Abstract
Rett syndrome (RTT) is an X-linked neurodevelopmental disorder that apparently is lethal in male embryos. RTT almost exclusively affects female offspring and, in 99.5% of all cases, is sporadic and due to de novo mutations in the MECP2 gene. Familial cases of RTT are rare and are due to X-chromosomal inheritance from a carrier mother. We analyzed the parental origin of MECP2 mutations in sporadic cases of RTT, by analysis of linkage between the mutation in the MECP2 gene and intronic polymorphisms in 27 families with 15 different mutations, and we found a high predominance of mutations of paternal origin in 26 of 27 cases (P<.001). The paternal origin was independent of type of mutation and was found for single-base exchanges as well as for deletions. Parents were not of especially advanced age. We conclude that de novo mutations in RTT occur almost exclusively on the paternally derived X chromosome and that this is most probably the cause for the high female:male ratio observed in patients with RTT. Affected males recently have been described in a few cases of familial inheritance. Identification of the parental origin may be useful to distinguish between the sporadic form of RTT and a potentially familial form. This distinction will allow geneticists to offer more-specific counseling and discriminate between higher (maternal origin) and lower (paternal origin) recurrence risk.
Introduction
Rett syndrome (RTT [MIM 312750]) is an X-linked neurodevelopmental disorder that almost exclusively affects female offspring. It is characterized by mental retardation, loss of acquired skills (especially purposeful hand use), and deceleration of head growth. A normal prenatal and postnatal development that lasts 8–30 mo is followed by developmental stagnation and regression of mental and motor abilities. Diagnostic criteria and disease stages for RTT were established in 1985 (Hagberg et al. 1985). Common clinical features include stereotypical hand movements, hyperventilation, seizures, growth retardation, scoliosis, and autonomic dysfunction. The prevalence of the disease is estimated to be 1/10,000–1/15,000 female births (Hagberg 1985).
Mutations in MECP2 (MIM 300005) recently have been described by several groups, with a detection rate of 25%–90% (Amano et al. 2000; Amir et al. 2000; Bienvenu et al. 2000; Cheadle et al. 2000; Huppke et al. 2000; Obata et al. 2000; Xiang et al. 2000). Interestingly, in all studies, a low rate of detection of mutations has been detected in familial cases of RTT (Amir et al. 2000; Cheadle et al. 2000; Xiang et al. 2000). No satisfactory explanation for the discrepancy between detection rates in sporadic cases and detection rates in familial cases is available. Until now, five reports had described mutations in the MECP2 gene in male patients (Wan et al. 1999; Clayton-Smith et al. 2000; Meloni et al. 2000; Orrico et al. 2000; Villard et al. 2000). Four of these reports describe the rare, documented familial cases of MECP2 mutations: the first describes a male child affected by congenital encephalopathy who survived to age ⩾1 year (Wan et al. 1999); the second describes four adult brothers affected by severe nonspecific mental retardation and movement disorders (resting tremors and slowness of movements) (Orrico et al. 2000); the third describes two men affected by severe mental retardation and progressive spasticity (Meloni et al. 2000); and the fourth describes two brothers affected by severe neonatal encephalopathy who died of severe apnea at age <1 year (Villard et al. 2000). The fifth report describes a sporadic case—a boy affected by a nonfatal neurodevelopmental disorder who has a somatic mosaicism in the MECP2 gene (Clayton-Smith et al. 2000). Owing to the paucity of surviving affected male offspring, mutations in the MECP2 gene are considered developmentally lethal in male embryos. This hypothesis is consistent with the results of a knockout mouse model for MECP2, in which no viable male animals were born (Tate et al. 1996). Thomas (1996), however, hypothesizes a high male:female ratio of germline mutations for RTT and other X-linked dominant genetic diseases; this hypothesis suggested that the lack of affected males is due merely to the fact that the male offspring do not inherit the paternal X chromosome. Thomas's hypothesis implies both that de novo mutations occur on the paternal X chromosome, which is inherited only by the female offspring, and that the affected females have a significant reproductive disadvantage; the few female carriers of a mutation in the MECP2 gene that are able to reproduce account for the rare familial cases. In this study, to determine the parental origin of the mutations, we investigated 27 females with RTT, affected because of de novo mutations in the MECP2 gene
Subjects and Methods
Subjects
A total of 144 patients with RTT who carried a mutation in the MECP2 gene were studied for the presence of frequent polymorphisms in intron 3. The identification of intragenic single-nucleotide polymorphisms (SNPs) relatively near the mutation sites allowed us to coamplify mutations and SNPs in the same amplicon. These were subsequently cloned to determine the phase of the mutation and parental SNP. The group of patients consisted of 132 German females, 2 Italian females, and 10 Turkish females. Of the 144 patients with RTT, 42 were found to be informative because of heterozygosity for at least one polymorphism in the MECP2 gene. For 25 patients with RTT, blood samples from both parents were obtained, with informed consent. In two cases, blood samples were available only from the mother. Parental ages at the time of childbirth were obtained from the clinical files of the patients and were available for 25 fathers and 24 mothers.
Mutation Detection
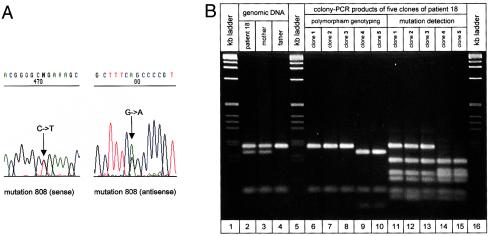
Total genomic DNA was prepared from peripheral blood leukocytes according to standard procedures (Miller et al. 1988). PCR amplification of a 2.3-kb DNA fragment containing exon 3, intron 3, and exon 4 of the MECP2 gene, as well as mutation detection in these products, were performed as described elsewhere (Huppke et al. 2000). Whenever a mutation created or abolished a cleavage site, it was confirmed by restriction-enzyme digestion (fig. 1).
Figure 1.
A, Sequence analysis of patient 18, who is heterozygous for mutation R270X (c.808C→T). The wild-type allele shows the recognition site (GCCG) of the NlaIV restriction enzyme; in the mutant allele, the NlaIV recognition site (GTCG) is abolished. B, Linkage analysis of mutation R270X in patient 18. The status, at the polymorphic region c.377+266C→T of patient 18 and her parents was determined by amplification of the respective region from genomic DNA and subsequent AluI digestion (lanes 2–4). The patient showed a DNA restriction pattern with fragments 195 and 169 bp in length (upper bands), indicating heterozygosity for the c.377+266C→T polymorphism. The patient's father showed a restriction pattern with a 195-bp fragment (missing the 169-bp fragment) indicating that he carries the cytosine variant of the c.377+266C→T polymorphism on his single X chromosome. The patient's mother showed the same restriction pattern as was seen in the patient and is also heterozygous for the c.377+266C→T polymorphism. Conclusively, in patient 18, the allele containing cytosine must be of paternal origin, and the allele containing thymine must be of maternal origin. Lanes 6–15 show polymorphism genotyping and mutation detection in five different clones of patient 18. Clones 1–3 show an AluI restriction pattern indicated by cytosine variant alleles of the c.377+266C→T polymorphism (lanes 6–8), and clones 4 and 5 show a pattern indicated by thymine variant alleles (lanes 9 and 10). Mutation detection in these five clones was done by second-round PCR and subsequent digestion with NlaIV. Mutant alleles miss an NlaIV cleavage site, at position 490 of the generated 590-bp PCR product, resulting in a DNA fragment that is 191 bp in length (upper band), instead of two smaller fragments. Clones 1–3 (lanes 11–13) show this 191-bp fragment, and clones 4 and 5 (lanes 14 and 15) do not. Mutation R270X is linked to the cytosine variant allele of the c.377+266C→T polymorphism and therefore is of paternal origin.
Identification of Polymorphic Variants
In a search for polymorphisms in the sequence of the MECP2 gene, we analyzed intron 3 in 10 patients, by direct sequencing on an ABI 377 automatic sequencer with the primer In-RT-F (table 1). In intron 3, at position c.377+266, we found a new frequent SNP (C/T) which creates a recognition site for restriction enzyme AluI (AGCC/AGCT). To screen our group of patients for individuals heterozygous for this polymorphism, we amplified the relevant DNA region from patient genomic DNA, with flanking intronic primers, and digested the product with AluI (table 1). The resulting PCR product, 248 bp in length, constitutively contains a recognition site for AluI, at positions 52–55. A second AluI recognition site, at positions 77–80 of the fragment, is selectively present in those products created from c.377+266T variant alleles. A further intronic polymorphism, c.378−17delT, was detected by sequencing of the 3′ part of intron 3, with the primer Rett Ex3-F1. In our laboratory, this primer is routinely used for mutation analysis of the amplicon containing exon 4. Screening for the c.378−17delT polymorphism was done by direct sequencing in all cases. To determine the allele frequencies of these two intronic polymorphisms, the allele status was determined for 56 unrelated, healthy white females, as indicated above. Other intragenic SNPs recently have been reported in the public dbSNP database and may be useful for analysis of the phase of a patient’s mutation in cases of noninformative SNPs (dbSNP accession numbers rs760103, rs1474485, rs1042870, rs1042873, and rs1474486).
Table 1.
Primers and PCR Conditions
| PCR |
|||
| Primer Sequence | Setup | Cycling Conditions | Product and Type of Detection |
| Sequencinga | |||
| Rett Ex2-F2: 5′-CTGCTCACTTGTTCTGCAGACTGG-3′ | … | … | … |
| In-RT-F: 5′-AGCAGGCCCTCTATCCTCTCCACA-3′ | … | … | … |
| Rett Ex3-F1: 5′-CTCGACATTGCTATGGAGAGCC-3′ | … | … | … |
| Rett Ex3-F2: 5′-GTGGCAGCCGCTGCCGCCGAGGCC-3′ | … | … | … |
| Rett Ex3-R2: 5′-GTCAGAGCCCTACCCATAAGGAGA-3′ | … | … | … |
| Screening for 377+266C/T Polymorphism | |||
| In-RT-F: 5′-AGCAGGCCCTCTATCCTCTCCACA-3′In-RT-R: 5′-CGGTGCTCAGTCTCTCCAGGAATC-3′ | HSTaq MasterMix, 0.2 pmol/μl (each primer), 500 ng of genomic DNA | 15 min at 97°C, 35 × (20 s at 94°C, 30 s at 65°C, 30 s at 72°C), 5 min at 72°C | 248 bp, AluI restriction |
| Colony PCR | |||
| Sp6: 5′-AGGTGACACTATAGAATAC-3′T7: 5′-GTAATACGACTCACTATAGGGC-3′ | HSTaq MasterMix, 0.2 pmol/μl (each primer), picked bacterial colony | 15 min at 97°C, 35 × (30 s at 96°C, 30 s at 55.5°C, 2 min 10 s at 72°C), 10 min at 72°C | 174 bp (vector religation) or 2,512 bp (successful cloning) |
| Identification of 377+266 C/T Polymorphisms in Colony-PCR Products | |||
| In-RT-F: 5′-AGCAGGCCCTCTATCCTCTCCACA-3′In-RT-R: 5′-CGGTGCTCAGTCTCTCCAGGAATC-3′ | HSTaq MasterMix, 0.2 pmol/μl (each primer), 1 μl of colony-PCR product (1:100) | 15 min at 97°C, 30 × (20 s at 94°C, 30 s at 65°C, 60 s at 72°C), 5 min at 72°C | 248 bp, AluI restriction |
| Identification of 378−16T/delT Polymorphisms in Colony-PCR Products | |||
| In-RT-F: 5′-AGCAGGCCCTCTATCCTCTCCACA-3′Rett Ex3-R3: 5′-GATGGGGAGTACGGTCTCCTGCAC-3′ | HSTaq MasterMix, 0.2 pmol/μl (each primer), 1 μl of colony-PCR product (1:100) | 15 min at 97°C, 30 × (20 s at 94°C, 30 s at 65°C, 1 min 15 s at 72°C), 5 min at 72°C | 1,102 bp, direct sequencing |
| Mutation Detection in Colony-PCR Products | |||
| 808C→T and 455C→G: 5′-CTCGACATTGCTATGGAGAGCC-3′ 5′-GATGGGGAGTACGGTCTCCTGCAC-3′ | HSTaq MasterMix, 0.2 pmol/μl (each primer), 1 μl of colony-PCR product (1:100) | 15 min at 97°C, 30 × (20 s at 94°C, 30 s at 65°C, 60 s at 72°C), 5 min at 72°C | 590 bp, NlaIV restriction |
| 502C→T: 5′-CTCGACATTGCTATGGAGAGCC-3′ 5′-GATGGGGAGTACGGTCTCCTGCAC-3′ | HSTaq MasterMix, 0.2 pmol/μl (each primer), 1 μl of colony-PCR product (1:100) | 15 min at 97°C, 30 × (20 s at 94°C, 30 s at 65°C, 60 s at 72°C), 5 min at 72°C | 590 bp, HphI restriction |
| 316C→T: 5′-CTGCTCACTTGTTCTGCAGACTGG-3′ 5′-GCACATACATTTTCCTGCTCCATG-3′ | HSTaq MasterMix, 0.2 pmol/μl (each primer), 1 μl of colony-PCR product (1:100) | 15 min at 97°C, 30 × (20 s at 94°C, 30 s at 65°C, 60 s at 72°C), 5 min at 72°C | 496 bp, NlaIII restriction |
| 916C→T: 5′-GTGGCAGCCGCTGCCGCCGAGGCC-3′ 5′-GTCAGAGCCCTACCCATAAGGAGA-3′ | HSTaq MasterMix, 0.2 pmol/μl (each primer), 1 μl of colony-PCR product (1:100) | 15 min at 97°C, 30 × (20 s at 94°C, 30 s at 65°C, 60 s at 72°C), 5 min at 72°C | 718 bp, HhaI restriction |
| Other point mutations in exon IV: 5′-AGCAGGCCCTCTATCCTCTCCACA-3′ 5′-GATGGGGAGTACGGTCTCCTGCAC-3′ | HSTaq MasterMix, 0.2 pmol/μl (each primer), 1 μl of colony-PCR product (1:100) | 15 min at 97°C, 30 × (20 s at 94°C, 30 s at 65°C, 60 s at 72°C), 5 min at 72°C | 1,102 bp, direct sequencing |
| Deletions in exon IV: 5′-GTGGCAGCCGCTGCCGCCGAGGCC-3′ 5′-GTCAGAGCCCTACCCATAAGGAGA-3′ | HSTaq MasterMix, 0.2 pmol/μl (each primer), 1 μl of colony-PCR product (1:100) | 15 min at 97°C, 3 × (30 s at 95°C, 30 s at 40°C, 30 s at 72°C), 5 × (30 s at 95°C, 30 s at 40°C + 1°C/cycle, 30 s at 72°C), 23 × (30 s at 96°C, 30 s at 55°C, 30 s at 72°C), 5 min at 72°C | 718 bp, direct sequencing |
DYEnemic ET Terminator cycle-sequencing kit and cycling conditions were according to the manufacturer’s instructions.
Allele Separation, Linkage Analysis, and Identification of Parental Origin
Only patients heterozygous for at least one intronic SNP were selected. Since RTT is an X-linked disorder, the unequivocal identification of the paternal alleles was obtained by comparison of the genotypes of the heterozygous patients with those of their hemizygous fathers. The identity of the maternal alleles was then simply deduced. To establish the phase of mutation and the parental marker allele, 2.3-kb PCR products ranging from exon 3 to exon 4 and including a patient’s mutation and informative SNP were cloned into the pGEM-T Easy Vector (Promega), according to the manufacturer’s instructions, and were transformed in pSure Escherichia coli (Stratagene). For each patient, several colonies were selected and screened, by colony PCR, for the presence of the insert with vector-specific primers (table 1). This procedure resulted in 2.5-kb PCR products in cases with successful ligation of the 2.3-kb fragment into the pGEM-T Easy Vector (2.3 kb of specific genomic DNA + 174 bp of vector sequences). Each 2.5-kb PCR product resulting from a single colony was then genotyped for the c.377+266C→T polymorphism, by a second-round PCR, followed either by restriction cutting with AluI or, in cases of polymorphism for c.378−17delT, by direct sequencing. The same 2.5-kb products were then tested for the presence of a patient’s mutation (table 1) to determine the linkage between polymorphism and mutation. All analyzed colony-PCR products were unequivocally hemizygous for the intronic polymorphisms and for a patient’s mutation or wild-type alleles, as expected. This highlights the 100% specificity of this approach, with virtually no mistakes. For each patient, we analyzed 5–10 clones. In all 27 patients, we were able to examine clones of both alleles. The parental allele status for the corresponding intronic polymorphisms was determined by amplification from genomic DNA, followed by either restriction-enzyme digestion or direct sequencing, as indicated in the “Identification of Polymorphic Variants” subsection (above).
Results
Allelic Frequencies
In our group of patients (288 alleles), the frequency of the cytosine variant of the c.377+266C→T polymorphism in intron 3 was found to be 83%, whereas the frequency of the thymine variant for that polymorphism was found to be 17%. This resulted in a calculated heterozygosity frequency of 28%; however, we counted 44 heterozygous females in our group of 144 screened patients (30.5%). The frequency of the TTT variant of the c.378−17delT polymorphism in intron 3 was found to be ∼99%, and, accordingly, the frequency of the TT variant was found to be ∼1%. Three patients in our group were heterozygous for the c.378−17delT polymorphism.
Fifty-six healthy control females were screened for both polymorphisms. In this sample (112 alleles), the frequency of the cytosine variant of the c.377+266C→T polymorphism was found to be 77%, whereas the frequency of the thymine variant was found to be 23%. None of the females in the control group carried the c.378−17delT allele. The differences, in allelic frequencies, between the control group and the group of patients are not statistically significant (P>.15 for the c.377+266C→T polymorphism, χ2=2.04; and P>.27 for the c.378−17delT polymorphism, χ2=1.18).
Spectrum of Analyzed Mutations—and Their Linkage to Intronic Polymorphisms
In a total of 27 patients, we analyzed 15 different mutations in the MECP2 gene to examine the parental origin of de novo mutations in RTT; 9 patients had missense mutations, 14 had nonsense mutations, 1 had a splice-site mutation, 1 had a small insertion, and 2 had a deletion in exon 4 (one deletion was 44 bp and the other was 156 bp). The analysis of linkage between the intronic polymorphism c.377+266C→T and the different mutations in the MECP2 gene revealed linkage of the mutation to the cytosine variant allele in 16 patients and to the thymine variant allele in 9 patients. On the basis of the allelic frequencies for the c.377+266C→T polymorphism in our control group, we predicted the linkage of mutations to the cytosine variant allele to be three times more frequent than that to the thymine variant allele. In fact, the proportion of mutations linked to thymine variant alleles was slightly higher than expected (i.e., nine, instead of six), but this difference did not reach statistical significance (P>.35, χ2=0.86). Thus, our results do not indicate a preference of de novo mutations, for either of the polymorphic variants. With respect to the c.378−17delT polymorphism, the mutation in the MECP2 gene was always detected on the TTT allele, which is the much more frequent allele; however, only two patients were analyzed for this polymorphism.
Origin of Mutation
Of the 27 patients, 26 showed a paternal origin of mutation. This predominance of paternal origin is highly significant (P<.001, χ2=13.89). Furthermore, the paternal origin was independent of the type and localization of mutation and was found in point mutations as well as in deletions ⩽156 bp. A detailed analysis of the origin of mutations for all patients analyzed is shown in table 2.
Table 2.
The Parental Origin of MECP2 Mutations in Sporadic RTT
|
SNP Genotypea |
|||||||
| Mutation Type andPatient Number | Patient Mutation | Intragenic SNP | Paternalb | Maternal | Patient | Phase of Mutation | Origin of Mutation |
| Missense: | |||||||
| 1 | 316C→T | 377+266C→T | C | T/T | C/T | C | Paternal |
| 2 | 397C→T | 377+266C→T | C | T/T | C/T | C | Paternal |
| 3 | 397C→T | 377+266C→T | n.a. | C/C | C/T | T | Paternal |
| 4 | 455C→G | 377+266C→T | T | C/C | C/T | T | Paternal |
| 5 | 467A→G | 377+266C→T | C | C/T | C/T | C | Paternal |
| 6 | 473C→T | 377+266C→T | T | C/C | C/T | T | Paternal |
| 7 | 473C→T | 378−17delT | TTT | TT/TTT | TT/TTT | TTT | Paternal |
| 8 | 916C→T | 377+266C→T | C | C/T | C/T | C | Paternal |
| Nonsense: | |||||||
| 9 | 502C→T | 377+266C→T | C | C/T | C/T | C | Paternal |
| 10 | 502C→T | 377+266C→T | T | C/T | C/T | T | Paternal |
| 11 | 502C→T | 377+266C→T | n.a. | C/C | C/T | T | Paternal |
| 12 | 502C→T | 377+266C→T | C | T/T | C/T | C | Paternal |
| 13 | 502C→T | 378−17delT | TTT | TT/TTT | TT/TTT | TTT | Paternal |
| 14 | 502C→T | 377+266C→T | T | C/C | C/T | T | Paternal |
| 15 | 613G→T | 377+266C→T | C | C/T | C/T | C | Paternal |
| 16 | 763C→T | 377+266C→T | C | C/T | C/T | C | Paternal |
| 17 | 763C→T | 377+266C→T | C | T/T | C/T | C | Paternal |
| 18 | 808C→T | 377+266C→T | C | C/T | C/T | C | Paternal |
| 19 | 808C→T | 377+266C→T | T | C/C | C/T | T | Paternal |
| 20 | 808C→T | 377+266C→T | C | C/T | C/T | C | Paternal |
| 21 | 880C→T | 377+266C→T | C | T/T | C/T | C | Paternal |
| 22 | 880C→T | 377+266C→T | T | C/C | C/T | T | Paternal |
| Splice site: | |||||||
| 23 | 378−2A→G | 377+266C→T | C | T/T | C/T | C | Paternal |
| Insertions/deletions: | |||||||
| 24 | 718insC | 377+266C→T | T | C/C | C/T | T | Paternal |
| 25 | 1154–1198del | 377+266C→T | C | C/T | C/T | C | Paternal |
| 26 | 1039–1195delinsGT | 377+266C→T | C | C/T | C/T | C | Paternal |
| Missense: | |||||||
| 27 | 397C→T | 377+266C→T | C | T/T | C/T | T | Maternal |
Only patients heterozygous for an intronic SNP were selected. Because RTT is an X-linked disorder, the unequivocal identification of a patient's paternal allele was obtained by comparison of her SNP genotypes with those of her father; the genotypes compared are in boldface italic type. The patient's second allele must then be maternally derived.
n.a. = Not available; however, in these cases, homozygosity for the cytosine variant allele in the patients' mothers allows paternally derived identification of the patients' thymine variant allele.
The only patient showing maternal origin revealed a missense mutation (R133C) in the MBD of the MECP2-gene product. The same mutation was found in two cases with paternal origin of mutations. Mixing up the DNA probes of father and mother was excluded by Y-specific PCR and X-specific amplification of the highly polymorphic androgen-receptor gene. The patient’s mother did not carry the mutation, at least in the DNA derived from blood leukocytes.
Parental Ages
The mean parental ages at the time of childbirth were determined for the families with paternal origin of mutation. The mean paternal age was 31.3±4.5 years, and the mean maternal age was 28.2±4.4 years, with ranges of 23–42 years and 22–36 years, respectively. In the single case of maternal origin, the mother was aged 25 years, and the father was aged 34. During the past 10 years, the range in mean maternal age at the time of childbirth for the German population has been 27–30 years, and the age at childbirth continuously rose during this period of time; also during the past 10 years, German men have been, on average, 2.86 years older than their partners at time of marriage (Statistisches Bundesamt, Statistische Jahrbücher 1985–99). In parents of patients with RTT, neither paternal age nor maternal age differed significantly from that for the German population in general.
Discussion
Paternal Origin of Mutations in RTT: Almost Exclusively Female Affection
Along with RTT, there are at least 13 X-linked dominant diseases that are caused by de novo mutations and that have a supposedly lethal phenotype in males and that result in reproductive disadvantage in affected females (Thomas 1996). Male lethality is the traditional explanation for the pronounced majority of affected females and, in some cases, the almost total absence of affected males (Wettke-Schäfer and Kantner 1983). In 1996, Thomas proposed a simple alternative to this theory: he suggested a very high male:female ratio of de novo mutations (Thomas 1996). According to this model, males are protected from X-linked dominant diseases because they do not inherit the mutation-prone paternal X chromosome. Our investigation of the parental origin of the MECP2 mutations in patients with sporadic RTT clearly gives strong evidence that, in almost all (26 of 27) cases, de novo mutations in the MECP2 gene arise on the paternal X chromosome. We suggest that males are, therefore, naturally protected from the sporadic form of RTT, which is caused by de novo mutations, and that RTT should no longer be considered lethal in males. A corollary of this hypothesis is that the affected males would inherit their X chromosome from their mothers, who are carriers, or that the mutation arises de novo on the maternal chromosome. In fact, mutations in the MECP2 gene recently have been described in males in four familial cases: a male child affected by congenital encephalopathy who survived to age ⩾1 year (Wan et al. 1999), four adult brothers affected by severe nonspecific mental retardation and movement disorders (resting tremors and slowness of movements) (Orrico et al. 2000), two men affected by severe mental retardation and progressive spasticity (Meloni et al. 2000), and two brothers affected by severe neonatal encephalopathy who died of severe apnea at age <1 year (Villard et al. 2000). In a further case, a postzygotic mutation most probably arose de novo on the maternal chromosome (Clayton-Smith et al. 2000) and confirms the viability of both possible causes accounting for affected males, inheritance of the affected alleles from carrier mothers and de novo mutations on maternal X chromosomes.
Studies on the parental origin of de novo mutations have been done both for autosomal dominant diseases, such as Apert syndrome (Moloney et al. 1996) and Crouzon syndrome (Glaser et al. 2000), and for tumor-associated diseases, such as MEN-2B (Carlson et al. 1994) and NF-1 (Jadayel et al. 1990). The exclusively paternal origin of de novo mutations has been proved for Apert syndrome, Crouzon syndrome, and MEN-2B, and, for NF-1, the paternal origin of de novo mutations has been proved to be highly predominant. An increased paternal age, however, has been documented only for Apert syndrome and Crouzon syndrome. In these diseases, the exclusively paternal mutation rate might correlate positively with the number of cell divisions of the spermatogonia. This mechanism cannot be responsible for the high male:female ratio of de novo mutations in RTT, because there is no increment in the paternal age in fathers of patients with RTT. The underlying mechanisms for the increased male mutation rate are not clear. The different activation state of the X chromosome in the oocytes and in the spermatocytes is a distinctive element for the two germ lines. The X chromosome in oocytes is undermethylated, whereas the X chromosome in sperm is hypermethylated because of the epigenetic modification associated with the process of chromatin inactivation (Goto and Monk 1998). It has been suggested that a spontaneous deamination of the 5m-cytosine residue at position 4 of the carbon ring may result in the conversion to a thymine (Goto and Monk 1998). As a matter of fact, ∼70% of the mutations in the MECP2 gene are cytosine-to-thymine transitions (Dragich et al. 2000), and this is consistent with the possibility of hypermutability at methylated CpG dinucleotides. However, this mechanism cannot account for the other types of mutations in MECP2, such as deletions or transversions, that also arise on the paternal X chromosome. It may be possible that the mutation rate, independently of the kind of mutation, does not differ as much as supposed in the female and male germ line. Recently, in fact, Bohossian et al. (2000) questioned the hypothesis of a higher mutation rate in males and found a male:female mutation rate of 1.7. If these findings are also applicable to mutations in the MECP2 gene, then the observed bias with respect to the parental origin of the mutations should be a postmutational event. It may be possible that mutations on the female X chromosome are detrimental to the oocytes expressing the mutated protein. This mechanism would reduce the likelihood that oocytes with a mutation of the MECP2 gene would be fertilized. Only a few oocytes escaping this selection would then account for cases of RTT that are due to de novo mutation on the maternal X chromosome. This hypothesis, however, is partially invalidated by the familial cases of RTT in which healthy mothers either are obligate carriers of a germline mutation or have a constitutive mutation of the MECP2 gene. The different epigenetic situation of the male and female X chromosomes in germ cells may, however, be a key point for understanding the different behavior regarding the mutation rate of the MECP2 gene.
Mutations of Maternal Origin—and the Consequences for Genetic Counseling
Until now, in all known familial cases with inheritance of MECP2 mutations, the mother has been found either to be carrier of the mutation (Amir et al. 1999, 2000; Wan et al. 1999; Cheadle et al. 2000; Meloni et al. 2000; Orrico et al. 2000) or to carry a mosaicism for the mutation (Amir et al. 1999). In contrast, a recurrent MECP2 mutation inherited through the paternal germ line had, until now, never been described in patients with RTT. Our findings demonstrate a very high predominance of paternal origin of MECP2 mutations in sporadic cases of RTT. This, along with the above-mentioned familial cases of MECP2 mutations due to exclusively maternal inheritance, suggests that mutations appearing on maternal alleles might have a higher risk to cause familial cases of RTT. A proportion of these alleles will represent de novo mutations on the maternal X chromosome, but some of them may be due to a maternally inherited mutation that, because of either skewed X inactivation or mosaicism, does not induce an evident phenotype in the carrier mother. We speculate that, in such cases of maternally inherited MECP2 mutations, the primary mutational event appeared on the mother's paternal X chromosome. The recent report by Villard et al. (2000) describes a family with two affected male children and with a female child with typical RTT, in all of whom the T151M mutation is due to inheritance of a mutated allele from the mother. Consistent with our hypothesis of a prevalent mutational event on the paternal chromosomes is the finding that the MECP2 mutation in this family is, in fact, located on the mother's paternal X chromosome.
We therefore suggest that MECP2 mutations of maternal origin should be considered to have a recurrence risk higher than that in MECP2 mutations of paternal origin. After exclusion of the respective mutation in maternal DNA derived from blood leukocytes, we suggest analysis of parental origin of the mutated allele, to estimate the possible recurrence risk due to potential maternal mosaicism. Then, if the analysis of polymorphic markers reveals a maternal origin of the mutated allele, it may be advisable to investigate DNA from the mother that is derived from additional tissues (such as gingiva cells and fibroblasts). The identification of mosaicism in the mother would, of course, lead to prediction of a recurrence risk of as much as 50%. However, independent of a detectable mosaicism, we consider the few cases in which the mutations arise on the maternal chromosome to be at a higher recurrence risk, because germ-cell mosaicism cannot be excluded. In contrast, if, as in the majority of cases, a paternal origin of mutation is found, then the recurrence risk may be low, since the paternal origin of MECP2 mutations has been shown to be the usual condition of the sporadic form of RTT and, until now, has never been described in familial cases of RTT. Nevertheless, the possibility of paternal germline mosaicism in the MECP2 gene cannot generally be excluded a priori. In some sporadic cases, a paternal germline mosaicism has been described for autosomal disorders, such as neurofibromatosis type 1 (Lazaro et al. 1994) and osteogenesis imperfecta (Cohn et al. 1990; Edwards et al. 1992; Namikawa et al. 1995). The genotyping of DNA from sperm cells of fathers of patients with RTT may be a useful approach for analysis of a potential male germline mosaicism. Although we consider the recurrence risk to be low for MECP2 mutations of paternal origin, prenatal diagnosis should be discussed and provided if requested.
In conclusion, our findings give strong evidence that there is male sparing in RTT—and not necessarily male lethality, as previously has been supposed. The identification of a mutation arising on the maternal chromosome may reveal a potentially familial form of RTT. The analysis of parental origin of the underlying mutations may therefore be beneficial to families of patients with RTT.
Acknowledgments
We thank the patients with RTT, as well as their parents, for their participation; S. Buth, S. Herlt, U. Lenz, A. Herwig, and K. Rücker for their technical contribution; and Elternhilfe für Kinder mit Rett Syndrom.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- dbSNP, http://www.ncbi.nlm.nih.gov/SNP/ (for intragenic SNPs, accession numbers rs760103, rs1474485, rs1042870, rs1042873, and rs1474486)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MECP2 [MIM 300005] and RTT [MIM 312750])
References
- Amano K, Nomura Y, Segawa M, Yamakawa K (2000) Mutational analysis of the MECP2 gene in Japanese patients with Rett syndrome. J Hum Genet 45:231–236 [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Schultz R, Malicki DM, Tran CQ, Dahle EJ, Philippi A, Timar L, Percy AK, Motil KJ, Lichtarge O, Smith EO, Glaze DG, Zoghbi HY (2000) Influence of mutation type and X chromosome inactivation on Rett syndrome phenotypes. Ann Neurol 47:670–679 [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY (1999) Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23:185–188 [DOI] [PubMed] [Google Scholar]
- Bienvenu T, Carrie A, de Roux N, Vinet MC, Jonveaux P, Couvert P, Villard L, Arzimanoglou A, Beldjord C, Fontes M, Tardieu M, Chelly J (2000) MECP2 mutations account for most cases of typical forms of Rett syndrome. Hum Mol Genet 9:1377–1384 [DOI] [PubMed] [Google Scholar]
- Bohossian HB, Skaletsky H, Page DC (2000) Unexpectedly similar rates of nucleotide substitution found in male and female homonids. Nature 406:622–625 [DOI] [PubMed] [Google Scholar]
- Carlson KM, Bracamontes J, Jackson CE, Clark R, Lacroix A, Wells SA Jr, Goodfellow PJ (1994) Parent-of-origin effects in multiple endocrine neoplasia type 2B. Am J Hum Genet 55:1076–1082 [PMC free article] [PubMed] [Google Scholar]
- Cheadle JP, Gill H, Fleming N, Maynard J, Kerr A, Leonard H, Krawczak M, Cooper DN, Lynch S, Thomas N, Hughes H, Hulten M, Ravine D, Sampson JR, Clarke A (2000) Long-read sequence analysis of the MECP2 gene in Rett syndrome patients: correlation of disease severity with mutation type and location. Hum Mol Genet 9:1119–1129 [DOI] [PubMed] [Google Scholar]
- Clayton-Smith J, Watson P, Ramsden S, Black GCM (2000) Somatic mutation in MECP2 as a non-fatal neurodevelopmental disorder in males. Lancet 356:830–832 [DOI] [PubMed] [Google Scholar]
- Cohn DH, Starman BJ, Blumberg B, Byers PH (1990) Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for dominant mutation in a human type I collagen gene (COL1A1). Am J Hum Genet 46:591–601 [PMC free article] [PubMed] [Google Scholar]
- Dragich J, Houwink-Manville I, Schanen C (2000) Rett syndrome: a surprising result of mutation in MECP2. Hum Mol Genet 9:2365–2375 [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Wenstrup RJ, Beyers PH, Cohen DH (1992) Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a mutation in the COL1A2 gene of type I collagen: the mosaic parent exhibits phenotypic features of mild form of the disease. Hum Mutat 1:47–54 [DOI] [PubMed] [Google Scholar]
- Glaser RL, Jiang W, Boyadjiev SA, Tran AK, Zachary AA, Van Maldergem L, Johnson D, Walsh S, Oldridge M, Wall SA, Wilkie AO, Jabs EW (2000) Paternal origin of FGFR2 mutations in sporadic cases of Crouzon syndrome and Pfeiffer syndrome. Am J Hum Genet 66:768–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Monk M (1998) Regulation of X-chromosome inactivation in development in mice and humans. Microbiol Mol Biol Rev 62:362–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg B (1985) Rett's syndrome: prevalence and impact on progressive severe mental retardation in girls. Acta Paediatr Scand 74:405–408 [DOI] [PubMed] [Google Scholar]
- Hagberg B, Goutieres F, Hanefeld F, Rett A, Wilson J (1985) Rett syndrome: criteria for inclusion and exclusion. Brain Dev 7:372–373 [DOI] [PubMed] [Google Scholar]
- Huppke P, Laccone F, Kramer N, Engel W, Hanefeld F (2000) Rett syndrome: analysis of MECP2 and clinical characterization of 31 patients. Hum Mol Genet 9:1369–1375 [DOI] [PubMed] [Google Scholar]
- Jadayel D, Fain P, Upadhyaya M, Ponder MA, Huson SM, Carey J, Fryer A, Mathew CG, Barker DF, Ponder BA (1990) Paternal origin of new mutations in von Recklinghausen neurofibromatosis. Nature 343:558–559 [DOI] [PubMed] [Google Scholar]
- Lazaro C, Ravella A, Gaona A, Volpini V, Estivill X (1994) Neurofibromatosis type 1 due to germ-line mosaicism in a clinically normal father. N Engl J Med 331:1403–1407 [DOI] [PubMed] [Google Scholar]
- Meloni I, Bruttini M, Longo I, Mari F, Rizzolio F, D'Adamo P, Denvriendt K, Fryns JP, Toniolo D, Renieri A (2000) A mutation in the Rett syndrome gene, MECP2, causes X-linked mental retardation and progressive spasticity in males. Am J Hum Genet 67:982–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney DM, Slaney SF, Oldrige M, Wall SA, Sahlin P, Stenman G, Wildie AO (1996) Exclusive paternal origin of new mutations in Apert syndrome. Nat Genet 13:9–10 [DOI] [PubMed] [Google Scholar]
- Namikawa C, Suzumori K, Fukushima Y, Sasaki M, Hata A (1995) Recurrence of osteogenesis imperfecta because of paternal mosaicism: Gly862→Ser substitution in a type I collagen gene (COL1A1). Hum Genet 95:666–670 [DOI] [PubMed] [Google Scholar]
- Obata K, Matsuishi T, Yamashita Y, Fukuda T, Kuwajima K, Horiuchi I, Nagamitsu S, Iwanaga R, Kimura A, Omori I, Endo S, Mori K, Kondo I (2000) Mutation analysis of the methyl-CpG binding protein 2 gene (MECP2) in patients with Rett syndrome. J Med Genet 37:608–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrico A, Lam C, Galli L, Dotti MT, Hayek G, Tong SF, Poon PM, Zappella M, Federico A, Sorrentino V (2000) MECP2 mutation in male patients with non-specific X-linked mental retardation. FEBS Lett 481:285–288 [DOI] [PubMed] [Google Scholar]
- Statistisches Bundesamt Wiesbaden, Statistisches Jahrbuch für die Bundesrepublik Deutschland, Metzler-Poeschel, Stuttgart, Editions 1985–99 [Google Scholar]
- Tate P, Skarnes W, Bird A (1996) The methyl-CpG binding protein MeCP2 is essential for embryonic development in the mouse. Nat Genet 12:205–208 [DOI] [PubMed] [Google Scholar]
- Thomas GH (1996) High male:female ratio of germ-line mutations: an alternative explanation for postulated gestational lethality in males in X-linked dominant disorders. Am J Hum Genet 58:1364–1368 [PMC free article] [PubMed] [Google Scholar]
- Villard L, Cardoso AK, Chelly PJ, Tardieu PM, Fontes M (2000) Two affected boys in a Rett syndrome family: clinical and molecular findings. Neurology 55:1188–1193 [DOI] [PubMed] [Google Scholar]
- Wan M, Lee SS, Zhang X, Houwink-Manville I, Song HR, Amir RE, Budden S, Naidu S, Pereira JL, Lo IF, Zoghbi HY, Schanen NC, Francke U (1999) Rett syndrome and beyond: recurrent spontaneous and familial MECP2 mutations at CpG hotspots. Am J Hum Genet 65:1520–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettke-Schäfer R, Kantner G (1983) X-linked dominant inherited diseases with lethality in hemizygous males. Hum Genet 64:1–23 [DOI] [PubMed] [Google Scholar]
- Xiang F, Buervenich S, Nicolao P, Bailey ME, Zhang Z, Anvret M (2000) Mutation screening in Rett syndrome patients. J Med Genet 37:250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]