Abstract
Birt-Hogg-Dubé syndrome (BHD), an inherited autosomal genodermatosis characterized by benign tumors of the hair follicle, has been associated with renal neoplasia, lung cysts, and spontaneous pneumothorax. To identify the BHD locus, we recruited families with cutaneous lesions and associated phenotypic features of the BHD syndrome. We performed a genomewide scan in one large kindred with BHD and, by linkage analysis, localized the gene locus to the pericentromeric region of chromosome 17p, with a LOD score of 4.98 at D17S740 (recombination fraction 0). Two-point linkage analysis of eight additional families with BHD produced a maximum LOD score of 16.06 at D17S2196. Haplotype analysis identified critical recombinants and defined the minimal region of nonrecombination as being within a <4-cM distance between D17S1857 and D17S805. One additional family, which had histologically proved fibrofolliculomas, did not show evidence of linkage to chromosome 17p, suggesting genetic heterogeneity for BHD. The BHD locus lies within chromosomal band 17p11.2, a genomic region that, because of the presence of low-copy-number repeat elements, is unstable and that is associated with a number of diseases. Identification of the gene for BHD may reveal a new genetic locus responsible for renal neoplasia and for lung and hair-follicle developmental defects.
In 1977, Birt, Hogg, and Dubé described a kindred in which 15 of 70 family members developed multiple, small, white or skin-colored papules on the face, neck, and upper trunk, after the age of 25 years (Birt et al. 1977). Histologic evaluation of the papules identified anastomosing strands of epithelial cells emanating from hair follicles surrounded by loose connective-tissue fibers and mucin-rich stroma. These aberrant hamartomas of the hair-follicle structure, called “fibrofolliculomas,” were often found associated with trichodiscomas and achrocordons (Cohen and Kurzrock 1995; Scalvenzi et al. 1998) and were inherited in an autosomal dominant manner. This triad of dermatologic lesions has become known as the “Birt-Hogg-Dubé syndrome” (BHD). Subsequent reports have described patients in which the cutaneous lesions of BHD are associated with renal tumors (Roth et al. 1993; Toro et al. 1999), spontaneous pneumothorax or lung cysts (Chung et al. 1996; Toro et al. 1999), and/or colonic polyps (Rongioletti et al. 1989). To date, a genetic locus for BHD has not been identified.
As part of an ongoing study of families with inherited renal cancer, we recently described a new type of inherited renal neoplasia, familial renal oncocytoma (FRO), in five families with multiple members affected with bilateral, multiple renal oncocytomas (Weirich et al. 1998). On dermatologic examination, 13 members of three of these five families with FRO were found to have cutaneous BHD lesions (Toro et al. 1999). Subsequently, through mass mailings to dermatologists, we identified 33 families with BHD skin lesions and associated renal tumors, lung cysts or pneumothorax, and/or colonic polyps, including the original Canadian family (family 172) described by Birt et al. (1977). In a separate study (B. Zbar, G. Alvord, G. Glenn, M. Turner, C. Pavlovich, J. Toro, M. Merino, L. Schmidt, M. Walther, P. Choyke, G. Weirich, S. Hewitt, P. Duray, F. Gibril, C. Greenberg, and W. M. Linehan, unpublished data), we have found evidence that BHD confers an increased risk for the development of renal tumors and lung cysts or spontaneous pneumothorax but not for the development of colonic polyps. In the present study, we used family 172 to perform an initial genomewide scan and two-point linkage analysis. We subsequently performed linkage analysis in an additional eight families to identify the disease-gene locus for BHD.
Families with two or three generations of affected members were recruited and evaluated on field trips and at the National Institutes of Health (NIH) Clinical Center. Skin and oral examinations were performed, and skin lesions suspicious for fibrofolliculomas were biopsied for histology. Blood samples were taken for DNA extraction by standard phenol/chloroform methods. Screening of patients included renal ultrasound and abdominal computed-tomography (CT) scan to detect renal neoplasms, colonoscopy to detect colonic polyps, high-resolution thoracic CT scan to detect air-filled lung cysts (blebs), and standard chest CT and chest x-ray examination to look for evidence of old or new pneumothoraces. Histology of previously resected renal tumors was reviewed and diagnosed according to criteria established by the National Cancer Institute (NCI) Laboratory of Pathology. Signed, informed consent was obtained from all participants in the study, according to the NCI institutional review board–approved protocol.
We chose family 172, a large three-generation family with 18 affected members, for the initial genome scan. Simulation of linkage was first performed to assess the power of data on this pedigree to produce a LOD score >3.0 at a disease-linked locus. Linkage analysis with simulated genotyping data, forcing linkage between the disease locus and a hypothetical polymorphic marker locus, produced a LOD score of 6.1 in family 172 (data not shown). Subsequently, we performed a genomewide scan in which 40 family members were genotyped with 185 polymorphic microsatellite markers (Dib et al. 1996), at a resolution of 10–15 cM, covering 19 autosomal chromosomes. Radiolabeled alleles were amplified from genomic DNA templates by PCR, separated on 6% polyacrylamide gels, exposed to x-ray films, and scored. Two-point LOD scores were calculated with MLINK in LINKAGE package version 5.1 (Lathrop et al. 1984) and the low-penetrance model (i.e., 10% risk of development of BHD at age ⩽40 years and maximum [40%] risk at age >40 years). Autosomal dominant inheritance was assumed, with a gene frequency of .0001 and three liability classes. Allele frequencies were estimated on the basis of the genotypes of unrelated spouses. An individual was considered to be affected when he or she displayed (a) >10 skin lesions with the clinical appearance of fibrofolliculoma and (b) a minimum of one histologically confirmed fibrofolliculoma. The affection status of asymptomatic obligate carriers was considered to be unknown.
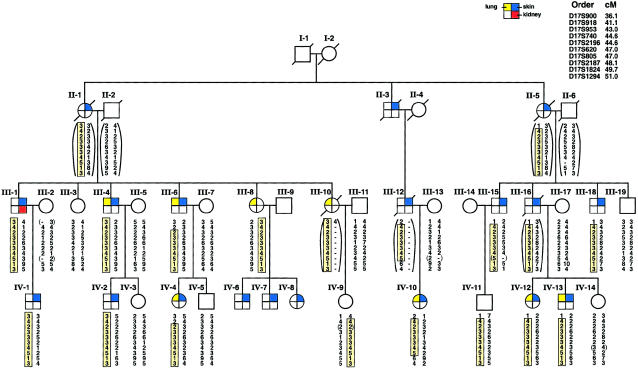
The results of the primary genomewide search for the BHD-gene locus strongly supported linkage to chromosome 17 in family 172. All of the 185 markers from other regions of the genome gave LOD scores <−1.2 at a recombination fraction (θ) of 0, with the exception of four markers. The low heterozygosity of D1S1665, D2S434, D9S915, and D8S1477 in family 172 gave weakly positive LOD scores (0.18–0.83); in all cases, however, negative LOD scores were obtained with markers immediately flanking these loci, ruling out linkage at these sites. The BHD-gene candidate interval spanned an 8.5-cM region located at chromosome 17p11.1-p12. Two -point linkage analysis for six microsatellite markers gave LOD scores >2.9 when the low-penetrance model was used (table 1). A maximum two-point LOD score (Zmax) of 4.98 at a maximum θ (θmax) of 0 was obtained with marker D17S740. Recombinants were identified at the flanking telomeric marker D17S918 (Zmax 1.77 at θmax=.05) and the centromeric marker D17S1824 (Zmax 4.17 at θmax=.05). Haplotype analysis (a) indicated that all individuals in family 172 who had fibrofolliculomas shared the affected haplotype from D17S953 to D17S2187 and (b) identified individuals IV-9, IV-11, and III-8 as asymptomatic carriers of the BHD haplotype (fig. 1). Individual III-8 is an obligate carrier of the disease gene, since three of her children are affected with fibrofolliculomas and have inherited the affected haplotype (data not shown). For purposes of linkage analysis, her affected status was considered to be unknown. Changing the affected status of asymptomatic haplotype carriers from unaffected to unknown raised the LOD score for D17S740 to 5.25. LOD scores generated with the less-permissive, high-penetrance model (i.e., 40% risk of development of BHD at age ⩽40 years of age and maximum [80%] risk at age >40 years) increased for all six nonrecombinant markers (data not shown).
Table 1.
Two-Point LOD Scores for Chromosome 17p Markers in Family 172
|
LOD Score at θ = |
||||||||
| Markera | .00 | .01 | .05 | .10 | .20 | .30 | .40 | Zmax (θmax) |
| D17S918 | −1.74 | 1.30 | 1.77 | 1.77 | 1.44 | .95 | .39 | 1.77 (.05) |
| D17S953 | 3.18 | 3.11 | 2.81 | 2.43 | 1.65 | .88 | .23 | 3.18 (.00) |
| D17S740 | 4.98 | 4.88 | 4.50 | 4.00 | 2.96 | 1.88 | .81 | 4.98 (.00) |
| D17S2196 | 4.07 | 3.99 | 3.69 | 3.30 | 2.49 | 1.66 | .84 | 4.07 (.00) |
| D17S620 | 2.93 | 2.87 | 2.61 | 2.29 | 1.63 | .97 | .35 | 2.93 (.00) |
| D17S805 | 3.75 | 3.68 | 3.40 | 3.04 | 2.28 | 1.47 | .61 | 3.75 (.00) |
| D17S2187 | 4.37 | 4.28 | 3.88 | 3.37 | 2.29 | 1.21 | .33 | 4.37 (.00) |
| D17S1824 | 2.51 | 3.87 | 4.17 | 3.98 | 3.21 | 2.22 | 1.06 | 4.17 (.05) |
Order is from telomere to centromere and is based on the Marshfield map (see the Center for Medical Genetics, Marshfield Medical Research Foundation web site).
Figure 1.
Abridged pedigree and haplotype analysis of family 172. Skin (blue), lung (yellow), and kidney (red) phenotypes are indicated by the colored quadrants in the symbol for each individual. For linkage analysis, individuals with histologically positive fibrofolliculomas were considered to be affected. Marker order and genetic distance from the chromosome 17p telomere are indicated. Haplotypes were generated under the assumption that the smallest number of recombination events was present. Shaded boxes represent the affected haplotype commonly shared by family members with BHD. Genotypes in parentheses were inferred on the basis of data on relatives. Individual III-8 is an obligate carrier of the disease gene, because three of her children are positive for fibrofolliculomas. Individuals IV-9 and IV-11 are asymptomatic carriers of the affected haplotype. Critical recombinants IV-10 and III-6 define the BHD minimal region of linkage to an 8.5-cM distance between D17S918 and D17S1824.
Subsequently, we performed linkage analysis, using the high-penetrance model, in eight additional families with BHD, with the markers from chromosome 17p, which cosegregated with BHD in family 172 (table 2). For all nine families, a Zmax of 16.06 at θmax=0 was obtained with the most informative marker, D17S2196. When the affected status of asymptomatic haplotype carriers was changed from unaffected to unknown, the total LOD score increased to 19.17. Multipoint linkage analysis performed with the VITESSE and FASTLINK programs (Cottingham et al. 1993; O’Connell et al. 1995), using the high-penetrance model and data from samples from all nine families, supported a disease-locus location between flanking recombinant markers D17S953 and D17S805, very near D17S2196, with a LOD score of 22.44 at θ=0. (Intermarker distances reported by Dib et al. [1996] are as follows: D17S953–1.6 cM–D17S2196–2.4 cM–D17S805.)
Table 2.
LOD Scores between BHD Locus and 17p11 Markers in Nine Families with BHD
|
LOD Score at θ = |
|||||||||
| Markera | Distance from pterb(cM) | .00 | .01 | .05 | .10 | .20 | .30 | .40 | Zmax (θmax) |
| D17S953 | 43.01 | 8.54 | 12.34 | 12.01 | 10.88 | 7.93 | 4.57 | 1.45 | 12.40 (.02) |
| D17S740 | 44.62 | 11.88 | 11.69 | 10.87 | 9.70 | 7.05 | 4.18 | 1.48 | 11.88 (.00) |
| D17S2196 | 44.62 | 16.06 | 15.81 | 14.77 | 13.30 | 9.94 | 6.19 | 2.48 | 16.06 (.00) |
| D17S620 | 47.00 | 8.96 | 8.79 | 8.05 | 7.09 | 5.03 | 2.93 | 1.02 | 8.96 (.00) |
| D17S805 | 47.00 | 3.68 | 6.99 | 7.28 | 6.89 | 5.41 | 3.47 | 1.38 | 7.29 (.03) |
| D17S1824 | 49.67 | .11 | 10.82 | 11.88 | 11.34 | 8.94 | 5.76 | 2.42 | 11.88 (.05) |
Nonrecombining markers are underlined.
Distance is based on the Marshfield map (see the Center for Medical Genetics, Marshfield Medical Research Foundation web site).
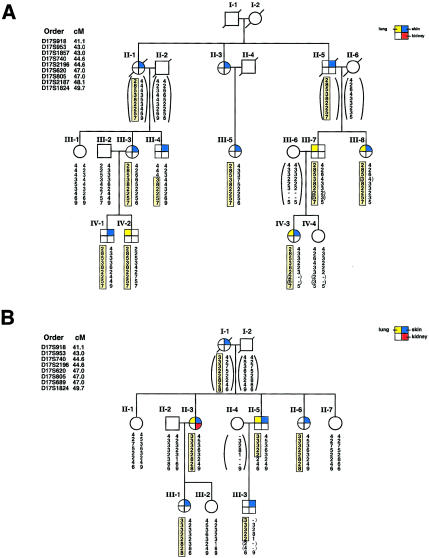
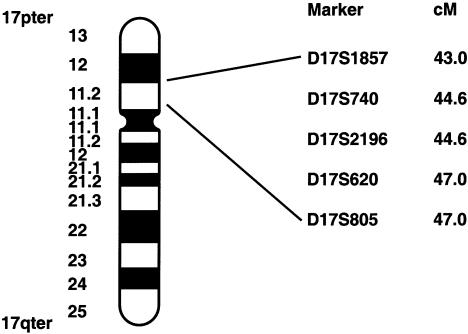
Haplotype analysis of the eight additional families with BHD showed that, within each family, all affected individuals shared the family’s disease haplotype from D17S918 to D17S1824 (data not shown), with two exceptions. An additional marker, D17S1857, was genotyped in these families, and two critical recombinants were identified that narrowed the BHD-gene candidate region. Individual III-4 in family 210 (fig. 2A) inherited his family’s affected haplotype from D17S740 to D17S1824 but had recombination between the disease haplotype and the distal markers D17S918, D17S953, and D17S1857. Individual II-5 in family 230 (fig. 2B) inherited the affected haplotype of his affected family members from D17S918 to D17S620 but had recombination at D17S805, D17S689, and D17S1824. The recombinants identified in families 210 and 230 narrowed the region of the BHD-gene locus to a distance of <4 cM, flanked by D17S1857 and D17S805 (fig. 3).
Figure 2.
Critical recombinants in families 210 and 230, which narrow the BHD nonrecombining region to <4.0 cM between D17S1857 and D17S805. A, Individual III-4 in family 210, who shows recombination at distal markers, D17S918, D17S953, and D17S1857 but shares the proximal affected haplotype (D17S740–D17S1824) with affected family members. Individual III-7 is an obligate carrier with an affected child, IV-3. Individual IV-2 is an asymptomatic carrier of the affected haplotype. B, Individual II-5 in family 230, who shares the distal affected haplotype with affected family members but shows recombination at proximal markers D17S805, D17S689, and D17S1824. The affected son, III-3, inherits the affected haplotype of his recombinant father. (For additional details, refer to the legend to fig. 1.)
Figure 3.
Ideogram of chromosome 17, showing genetic-map location of microsatellite markers linked to the BHD locus. Map distances (in cM), from the 17p telomere, were obtained from the Marshfield map (see the Center for Medical Genetics, Marshfield Medical Research Foundation web site). The BHD interval, determined on the basis of recombination events, is flanked by D17S1857 and D17S805 (3.99 cM).
Linkage to chromosome 17p11 was established for all families with BHD that were included in this study, except for family 171, described previously by Toro et al. (1999). Four members of this family were diagnosed with fibrofolliculomas; however, linkage in this family was excluded at chromosome 17p markers in the critical nonrecombinant region: D17S2196 (LOD −3.13), D17S740 (LOD −2.89), D17S620 (LOD −3.0), D17S805 (LOD −3.21), and D17S1824 (LOD −6.79), suggesting genetic heterogeneity for BHD. When we identify additional families with BHD that do not show evidence of linkage to 17p, we will perform a genomewide scan to search for additional BHD-gene loci.
Both low- and high-penetrance models were used for the calculation of LOD scores in the nine families with BHD; and significant Zmax values were obtained with both models. The most suitable model for BHD appears to be the high-penetrance model, in light of the fact that, in the 33 families with BHD that were recruited for our study, 88% (98/111) of family members of age >25 years who carry the affected haplotype were positive for fibrofolliculomas (B. Zbar, G. Alvord, G. Glenn, M. Turner, C. Pavlovich, J. Toro, M. Merino, L. Schmidt, M. Walther, P. Choyke, G. Weirich, S. Hewitt, P. Duray, F. Gibril, C. Greenberg, and W. M. Linehan, unpublished data). It is of interest to note the variability of phenotypic expression of this syndrome in the nine families with BHD. Although a single gene defect is presumed to be inherited within each family with BHD, phenotypic features within a family varied from member to member—some individuals harbored only cutaneous lesions, others had associated renal tumors, and still others presented with pneumothorax. Phenotypic heterogeneity within a family with BHD suggests the involvement of other factors, such as modifier genes and/or environmental factors, in the expression of phenotype.
The region of linkage for the BHD locus—between D17S1857 and D17S805, within chromosomal band 17p11.2—is the site of chromosomal breakpoints in medulloblastomas (Seranski et al. 1999) and in primitive neuroectodermal tumors (Scheurlen et al. 1997). This region is associated with the presence of complex repeat elements, which cause the frequent occurrence of interstitial deletions, duplications, and isochromosome formation (Chen et al. 1997; Wilgenbus et al. 1997; Potocki et al. 1999, 2000; Scheurlen et al. 1999), leading to microdeletion/duplication syndromes such as Smith-Magenis syndrome (SMS) (for review, see Chen et al. 1996) Using microsatellite and FISH analysis, we have begun to evaluate renal tumors and lymphoblastoid cell lines in patients with BHD, for interstitial deletions and DNA rearrangements.
This region of chromosome 17p is very gene rich, with >30 known genes and many uncharacterized expressed-sequence tags identified by the human-genome–sequencing effort and available from the Human Genome Browser (version 4), maintained as the “Golden Path” by University of California, Santa Cruz (see the UCSC Human Genome Project Working Draft web site). Many of these genes play a role in the regulation of either cell proliferation and differentiation or carcinogenesis and, therefore, are considered to be candidate genes for BHD. They include lethal giant larvae, the human orthologue of the Drosophila tumor-suppressor gene (Strand et al. 1995), flightless, the human orthologue of the Drosophila gene (Chen et al. 1995), the gene for topoisomerase III, the homologue of the yeast gene (Hanai et al. 1996), the gene for subunit 3 of the COP9 signalosome, the gene for part of a multiprotein complex involved in the ubiquitin-proteosome pathway (Schwechheimer et al. 2001), and two genes for Ras signaling-pathway molecules, Grb-2–related adaptor protein (Feng et al. 1996) and Ran-binding protein-1 (Bischoff et al. 1995). Housekeeping genes in the region include those for serine hydroxymethyltransferase (Elsea et al. 1995) and phosphatidylethanolamine N-methyltransferase (Walkey et al. 1999). Screening of these and other candidate genes from the region of BHD linkage is in progress. Identification of the locus for this genodermatosis will reveal a gene with a novel role in kidney, lung, and hair-follicle development, whose alteration can lead to renal neoplasia.
Acknowledgments
We thank the familie for their cooperation. We thank Alejandro Schäffer (National Center for Biotechnology Information, National Library of Medicine, NIH) and James Tomlin (Center for Information Technology, Computational Bioscience and Engineering Lab, Bioinformatics and Molecular Analysis Section, NIH) for running the multipoint linkage computations. We thank Michele Moody for expert technical assistance. This publication has been funded in whole or in part by Federal funds from the National Cancer Institute, NIH, under contract N01-C0-56000. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Electronic-Database Information
URLs for data in this article are as follows:
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://research.marshfieldclinic.org/genetics (for map distances)
- UCSC Human Genome Project Working Draft, http://genome.ucsc.edu/goldenPath/hgTracks.html (for Human Genome Browser, version 4 [“Golden Path”])
References
- Birt AR, Hogg GR, Dubé WJ (1977) Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol 113:1674–1677 [PubMed] [Google Scholar]
- Bischoff FR, Krebber H, Smirnova E, Dong W, Ponstingl H (1995) Coactivation of Ran GTPase and inhibition of GTP association by Ran-GTP binding protein RanBP1. EMBO J 14:705–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KS, Gunaratne PH, Hoheisel JD, Young IG, Miklos GL, Greenberg F, Shaffer LG, Campbell HD, Lupski JR (1995) The human homologue of the Drosophila melanogaster flightless-I gene (fli1) maps within the Smith-Magenis microdeletion critical region in 17p11.2. Am J Hum Genet 56:175–182 [PMC free article] [PubMed] [Google Scholar]
- Chen KS, Potocki L, Lupski JR (1996) The Smith-Magenis syndrome [del(17)p11.2]: critical review and molecular advances. Ment Retard Dev Disabil Res Rev 2:122–129 [Google Scholar]
- Chen KS, Manian P, Koeuth T, Potocki L, Zhao Q, Chinault AC, Lee CC, Lupski J (1997) Homologous recombination of a flanking repeat gene cluster is a mechanism for a common contiguous gene deletion syndrome. Nat Genet 17:154–163 [DOI] [PubMed] [Google Scholar]
- Chung JY, Ramos-Caro FA, Beers B, Ford MJ, Flowers F (1996) Multiple lipomas, angiolipomas and parathyroid adenomas in a patient with Birt-Hogg-Dubé syndrome. Int J Dermatol 35:365–367 [DOI] [PubMed] [Google Scholar]
- Cohen PR, Kurzrock R (1995) Miscellaneous genodermatoses: Beckwith-Weidemann syndrome, Birt-Hogg-Dubé syndrome, familial atypical multiple mole melanoma syndrome, hereditary tylosis, incontinentia pigmenti and supernumerary nipples. Dermatol Clin 13:211–229 [PubMed] [Google Scholar]
- Cottingham RW, Idury RM, Schäffer AA (1993) Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263 [PMC free article] [PubMed] [Google Scholar]
- Dib C, Faure S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Marc S, Hazan J, Seboun E, Lathrop M, Gyapay G, Morissette J, Weissenbach J (1996) A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152–154 [DOI] [PubMed] [Google Scholar]
- Elsea SH, Juyal RC, Jiralerspong S, Finucane BM, Pandolfo M, Greenberg F, Baldini A, Stover P, Patel PI (1995) Haploinsufficiency of cytosolic hydroxymethyltransferase in the Smith-Magenis syndrome. Am J Hum Genet 57:1342–1350 [PMC free article] [PubMed] [Google Scholar]
- Feng GS, Ouyang YB, Hu DP, Shi ZQ, Gentz R, Ni J (1996) Grap is a novel SH3-SH2-SH3 adaptor protein that couples tyrosine kinases to the Ras pathway. J Biol Chem 271:12129–12132 [DOI] [PubMed] [Google Scholar]
- Hanai R, Caron PR, Wang JC (1996) Human TOP3: A single-copy gene encoding DNA topisomerase III. Proc Natl Acad Sci USA 93:3653–3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell JR, Weeks DE (1995) The VITESSE algorithm for rapid exact multilocus linkage analysis via genotype set-recording and fuzzy inheritance. Nat Genet 11:402–408 [DOI] [PubMed] [Google Scholar]
- Potocki L, Chen KS, Koeuth T, Killian J, Iannaccone ST, Shapira SK, Kashork CD, Spikes AS, Shaffer LG, Lupski J (1999) DNA rearrangements on both homologues of chromosome 17 in a mildly delayed individual with a family history of autosomal dominant carpal tunnel syndrome. Am J Hum Genet 64:471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocki L, Chen KS, Park SS, Osterholm DE, Withers MA, Kimonis V, Summers AM, Meschino WS, Ayane-Yeboa K, Kashork CD, Shaffer LG, Lupski JR (2000) Molecular mechanism for duplication 17p11.2—the homologous recombination reciprocal of the Smith-Magenis microdeletion. Nat Genet 24:84–87 [DOI] [PubMed] [Google Scholar]
- Rongioletti F, Hazzini R, Gianotti G, Rebora A (1989) Fibrofolliculomas, trichodiscomas and achrochordons (Birt-Hogg-Dubé) associated with intestinal polyposis. Clin Exp Dermatol 14:72–74 [DOI] [PubMed] [Google Scholar]
- Roth JS, Rabinowitz AD, Benson M, Grossman ME (1993) Bilateral renal cell carcinoma in the Birt-Hogg-Dubé syndrome. J Am Acad Dermatol 29:1055–1056 [DOI] [PubMed] [Google Scholar]
- Scalvenzi M, Argenziano G, Sammarco E, Delfino M (1998) Hereditary multiple fibrofolliculomas, trichodiscomas and acrochordons: syndrome of Birt-Hogg-Dubé. J Eur Acad Dermatol Venereol 11:45–47 [PubMed] [Google Scholar]
- Scheurlen WG, Schwabe GC, Seranski P, Joos S, Harbott J, Metzke S, Dohner H, Poustka A, Wilgenbus K, Haas OA (1999) Mapping of the breakpoints on the short arm of chromosome 17 in neoplasms with an i(17q). Genes Chromosomes Cancer 25:230–240 [DOI] [PubMed] [Google Scholar]
- Scheurlen WG, Seranski P, Mincheva A, Kuhl J, Sorenson N, Krauss J, Lichter P, Poustka A, Wilgenbus KK (1997) High-resolution deletion mapping of chromosome arm 17p in childhood primitive neuroectodermal tumors reveals a common chromosomal disruption within the Smith-Magenis region, an unstable region in chromosome band 17p11.2. Genes Chromosomes Cancer 18:50–58 [DOI] [PubMed] [Google Scholar]
- Schwechheimer C, Serino G, Callis J, Crosby WL, Lyapina S, Deshaies RJ, Gray WM, Estelle M, Deng X-W (2001) Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIR1 in mediating auxin response. Science 292:1379–1382 [DOI] [PubMed] [Google Scholar]
- Seranski P, Heiss NS, Dhorne-Pollet S, Radelof U, Korn B, Hennig S, Backes E, Schmidt S, Wiemann S, Schwartz CE, Lehrach H, Poustka A (1999) Transcription mapping in a medulloblastoma breakpoint interval and Smith-Magenis syndrome candidate region: identification of 53 transcriptional units and new candidate genes. Genomics 56:1–11 [DOI] [PubMed] [Google Scholar]
- Strand D, Unger S, Corvi R, Hartenstein K, Schenkel H, Kalmes A, Merdes G, Neumann B, Krieg-Schneider F, Coy JF, Poustka A, Schwab M, Mechler BM (1995) A human homologue of the Drosophila tumour suppressor gene l(2)gl maps to chromosome 17p11.2-12 and codes for a cytoskeletal protein that associates with nonmuscle myosin II heavy chain. Oncogene 11:291–301 [PubMed] [Google Scholar]
- Toro J, Glenn G, Duray P, Darling T, Weirich G, Zbar B, Linehan M, Turner M (1999) Birt-Hogg-Dubé syndrome: a novel marker of kidney neoplasia. Arch Dermatol 135:1195–1202 [DOI] [PubMed] [Google Scholar]
- Walkey CJ, Shields DJ, Vance DE (1999) Identification of three novel cDNAs for human phosphatidylethanolamine N-methyltransferase and localization of the human gene on chromosome 17p11.2. Biochim Biophys Acta 1436:405–412 [DOI] [PubMed] [Google Scholar]
- Weirich G, Glenn G, Junker K, Merino M, Storkel S, Lubensky I, Choyke P, Pack S, Amin M, Walther MM, Linehan WM, Zbar B (1998) Familial renal oncocytoma: clinicopathological study of 5 families. J Urol 160:335–340 [DOI] [PubMed] [Google Scholar]
- Wilgenbus KK, Seranski P, Brown A, Leuchs B, Mincheva A, Lichter P, Poustka A (1997) Molecular characterization of a genetically unstable region containing the SMS critical area and a breakpoint cluster for human PNETs. Genomics 42:1–10 [DOI] [PubMed] [Google Scholar]