for markers D5S1969, D5S1988, D5S2103, D5S495, D5S2117, D5S1983, D5S658, D5S2010, D5S2013, D5S673, D7S2497, D7S485, D7S691, D7S2427, D7S2422, D7S499, D7S2415, D7S2472, D7S2421, D7S2443, D7S2410, D7S479, D7S2504, D7S2459, D14S75, D14S989, D14S980, D14S1011, D14S77, D14S1025, D14S1036, D14S1037, and D14S1044)
References
- Anidjar S, Salzmann JL, Gentric D, Lagneau P, Camilleri JP, Michel JB (1990) Elastase-induced experimental aneurysms in rats. Circulation 82:973–981 [DOI] [PubMed] [Google Scholar]
- Calabretta B, Robberson DL, Barrera-Saldana HA, Lambrou TP, Saunders GF (1982) Genome instability in a region of human DNA enriched in Alu repeat sequences. Nature 296:219–225 [DOI] [PubMed] [Google Scholar]
- Carmichael R (1945) Gross defects in the muscular and elastic coats of the larger cerebral arteries. J Pathol Bacteriol 57:345–351 [Google Scholar]
- ——— (1950) The pathogenesis of non-inflammatory cerebral aneurysms. J Pathol Bacteriol 62:1–19 [DOI] [PubMed] [Google Scholar]
- Curran ME, Atkinson DL, Ewart AK, Morris CA, Leppert MF, Keating MT (1993) The elastin gene is disrupted by a translocation associating with supravalvular aortic stenosis. Cell 73:159–168 [DOI] [PubMed] [Google Scholar]
- Elston R, Bailey-Wilson J, Bonney G, Tran L, Keats B, Wilson A (1997) Sib-pair linkage program (SIBPAL). In: S.A.G.E., Statistical Analysis for Genetic Epidemiology, release 3.1. Case Western Reserve University, Cleveland [Google Scholar]
- Foster K, Ferrell R, King-Underwood L, Povey S, Attwood J, Rennick R, Humphries SE, Henney AM (1993) Description of a dinucleotide repeat polymorphism in the human elastin gene and its use to confirm assignment of the gene to chromosome 7. Ann Hum Genet 57:87–96 [DOI] [PubMed] [Google Scholar]
- Glynn L (1940) Medial defects in the circle of Willis and their relation to aneurysm formation. J Pathol Bacteriol 51:213–222 [Google Scholar]
- Hashimoto N, Handa H, Hazama F (1978) Experimentally induced cerebral aneurysms in rats. Surg Neurol 10:3–8 [PubMed] [Google Scholar]
- Hill WG, Robertson A (1968) Linkage disequilibrium in finite populations. Theor Appl Genet 38:226–231 [DOI] [PubMed] [Google Scholar]
- Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M, Hinokio Y, et al (2000) Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat Genet 26:163–175 [DOI] [PubMed] [Google Scholar]
- Inagawa T, Ishikawa S, Aoki H, Takahashi M, Yoshimoto H (1988) Aneurysmal subarachnoid hemorrhage in Izumo City and Shimane Prefecture of Japan: incidence. Stroke 19:170–175 [DOI] [PubMed] [Google Scholar]
- Inagawa T, Tokuda Y, Ohbayashi N, Takaya M, Moritake K (1995) Study of aneurysmal subarachnoid hemorrhage in Izumo City, Japan. Stroke 26:761–766 [DOI] [PubMed] [Google Scholar]
- Iwamoto H, Kiyohara Y, Fujishima M, Kato I, Nakayama K, Sueishi K, Tsuneyoshi M (1999) Prevalence of intracranial saccular aneurysms in a Japanese community based on a consecutive autopsy series during a 30-year observation period: the Hisayama study. Stroke 30:1390–1395 [DOI] [PubMed] [Google Scholar]
- Kahari VM, Fazio MJ, Chen YQ, Bashir MM, Rosenbloom J, Uitto J (1990) Deletion analyses of 5′-flanking region of the human elastin gene: delineation of functional promoter and regulatory cis-elements. J Biol Chem 265:9485–9490 [PubMed] [Google Scholar]
- Kasuya H, Onda H, Takeshita M, Hori T, Takakura K (2000) Clinical features of intracranial aneurysms in siblings. Neurosurgery 46:1301–1305 [DOI] [PubMed] [Google Scholar]
- Kiyohara Y, Ueda K, Hasuo Y, Wada J, Kawano H, Kato I, Sinkawa A, Ohmura T, Iwamoto H, Omae T, Fujishima M (1989) Incidence and prognosis of subarachnoid hemorrhage in a Japanese rural community. Stroke 20:1150–1155 [DOI] [PubMed] [Google Scholar]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- Lewontin RC (1964) The interaction of selection and linkage. I. General considerations: heterotic models. Genetics 49:49–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Toland AE, Boak BB, Atkinson DL, Ensing GJ, Morris CA, Keating MT (1997) Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol Genet 6:1021–1028 [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Nelson LM, Koepsell TD, van Belle G (1993) Clinical course of spontaneous subarachnoid hemorrhage: a population-based study in King County, Washington. Neurology 43:712–718 [DOI] [PubMed] [Google Scholar]
- Miskolczi L, Guterman LR, Flaherty JD, Hopkins LN (1998) Saccular aneurysm induction by elastase digestion of the arterial wall: a new animal model. Neurosurgery 43:595–600 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Hashi K (1994) The incidence and treatment of asymptomatic, unruptured cerebral aneurysms. J Neurosurg 80:217–223 [DOI] [PubMed] [Google Scholar]
- Peoples R, Franke Y, Wang YK, Perez-Jurado L, Paperna T, Cisco M, Francke U (2000) A physical map, including a BAC/PAC clone contig, of the Williams-Beuren syndrome–deletion region at 7q11.23. Am J Hum Genet 66:47–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LH, Whisnant JP, O'Fallon WM, Sundt TM (1980) The unchanging pattern of subarachnoid hemorrhage in a community. Neurology 30:1034–1040 [DOI] [PubMed] [Google Scholar]
- Ronkainen A, Hernesniemi J, Puranen M, Niemitukia L, Vanninen R, Ryynanen M, Kuivaniemi H, et al (1997) Familial intracranial aneurysms. Lancet 349:380–384 [DOI] [PubMed] [Google Scholar]
- Sacco RL, Wolf PA, Bharucha NE, Meeks SL, Kannel WB, Charette LJ, McNamara PM, Palmer EP, D'Agostino R (1984) Subarachnoid and intracerebral hemorrhage: natural history, prognosis, and precursive factors in the Framingham Study. Neurology 34:847–854 [DOI] [PubMed] [Google Scholar]
- Schievink WI (1997) Genetics of intracranial aneurysms. Neurosurgery 40:651–662 [DOI] [PubMed] [Google Scholar]
- Schievink WI, Schaid DJ, Michels VV, Piepgras DG (1995a) Familial aneurysmal subarachnoid hemorrhage: a community-based study. J Neurosurg 83:426–429 [DOI] [PubMed] [Google Scholar]
- Schievink WI, Schaid DJ, Rogers HM, Piepgras DG, Michels VV (1994) On the inheritance of intracranial aneurysms. Stroke 25:2028–2037 [DOI] [PubMed] [Google Scholar]
- Schievink WI, Wijdicks EF, Parisi JE, Piepgras DG, Whisnant JP (1995b) Sudden death from aneurysmal subarachnoid hemorrhage. Neurology 45:871–874 [DOI] [PubMed] [Google Scholar]
- Stebens WE (1963) Histopathology of cerebral aneurysms. Arch Neurol 8:272–285 [DOI] [PubMed] [Google Scholar]
- Szabo Z, Levi-Minzi SA, Christiano AM, Struminger C, Stoneking M, Batzer MA, Boyd CD (1999) Sequential loss of two neighboring exons of the tropoelastin gene during primate evolution. J Mol Evol 49:664–671 [DOI] [PubMed] [Google Scholar]
- Tassabehji M, Metcalfe K, Donnai D, Hurst J, Reardon W, Burch M, Read AP (1997) Elastin: genomic structure and point mutations in patients with supravalvular aortic stenosis. Hum Mol Genet 6:1029–1036 [DOI] [PubMed] [Google Scholar]
- Tromp G, Christiano A, Goldstein N, Indik Z, Boyd C, Rosenbloom J, Deak S, Prockop D, Kuivaniemi H (1991) A to G polymorphism in ELN gene. Nucleic Acids Res 19:4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujiie H, Sato K, Onda H, Oikawa A, Kagawa M, Takakura K, Kobayashi N (1993) Clinical analysis of incidentally discovered unruptured aneurysms. Stroke 24:1850–1856 [DOI] [PubMed] [Google Scholar]
- Urban Z, Csiszar K, Fekete G, Boyd CD (1997) A tetranucleotide repeat polymorphism within the human elastin gene (ELNi1). Clin Genet 51:133–134 [DOI] [PubMed] [Google Scholar]
- Urban Z, Michels VV, Thibodeau SN, Donis-Keller H, Csiszar K, Boyd CD (1999) Supravalvular aortic stenosis: a splice site mutation within the elastin gene results in reduced expression of two aberrantly spliced transcripts. Hum Genet 104:135–142 [DOI] [PubMed] [Google Scholar]