Abstract
This article describes the first patient with a deficiency of transaldolase (TALDO1 [E.C.2.2.1.2]). Clinically, the patient presented with liver cirrhosis and hepatosplenomegaly during early infancy. In urine and plasma, elevated concentrations of ribitol, d-arabitol, and erythritol were found. By incubating the patient's lymphoblasts and erythrocytes with ribose-5-phosphate and subsequently analyzing phosphate sugar metabolites, we discovered a deficiency of transaldolase. Sequence analysis of the transaldolase gene from this patient showed a homozygous deletion of 3 bp. This deletion results in absence of serine at position 171 of the transaldolase protein. This amino acid is invariable between species and is located in a conserved region, indicating its importance for enzyme activity. The detection of this new inborn error of pentose metabolism has implications for the diagnostic workup of liver problems of unknown etiology.
Introduction
Polyols, or polyhydric alcohols, are sugar-derived metabolites that are ubiquitous in humans. The origin, metabolic fate, and function of most of the polyols are unknown. Recently, we described a patient suffering from a cerebral-white-matter disorder and peripheral neuropathy of unknown origin (van der Knaap et al. 1999). In brain and body fluids of the patient, highly elevated levels of d-arabitol and ribitol were found. The strong brain/cerebrospinal fluid (CSF)/plasma gradient, with highest levels in brain and lowest levels in plasma, suggested a neurometabolic disorder. The biochemical basis of this inborn error of polyol metabolism has not yet been elucidated.
In a research project concerning human polyol metabolism and associated inborn errors, we investigated polyols in urine samples from patients suspected of having a metabolic disorder. In addition, we reviewed all results of the past 10 years of analyses of sugars and polyols. In the present study, we report on a new disorder with involvement of polyol metabolism. The patient, who was 10 years of age at the time of this study, presented in early childhood with liver cirrhosis of unknown origin. Biochemical and molecular-biological studies revealed a deficiency of transaldolase (TALDO1 [MIM 602063]) and a mutation in the human TALDO1 gene.
Patient and Methods
Case History
The patient was the first child of healthy, consanguineous Turkish parents. She was born after an uncomplicated pregnancy of 39 wk and had a birth weight of 1,910 g. Soon after birth, she underwent surgical correction of aortic coarctation. After the surgical procedure, she manifested mild bleeding problems. An enlarged clitoris was noted. Within several months, she developed hepatosplenomegaly. Liver function remained normal (normal levels of aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), lactate dehydrogenase, and γ-glutamyltransferase [γ-GT] in plasma), apart from a persisting bleeding tendency with mildly prolonged prothrombin time (PT) and activated partial thromboplastin time (APTT). Increase in height was normal, but weight for height remained just under the 3d percentile. Her mental and motor development was normal. Metabolic investigations of her urine showed mildly elevated concentrations of glycine and glutamine. Concentrations of organic acids in urine were normal. Gas-chromatographic analysis of sugars and polyols, performed to exclude galactosemia, showed normal concentrations of galactose and galactitol in urine. In the same analysis, elevated concentrations of arabitol, ribitol, and erythritol were observed. The analysis of sugars and polyols in urine was repeated in other urine samples, always revealing the same abnormalities.
When the child was 2 years of age, additional laboratory tests were performed because of the hepatosplenomegaly. Again, levels of ASAT, ALAT, and γ-GT in plasma were normal. Bile acids were slightly elevated (19 μmol/liter [control values <6 μmol/liter]). Ceruloplasmin was normal. Complement factors were normal. Results of cytomegalovirus culture in urine, of the Venereal Disease Research Laboratories test, of tests for hepatitis A, B, and C, and of tests for anti-HIV antibodies were all negative. Debranching-enzyme deficiency was excluded in leukocytes. A liver biopsy showed micronodular liver cirrhosis consisting of nodes of regenerating liver cells separated by fibrous septa, little inflammatory reaction, and no specific characteristics. Immunohistochemical staining provided no evidence of Wilson disease, hemochromatosis, α1-antitrypsin deficiency, or hepatitis B. Electron microscopy showed no inclusions in the cytosol, therefore providing no evidence of either a virus infection or a storage disease. Endocrine studies (dehydroepiandrosterone, androstenedione, and testosterone in serum) were performed because of the abnormal clitoris and were normal.
The patient was lost to follow-up. At the age of almost 10 years, she was readmitted to the hospital. Her height was at the 10th percentile, her weight for height at the 2d percentile. Physical examination revealed many telangiectasias of her skin and an unchanged hepatosplenomegaly and enlarged clitoris. The liver had a rock-hard consistency, and liver size was 7 cm below the costal margin. Results of neurological examination were normal. Laboratory investigations, performed over a period of 1 year, revealed a persistent trombocytopenia (60–63 × 109/liter [control values 150–400 × 109/liter]), which probably was caused by splenic pooling due to the hepatosplenomegaly. Both APTT and PT were mildly increased, which could be ascribed to decreased production of coagulation factors that was due to liver cirrhosis. Except for nosebleeds, there were no clinical manifestations of the prolonged APTT and PT. Iron-deficiency anemia was diagnosed, which was corrected by iron supplementation. Cholesterol was found to be low (2.1–2.5 mmol/liter [control values 3.1–5.2 mmol/liter]) on several occasions, with HDL being 1.25 mmol/liter and with LDL being 0.7 mmol/liter. Alkaline phosphatase in plasma was normal (125–160 U/liter [control values 60–325 U/liter), γ-GT was elevated (106 U/liter [control values 5–35 U/liter]) on one occasion but normalized. Transaminases in plasma were normal (ASAT, 22 U/liter [control values <40 U/liter]; ALAT, 15 U/liter [control values <45 U/liter]). Bile acids were elevated (23 and 24 μmol/liter [control values <6 μmol/liter), whereas bilirubin was normal. On two occasions elevated ammonia (103 and 89 μmol/liter [control values <50 μmol/liter]) was found , whereas on another occasion the ammonia level was normal (32 μmol/liter). Echo-doppler investigations showed an enlarged liver with a reflection pattern consistent with cirrhosis. The flow in the portal area and in the hepatic vein was normal. Spleen size was 14.5 cm.
A range of inborn errors of metabolism—peroxisomal diseases, cholesterol biosynthesis defects, congenital disorders of glycosylation, and fatty-acid–oxidation defects—were excluded. Analysis of sugars and polyols in urine revealed the same abnormalities as had been found both during the neonatal period and at the age of 2 years (table 1). Proton–magnetic-resonance spectroscopy of the brain at the age of 10 years revealed no abnormalities; in particular, there were no abnormal resonances in the sugar-and-polyol region of the spectrum (3.5–4.0 parts/million). Informed consent for the biochemical and molecular studies was obtained from the patient's father.
Table 1.
Concentrations of Pentoses, Pentitols, and Erythritol in Body Fluids of the Patient, Compared with Reference Values
| Patient <2 Years of Age (3 Samples) | Controls 0–2 Years of Age (n=31) | Patient 10–11 Years of Age (4 Samples) | Controls 6–15 Years of Age (n=10) | |
| Urine (mmol/mol creatinine): | ||||
| Xylulose | 18–61 | <5 | <5–11 | <5 |
| Ribose | 21–38 | <5 | 8–14 | <5 |
| Arabitol | 403–757 | 27–99 | 89–542 (d), 10–41 (l) | 28–87 (d), 23–43 (l) |
| Ribitol | 235–338 | 7–24 | 54–313 | 4–11 |
| Erytritol | 470–883 | 58–192 | 90–629 |
35–179 |
| Patient 10–11 Years of Age (2 Samples) |
Controls (n=10) |
|||
| Plasma (μmol/liter): | ||||
| Xylulose | <5 | <5 | ||
| Ribose | <5 | <5 | ||
| d-arabitol | 16a | <5 | ||
| l-arabitol | 3a | <5 | ||
| Ribitol | 8–12 | <5 | ||
| Erythritol | 15–17 |
<5 |
||
| Patient 10 Years of Age (1 Sample) |
Controls (n=45) |
|||
| CSF (μmol/liter): | ||||
| Xylulose | <5 | <5 | ||
| Ribose | <5 | <5 | ||
| d-arabitol | 28 | 7–30b | ||
| l-arabitol | 6 | 1–6b | ||
| Ribitol | 19 | <5 | ||
| Erythritol | 27 | 12–33 |
Analyzed in only 1 sample; in the other sample, total arabitol was 16 μmol/liter (that in controls was <5 μmol/liter).
Analyzed in only 5 samples. Total arabitol in the patient was 34 μmol/liter (that in 45 controls was 9–39 μmol/liter).
Analysis of Sugars and Polyols in Body Fluids
Sugars and polyols in urine, plasma, and CSF were assessed according to a method described elsewhere (Jansen et al. 1986). For urine, reference ranges were obtained from the literature (Jansen et al. 1986). The age groups 0–3 mo, 3–12 mo, and 1–2 years were combined into one group, since no significant age dependence up to the age of 2 years was reported. Reference ranges for ribose, xylulose, ribitol, arabitol, and erythritol in plasma and CSF were determined in our laboratory. The plasma samples were obtained from children of different ages. In none of the plasma samples were the sugars and polyols detectable, a result that is in line with that in the literature (Kusmierz et al. 1989). We used 42 CSF samples from children under investigation for an inborn error of metabolism, to determine control values for sugars and polyols. We included only samples from patients in whom no biochemical abnormalities were found. Neither age dependence nor sex dependence was found for ribose, xylulose, arabitol, and erythritol. Separate quantification of d- and l-arabitol was achieved by gas-chromatography mass spectrometry using a chiral chromatography column (Chiraldex; Chrompack). Reference values for d- and l-arabitol were obtained in our laboratory.
Transketolase and Transaldolase Assays
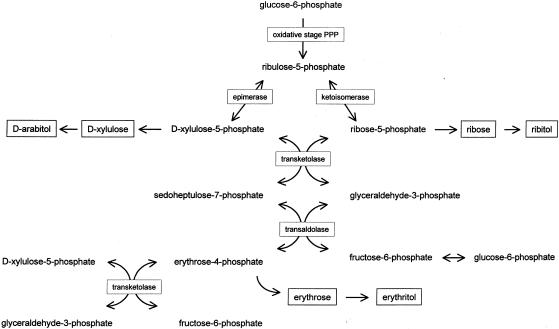
To evaluate the possibility of a defect in the pentose phosphate pathway, we developed an assay to determine the activities of both transketolase and transaldolase in erythrocytes and cultured lymphoblasts. Ribose-5-phosphate, which was used as the substrate, is rapidly converted into ribulose-5-phosphate and xylulose-5-phosphate by the cell system, providing the second substrate of transketolase (fig. 1) (Brownstone and Denstedt 1961). In the transketolase reaction, sedoheptulose-7-phosphate and glyceraldehyde-3-phosphate are formed, which are further converted by transaldolase. The reaction products of the transaldolase reaction are erythrose-4-phosphate and fructose-6-phosphate. Further conversion of fructose-6-phosphate by phosphohexose isomerase yields glucose-6-phosphate. Gas chromatography with nitrogen phosphorous detection was used to monitor the reaction products.
Figure 1.
Schematic representation of pentose phosphate pathway (PPP) and presumed reactions leading to pentoses (ribose and xylulose), pentitols (d-arabitol and ribitol), erythrose, and erythritol.
EDTA blood was centrifuged for 10 min at 1,500 g at room temperature. Plasma was removed, and erythrocytes were washed with an equal volume of saline. To 400 μl of washed erythrocytes, 800 μl of ice-cold demineralized water was added for lysis. After 15 min incubation at room temperature, an aliquot of 225 μl was taken for the enzyme assay. Lymphoblasts were obtained by Epstein-Barr–virus transformation of isolated peripheral blood B-lymphocytes. The lymphoblasts were cultured under standard conditions. For the transketolase-transaldolase enzyme assay, lymphoblasts from a 75-cm2 culture flask were harvested and were homogenized by sonification. The protein content of the lymphoblast homogenate was determined by the bicinchoninic acid method (Smith et al. 1985).
The transketolase and transaldolase reaction was performed in a final volume of 600 μl, with final concentrations of 45 mM Tris HCl buffer, 21 mM Mg2+, 0.1 mM thiamine pyrophosphate, and 4 mM ribose-5-phosphate (all from Sigma). The incubation mixture was placed at 37°C, and 50-μl samples were taken at 0 and 120 min. To the 50-μl incubation samples, 50 μl of 1 mM deoxyribose-5-phosphate (internal standard) (Sigma) in methanol was added, after which the samples were stored for ⩾30 min at −20°C. The samples were centrifuged for 10 min at 1,500 g at room temperature. The supernatants were evaporated to dryness, at 40°C, under a gentle stream of nitrogen. Then 200 μl of pyridine (Merck) and 4 mg of methoxyamine (Sigma) were added, after which the samples were left for 30 min at 40°C. The samples then were evaporated again, and 100 μl of N,O-bis(trimethylsilyl)-trifluoroacetamide (Pierce) was added. After derivatization at 90°C for 30 min, the samples were subjected to gas chromatography (CP-sil 19 CB column; Chrompack). The temperature program was 150°C for 1 min, increasing by 10°C/min to 325°C. The temperature of the nitrogen phosphorus detector was 325°C. This detector was used because its high sensitivity to compounds containing nitrogen and/or phosphor results in selective signals for sugar phosphates. The identities of the signals in the chromatogram were determined by use of commercially available standard compounds (glyceraldehyde-3-phosphate, fructose-6-phosphate, erythrose-4-phosphate, and sedoheptulose-7-phosphate [all from Sigma] and glucose-6-phosphate [Boehringer-Mannheim]). The enzyme assay was validated by use of commercially available transketolase and transaldolase enzymes (Sigma).
Molecular Analysis of Transaldolase
The cDNA sequence as well as the gene structure of human TALDO1 have recently been reported (Banki et al. 1994) (GenBank accession number NM_006755). cDNA was synthesized from total RNA derived from lymphoblasts from the patient, by Omniscript reverse transcriptase (RT) (Qiagen). One set of transaldolase-specific primers was used to amplify TALDO1 cDNA, by RT-PCR using Hot Startaq (Qiagen). The 1,100-bp cDNA was bidirectionally sequenced by use of a sequencing kit (BigDye terminator; PE Biosystems) and was analyzed on an automated DNA sequencer (ABI 310; PE Biosystems), according to the manufacturer's protocols. The mutation was confirmed by direct sequencing of PCR-amplified exon 5. (Primer sequences will be provided on request from the corresponding author.)
Results
Metabolite Analyses
Table 1 shows the ranges of concentrations of pentoses, arabitol, ribitol, and erythritol in urine, plasma, and CSF samples from our patient. In urine samples obtained during the neonatal period, elevated concentrations of the pentitols d-arabitol and ribitol and of the tetritol erythritol were noted. The presumed pentose precursors ribose and xylulose were slightly elevated. Other sugars and polyols, including sorbitol, galactose, galactitol, and mannitol, were present in normal amounts.
Repeated analysis of sugars and polyols at the ages of 3 and 10 years revealed the same profile of abnormalities. A consistent finding was a large, unknown peak in the chromatogram. The mass spectrum of this compound, obtained in the chemical-ionization mode, showed a signal at a mass:charge ratio of 660, which corresponds to the expected signal of the trimethylsilyl derivative of sedoheptulose. Since sedoheptulose is not commercially available, the exact nature of the unknown peak could not be established.
In both of two plasma samples obtained when the patient was 10 years of age, erythritol, d-arabitol, and ribitol were elevated. The concentrations of pentoses in plasma were normal.
In CSF, obtained at the age of 10 years, erythritol was normal, whereas ribitol and d-arabitol were mildly elevated.
Enzyme Studies
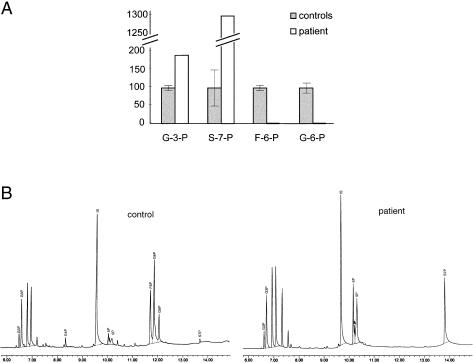
Figure 2 shows the formation of phosphate sugars by lymphoblasts from the patient and from a control, after 2 h of incubation with ribose-5-phosphate. Sedoheptulose-7-phosphate and glyceraldehyde-3-phosphate formation could be observed in both the patient and the controls, showing that transketolase activity is present. In the controls, formation of fructose-6-phosphate was found, whereas this product of the transaldolase reaction remained absent in the patient. Furthermore, the signal of sedoheptulose-7-phosphate in the patient was significantly higher than that in the controls, suggesting a decreased conversion of this compound in the patient’s cells. Also in erythrocytes, a deficiency of transaldolase was found (results not shown).
Figure 2.
A, Transketolase and transaldolase activity in cultured lymphoblast homogenates. Activity was measured by monitoring the production of glyceraldehyde-3-phosphate (G3P), sedoheptulose-7-phosphate (S7P), fructose-6-phosphate (F6P), and glucose-6-phosphate (G6P) from ribose-5-phosphate, by gas chromatography with nitrogen phosphorus detection. Five different control cell lines were analyzed. For the patient, the mean value of two independent experiments is presented. Results are expressed as percentage of the signals obtained in the controls. Error bars indicate SD in the results of the control cell lines. G3P = glyceraldehyde-3-phosphate; G6P = glucose-6-phosphate. B, Chromatogram showing results obtained in transketolase-transaldolase assay in control lymphoblasts and lymphoblasts from the patient, illustrating the formation of G3P and S7P and the absence of formation of F6P and G6P in the patient. E4P = erythrose-4-phosphate; 5P = ribose-5-phosphate.
Detection of the Mutated Transaldolase Gene and mRNA
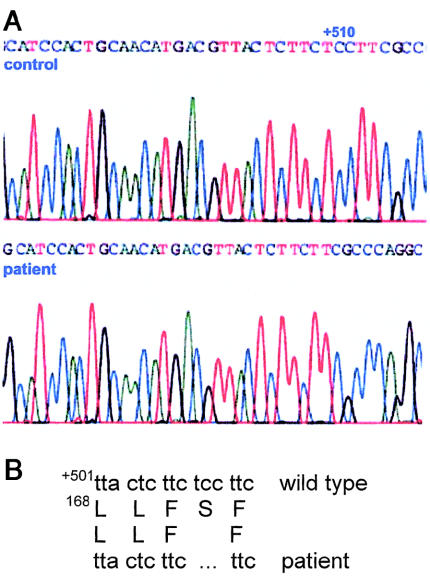
Sequence analysis of full-length TALDO1 cDNA amplified by RT-PCR demonstrated a homozygous deletion of 3 bp (nucleotides 561–563) in the patient, which was confirmed by DNA sequence analysis of exon 5. The deletion results in loss of serine 171 in the transaldolase protein, as shown in figure 3. Sequence analysis of DNA from the father showed the presence of both the wild-type allele and an allele with the same deletion as was found in the patient. Thus, the father was a carrier of TALDO1 deficiency. The mother was not available for investigations.
Figure 3.
A, Mutation in TALDO1 cDNA (GenBank accession number NM_006755; gi 5803186) of the patient. B, Deletion of 3 bp, resulting in absence of serine 171 in the transaldolase protein.
Discussion
This article has described a new inborn error in the pentose phosphate pathway: transaldolase deficiency. The pentose phosphate pathway has two metabolic functions: (1) generation of nicotinamide adenine dinucleotide phosphate (reduced), for reductive biosynthesis, and (2) formation of ribose, a component of essential biomolecules such as ATP, DNA, and RNA. Two enzymes, transketolase and transaldolase, reversibly link the pentose phosphate pathway to glycolysis. The net result of the concerted action of these two enzymes is the conversion of pentose-5-phosphate into glycolytic intermediates.
In the patient, deficiency of transaldolase resulted in accumulation of erythritol, d-arabitol, and ribitol. Apparently, inadequate conversion of sugar phosphates leads to polyol accumulation. This phenomenon is also seen in galactose-1-phosphate uridyltransferase (MIM 230400) deficiency. In the latter disorder, deficient conversion of galactose-1-phosphate results in a secondary accumulation of galactitol (Jakobs et al. 1995). The accumulated erythritol in our patient is probably derived from erythrose-4-phosphate. No polyols derived from one of the other substrates (i.e., fructose-6-phosphate, sedoheptulose-7-phosphate, and glyceraldehyde-3-phosphate) were observed. Fructose-6-phosphate and glyceraldehyde-3-phosphate can be further metabolized by glycolysis, which prevents accumulation. Accumulation of sedoheptulose and sedoheptitol could not be proved, because of analytical limitations. Elevations of d-arabitol and ribitol in the patient are probably caused by product inhibition of transketolase.
We investigated TALDO1 cDNA and DNA from the patient and found a homozygous mutation leading to the deletion of one amino acid (serine 171). This amino acid is part of a highly conserved region (Thorell et al. 2000), suggesting that the mutation causes the transaldolase deficiency that is found in erythrocytes and lymphoblasts. The father of the patient was found to be heterozygous for the deletion. This observation, in combination with the consanguinity of the parents, suggests an autosomal recessive inheritance pattern for transaldolase deficiency.
Transaldolase is present in many tissues, including brain (Adams et al. 1995). No isoforms of transaldolase are known in humans. This suggests that, also in brain tissue of our patient, transaldolase is mutated and thus inactive. Surprisingly, this does not result in highly elevated concentrations of polyols in brain tissue (as investigated by proton–magnetic-resonance spectroscopy) and CSF.
The patient affected with transaldolase deficiency is distinct from the patient whom we had reported elsewhere (Van der Knaap et al. 1999). Clinically, the latter patient had a cerebral-white-matter disease and polyneuropathy. Concentrations of polyols in brain and CSF were highly elevated, higher than those in plasma and urine. The transaldolase-deficient patient had no neurological involvement and presented with hepatic symptoms. polyol concentrations were most elevated in urine, whereas they were close to normal in both plasma and CSF.
So far, our patient has been the only individual reported with transaldolase deficiency. Therefore, the possible spectrum of clinical symptoms of this disease cannot yet be determined. It is highly probable that the liver cirrhosis and persistent hepatosplenomegaly are related to the enzyme deficiency. In another inborn error of metabolism—galactose-1-phosphate uridyltransferase deficiency—accumulation of both a polyol (galactitol) and a sugar phosphate (galactose-1-phosphate) occurs. This disorder is associated with liver cirrhosis. The accumulation of the sugar phosphates, rather than the accumulation of polyols, is thought to be important in the pathophysiology of liver cirrhosis (Holton et al. 2001). By analogy, we hypothesize that, in the transaldolase-deficient patient, accumulation of sugar phosphates occurs, which may result in cirrhosis.
At present, routine diagnostic workup of patients with liver cirrhosis does not include assessment of urinary tetritols and pentitols, implying that other patients with transaldolase deficiency may remain undiagnosed. We recommend that transaldolase deficiency be included in the differential diagnosis of children affected with liver disease of unknown origin.
In conclusion, we present the first patient affected with a deficiency of transaldolase. The discovery of this inborn error has implications for the diagnosis of liver problems of unknown etiology
Acknowledgments
Silvy van Dooren, Erwin Jansen, and Yvonne Koot are kindly acknowledged for their contributions. We thank Dr. G. Ruijter (Wageningen University) for stimulating discussions, and we thank Dr. S. Zweegman (Department of Haematology, VU Medical Center) and Dr. L. Jaspars (Department of Pathology, VU Medical Center) for their expert comments.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank Overview, http://www.ncbi.nlm.nih.gov/Genbank/GenbankOverview.html
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for TALDO1 [MIM 602063] and galactose-1-phosphate uridyltransferase [MIM 230400])
References
- Adams MD, Kerlavage AR, Fleischmann RD, Fuldner RA, Bult CJ, Lee NH, Kirkness EF, Weinstock KG, Gocayne JD, White O (1995) Initial assessment of human gene diversity and expression patterns based upon 83 million nucleotides of cDNA sequence. Nature 377:3–174 [PubMed] [Google Scholar]
- Banki K, Halladay D, Perl A (1994) Cloning and expression of the human gene for transaldolase: a novel highly repetitive element constitutes an integral part of the coding sequence. J Biol Chem 269:2847–2851 [PubMed] [Google Scholar]
- Brownstone YS, Denstedt OF (1961) The pentose phosphate metabolic pathway in the human erythrocyte. Can J Physiol 39:527–532 [Google Scholar]
- Holton JB, Walter JH, Tyfield LA (2001) Galactosemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The molecular and molecular bases of inherited disease. McGraw-Hill, New York, pp 1553–1588 [Google Scholar]
- Jakobs C, Schweitzer S, Dorland B (1995) Galactitol in galactosemia. Eur J Pediatr 154 Suppl 2:S50–S52 [DOI] [PubMed] [Google Scholar]
- Jansen G, Muskiet FA, Schierbeek H, Berger R, van der Slik W (1986) Capillary gas chromatographic profiling of urinary, plasma and erythrocyte sugars and polyols as their trimethylsilyl derivatives, preceded by a simple and rapid prepurification method. Clin Chim Acta 157:277–293 [DOI] [PubMed] [Google Scholar]
- Kusmierz J, DeGeorge JJ, Sweeney D, May C, Rapoport SI (1989) Quantitative analysis of polyols in human plasma and cerebrospinal fluid. J Chromatogr 497:39–48 [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85 [DOI] [PubMed] [Google Scholar]
- Thorell S, Gergely PJ, Banki K, Perl A, Schneider G (2000) The three-dimensional structure of human transaldolase. FEBS Lett 475:205–208 [DOI] [PubMed] [Google Scholar]
- Van der Knaap MS, Wevers RA, Struys EA, Verhoeven NM, Pouwels PJ, Engelke UF, Feikema W, Valk J, Jakobs C (1999) Leukoencephalopathy associated with a disturbance in the metabolism of polyols. Ann Neurol 46:925–928 [DOI] [PubMed] [Google Scholar]