Abstract
Wnt-4, a member of the Wnt family of locally acting secreted growth factors, is the first signaling molecule shown to influence the sex-determination cascade. In mice, a targeted deletion of Wnt-4 causes the masculinization of XX pups. Therefore, WNT-4, the human homologue of murine Wnt-4, is a strong candidate gene for sex-reversal phenotypes in humans. In this article, we show that, in testicular Sertoli and Leydig cells, Wnt-4 up-regulates Dax1, a gene known to antagonize the testis-determining factor, Sry. Furthermore, we elucidate a possible mechanism for human XY sex reversal associated with a 1p31-p35 duplication including WNT-4. Overexpression of WNT-4 leads to up-regulation of DAX1, which results in an XY female phenotype. Thus, WNT-4, a novel sex-determining gene, and DAX1 play a concerted role in both the control of female development and the prevention of testes formation. These observations suggest that mammalian sex determination is sensitive to dosage, at multiple steps in its pathway.
Introduction
To date, Wnt-4 is the only signaling molecule known to be involved in sex determination. Wnt-4 is a member of the Wnt family of secreted signals, which act locally, via Frizzled receptors and a cascade of intracellular signals leading to the transcriptional activation of a host of genes (Dale 1998). Wnt-4 expression is down-regulated in the developing male gonad after E11.5, although it persists in the developing ovary (Vainio et al. 1999). In addition, targeted deletion of Wnt-4 results in the masculinization of XX mice, with rudimentary development of the masculine internal (Wolffian) ducts and with degeneration of the female (Müllerian) reproductive tract (Vainio et al. 1999). These observations suggest that Wnt-4 plays an important role in the developing ovary.
In humans, sex is determined by a regulatory cascade of sex-linked and autosomal genes (Vilain and McCabe 1998; Koopman 1999; Swain and Lovell-Badge 1999). Most encode transcription factors such as SRY, SOX9, DAX1, and SF1, which are expressed, in a sexually dimorphic fashion, during gonadal development. The timing and dosage of the expression of these genes (along with many others yet to be identified) directs the bipotential urogenital ridge to develop either as testes, in males, or as ovaries, in females, but the particulars of this regulatory cascade remain unclear. Mutations in either of the high-mobility group (HMG)–box transcription factors, SRY (SRY [MIM 480000]) and SOX9 (SOX9 [MIM 114290]), cause the feminization of XY fetal gonads, leading to XY sex reversal with gonadal dysgenesis (Koopman 1999). DAX1, a member of the nuclear hormone-receptor superfamily (Zanaria et al. 1994), maps to the dosage-sensitive sex reversal (DSS) locus on Xp21.3. In humans, duplications of DSS (DSS [MIM 300018]) have been shown to cause XY sex reversal (Bardoni et al. 1994; Swain et al. 1996). SF1, another nuclear hormone receptor, has been shown to regulate Dax1 expression (Vilain et al. 1997), and mutation of SF1 (SF1 [MIM 184757]) has been associated with XY sex reversal and with adrenal insufficiency (Achermann et al. 1999).
In mice, Dax1 exhibits an expression pattern similar to that of Wnt-4—that is, down-regulated in males after the beginning of testis differentiation (at E12.5) but persistent in the ovary throughout fetal development (Swain et al. 1996). Overexpression of DAX1 in mice leads to XY sex reversal, suggesting that it antagonizes SRY (Swain et al. 1998). DAX1 may therefore contribute to ovarian development, as well as prevent testicular formation (Goodfellow and Camerino 1999). This study provides evidence that, by up-regulating DAX1 expression, WNT-4 may act in concert with DAX1, to accomplish these developmental goals.
Material and Methods
Gene Structure and Localization
The chromosomal localization of WNT-4 was determined by use of primers flanking exon 2 (forward primer, 5′-ATCCAGAGAGAAGTCGGCAGCC-3′; reverse primer, 5′-CCCGTTGCTCACGAGCGTCTC-3′) and of the GeneBridge 4 radiation-hybrid panel (Research Genetics) as a template for PCR. The radiation-hybrid localization was confirmed by FISH using a bacterial artificial chromosome (BAC) probe containing WNT-4 obtained by PCR screening of a human BAC library (Genome Systems), by use of the primers listed above. The sequence of exon 1 was verified by 5′ RACE-PCR version 2.0 (Gibco), on RNA from MDA-415 cells (American Type Culture Collection), a human mammary cell line, by use of a primer in exon 3 (5′-CCGAAGAGATGGCGTACACGAAGG-3′), for the reverse transcription, and by use of two primers in exon 2 (5′-ACCTTGCCGAAGACGGGCAAGG-3′ and 5′-CCAGCGCCGGTTCCGGAACTGG-3′), for nested PCR. An annealing temperature of 55°C was used for the 35 cycles in each nested PCR.
Molecular Cytogenetic Analysis
We identified an XY female patient carrying a duplication of 1p31-p35. This patient’s duplication was characterized by use of BAC probes mapping to several cytogenetic bands between 1p31 (BAC 395G6) and the 1p terminus (BAC 397E07) and by use of a pool of 3–4-kb genomic probes specific to various exons of WNT-4. Whole chromosome 1–specific paint probes (Coatasome 1 Total Chromosome Probe; Oncor) were used for painting.
Expression of WNT-4 in Fibroblasts
Total RNA was extracted, by use of Trizol reagent (Gibco), from cultures of skin fibroblasts isolated from the patient and from normal human foreskin (American Type Culture Collection). One microgram of total RNA was used as the template for reverse transcriptase–PCR (RT-PCR), to amplify a 360-bp fragment of WNT-4 containing exons 2–5 (forward primer, 5′-GCTGGAACTGCTCCACACTCG-3′; reverse primer, 5′-CCCGCATGTGTGTCAGGATGG-3′) and a 587-bp fragment of β-actin, by use of primers purchased from Clontech. The reverse transcription was performed at 42°C for 1 h, followed by a PCR protocol including 35 cycles at an annealing temperature of 58°C.
Transfections
MLTC-1 and TM4 cell lines (American Type Culture Collection) were transfected by LipofectAmine (Gibco), with Wnt-4 (a gift from Dr. A. McMahon), SF-1 (Vilain et al. 1997), and pcDNA3.1 (Invitrogen), the expression vector in which Wnt-4 and SF1 are cloned. In each experiment, 106 cells were transfected with 4 μg of each of the specified plasmids. A vector expressing Renilla luciferase (Promega) was used as a control with regard to transfection efficiency. Total RNA was extracted from these transfections after 24–36 h, as described above, and was examined by either RT-PCR or northern analysis. RT-PCR was performed with primers to amplify a 585-bp fragment of Dax1, which spans the intron/exon splice site (Tamai et al. 1996), with β-actin used as a control. The reverse transcription was performed on 1 μg of total RNA from each sample, at 42°C for 1 h, followed by a PCR protocol including 25 cycles at an annealing temperature of 57°C. Ten micrograms of total RNA were used in each lane, for the northern analysis, and full-length Dax1 and β-actin cDNAs were used as probes.
Results
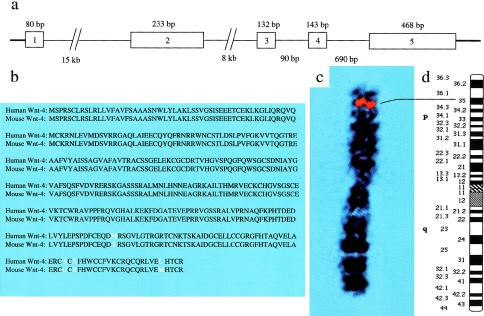
To assess the role that WNT-4 (WNT-4 [MIM 603490]) plays in human sex determination, we cloned the human homologue of murine Wnt-4, by searching the GenBank database for human BAC sequences with high homology to murine Wnt-4. This search was assisted by the sequencing of the human genome, which allowed the identification of a human BAC clone containing most of the genomic sequence of WNT-4, lacking only exon 1 and the 5′ end of intron 1. In addition, this BAC clone demonstrated the physical proximity of WNT-4 to the CDC42 gene, which is located ∼25 kb downstream. Using 5′ RACE-PCR on RNA from human mammary cells, we amplified and sequenced exon 1 of WNT-4. We confirmed all coding regions of exons 2–5 by PCR amplification and sequencing of normal human DNA. After determining the length of intron 1 by PCR amplification between exon 1 and the 3′ end of intron 1 (data not shown), we completed the map of the genomic structure of WNT-4 (fig. 1a). Alignment of the predicted mouse and human proteins revealed high evolutionary conservation, with 98.9% sequence identity and 99.7% sequence similarity. In fact, these homologues differ at only four amino acid residues, clustered near the C-terminus (fig. 1b). By radiation-hybrid mapping, we localized WNT-4 to the distal end of chromosome 1p, between markers WI-3177 and WI-5581 (data not shown). To confirm these data, we identified a BAC clone containing the full sequence of WNT-4, by PCR screening of a human BAC library, by using the primers listed above. Using this BAC as a probe, we localized WNT-4 to cytogenetic band 1p35, by FISH (fig. 1c and d). This cytogenetic localization is in good agreement with the results of the radiation-hybrid mapping. According to the Human-Mouse Homology Map of the National Center for Biotechnology Information, this region of human chromosome 1p is highly syntenic to mouse chromosome 4q, where the murine homologue of CDC42 is located between markers D4Mit31 and D4Mit13. These data, in combination with the physical proximity of the CDC42 and WNT-4 loci in the human genome, suggest that murine Wnt-4 also maps to mouse chromosome 4q.
Figure 1.
Genomic structure and localization of WNT-4. a, Genomic structure of WNT-4. The lengths of the exons appear above corresponding boxes, and the lengths of the introns appear below. b, Alignment of human and mouse Wnt-4 predicted protein sequences. The differences are denoted by white letters. c, FISH with a BAC probe containing WNT-4, on a normal metaphase spread. For easier comparison with the chromosome 1 ideogram, only chromosome 1 is shown. d, Ideogram of chromosome 1, illustrating normal GPG-banding. The guideline indicates the locus that corresponds to the WNT-4 FISH signal on chromosome 1.
Four XY patients with a duplication of part of chromosome 1p have been described as having sexual phenotypes ranging from cryptorchidism (Cousineau et al. 1981; Mohammed et al. 1989) to sex reversal (Elejalde et al. 1984; Wieacker et al. 1996). We obtained fibroblasts from one such XY sex-reversed patient carrying a cytogenetic duplication of 1p31-p35, who presented with ambiguous external genitalia accompanied by severe hypospadias, fibrous gonads with rudimentary tubules, and remnants of both Müllerian and Wolffian structures (Elejalde et al. 1984). In addition, the presence of a rudimentary uterus and vagina in this patient suggests that secretion of Müllerian inhibiting substance (MIS) was absent from the gonads during embryogenesis. Since MIS secretion is an early indication of testes differentiation, it is likely that testes never developed in this XY individual. The cause of this sex reversal is likely to be a gene within the duplication that this patient carries.
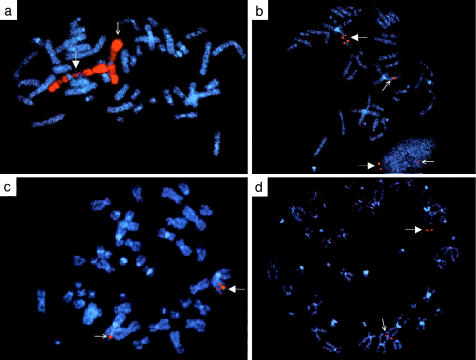
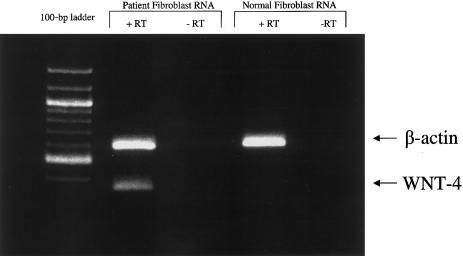
To better characterize the duplication found in this patient, we performed FISH with BAC probes known to map to cytogenetic bands 1p31 and 1pter (Korenberg et al. 1999), as well as with a pool of probes specific to WNT-4. Using whole chromosome 1 paint probes, we verified that this cytogenetic abnormality was a classic duplication and not a translocation (fig. 2a). We confirmed that the duplicated region includes 1p31 (fig. 2b) and extends to a distal breakpoint beyond the WNT-4 locus at 1p35 (fig. 2c). Figure 2c demonstrates that the WNT-4 locus is duplicated in this patient. We also found that the terminal band of 1p is not involved in the duplication (fig. 2d). To study the effects that this duplication has on WNT-4 expression, RT-PCR studies were performed. WNT-4 was expressed in the fibroblasts of this XY sex-reversed patient, but it was not expressed in normal male fibroblasts (fig. 3). Although this WNT-4 expression in the patient’s fibroblasts would probably not be physiologically relevant to the sex reversal, it does demonstrate that WNT-4 expression was misregulated in at least one tissue type, and it also raises the possibility that WNT-4 may have been misregulated in the developing gonads, where its expression could have altered the gonadal phenotype. However, since tissue from the developing gonad of this patient was not available for study, we cannot formally conclude that WNT-4 was misexpressed in this tissue.
Figure 2.
FISH mapping of the patient’s duplication. Larger, filled white arrows indicate the chromosome 1 carrying the duplication; smaller arrows indicate the normal chromosome 1. a, Metaphase spread hybridized with whole chromosome 1–specific paint (Coatasome 1 Total Chromosome Probe; Oncor). b, Metaphase spread and interphase nucleus hybridized with probes specific to cytogenetic band 1p31 (BAC 395G6). c, Metaphase spread hybridized with nonoverlapping 3–4-kb probes specific to genomic sequences of WNT-4. d, Metaphase spread hybridized with probes specific to the 1p terminus (BAC 397E07).
Figure 3.
Comparison of expression of WNT-4 in RNA from patient fibroblasts and from control fibroblasts. Lanes denoted “+RT” contain RT; lanes denoted “−RT” lack RT. Arrows indicate the 587-bp β-actin RT-PCR product and the 360-bp WNT-4 RT-PCR product.
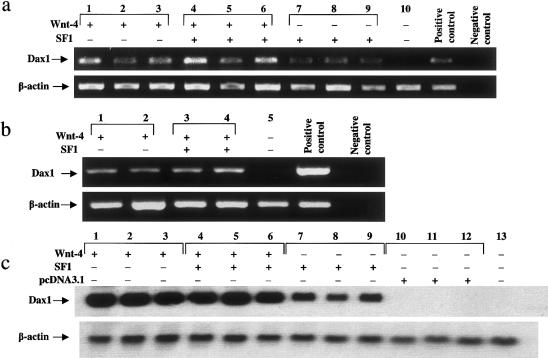
Because of the phenotypic similarities between this patient with an additional dose of WNT-4 and patients with an additional dose of DAX1, we hypothesized that these two genes may act in series in the sex-determination cascade. To evaluate the effect of Wnt-4 overexpression on Dax1 gene expression in vitro, full-length Wnt-4 cDNA was transfected into cultured mouse Leydig cells (MLTC-1), in which steroidogenesis takes place, and into Sertoli cells (TM4), the supporting cell lineage of the testis. Because of its previously described role in the up-regulation of Dax1 (Vilain et al. 1997; Kawabe et al.1999), SF1 was included in these transfection experiments, as a positive control. In both Leydig and Sertoli cells, transfection of Wnt-4 and cotransfection of Wnt-4 and SF1 resulted in a strong up-regulation of endogenous Dax1 (fig. 4a–c).
Figure 4.
Regulation of Dax1 by Wnt-4 and SF1. In a, the upper panel shows expression of Dax1 in Sertoli (TM4) cells, by RT-PCR after transfection with Wnt-4 (lanes 1–3), Wnt-4 and SF1 (lanes 4–6), SF1 (lanes 7–9), and Renilla luciferase control vector (lane 10), and the lower panel shows expression of β-actin from the same samples. In b, the upper panel shows expression of Dax1 in Leydig (MLTC-1) cells, by RT-PCR after transfection with Wnt-4 (lanes 1 and 2), Wnt-4 and SF1 (lanes 3 and 4), and Renilla luciferase control vector (lane 5), and the lower panel shows expression of β-actin from the same samples. The templates for positive and negative controls are mouse ovarian RNA and water, respectively. In c, the upper panel shows expression of Dax1 in Sertoli (TM4) cells, by northern blot after transfection with Wnt-4 (lanes 1–3), Wnt-4 and SF1 (lanes 4–6), SF1 (lanes 7–9), and pcDNA3.1 (lanes 10–12), as well as RNA from a mock-transfected control (lane 13), and the lower panel shows expression of β-actin on the same blot.
Discussion
In this study, we have cloned human WNT-4 and have localized it to 1p35. Furthermore, we have identified an XY sex-reversed patient carrying a duplication of WNT-4 in whom WNT-4 is overexpressed. In addition, we have proposed that WNT-4 plays a role in the sex-determination cascade, by demonstrating that Dax1 expression is up-regulated by Wnt-4.
Wnt molecules trigger a cascade of intracellular signals resulting in transcriptional activation of a variety of target genes. In vertebrates, two pathways for Wnt signaling have been identified. In one Wnt pathway, the transcriptional activation is thought to occur as a result of G-protein–mediated modulation of internal calcium concentrations (Kühl et al. 2000). In the canonical Wnt pathway, the activation is accomplished through an interaction between β-catenin and TCF (Dale 1998). This transcriptionally active complex binds to a TCF-response element and activates the transcription of target genes. Several sequences closely related to the TCF-response element can be found within the promoter of Dax1, at nucleotide positions −150, −2200, −3300, and −4000 in the mouse (data not shown). Although these TCF sites could mediate a direct control of Dax1 by the Wnt-4 signaling pathway, further study of this interaction will be necessary to determine whether Dax1 is a direct or an indirect target of Wnt-4 signaling.
Control of Dax1 expression by Wnt-4 should result in the reduction of Dax1 expression in Wnt-4–knockout mice. Although this was not reported in the original description (Vainio et al. 1999), subsequent in situ hybridization with Dax1 in these Wnt-4–knockout mice revealed a lower level of Dax1 expression in the gonads than in normal controls (A. Swain, unpublished observation). We observed control of Dax1 expression by Wnt-4 signaling in both Leydig and Sertoli cell lines, suggesting a concerted role for these two genes in steroidogenesis as well as in the initial steps of sex determination. Both Wnt-4 and Dax1 are turned off in the developing testis (at E11.5 and E12.5, respectively) and remain expressed in the developing ovary (Swain et al. 1996; Vainio et al. 1999), and both genes, in excess, can feminize XY embryos (Bardoni et al. 1994; Swain et al. 1998). These findings suggest that Dax1 and Wnt-4 play a common role in the prevention of testis formation. However, although chromosomal duplications involving either WNT-4 or DAX1 result in XY sex reversal, targeted deletions of these genes in mice give rise to different phenotypes. Absence of Wnt-4 results in the masculinization of XX pups, including the presence of Leydig cell markers (Vainio et al. 1999). On the other hand, absence of Dax1 from XX pups has no effect on sexual development (Yu et al. 1998). The more severe phenotype observed in the Wnt-4–knockout mice is consistent with our finding that Wnt-4 acts upstream from Dax1. Also, it suggests that Wnt-4 plays additional roles in female development, such as in the suppression of Leydig cell formation. Alternatively, the Dax1-knockout phenotype may be the result of a hypomorphic allele, since the adrenal hypoplasia that is caused by DAX1 mutations in humans was not observed in this model either (Yu et al. 1998). It is also possible that WNT-4 may act independently of DAX1 to cause sex reversal. A WNT-4-transgenic-mouse model would be necessary for elucidation of the interaction between WNT-4 and DAX1 and to test directly the hypothesis that WNT-4 causes sex reversal via its control of DAX1 expression.
Transgenic experiments have shown that, in XY mice, Sry action is antagonized by Dax1 (Swain et al. 1998). Wnt-4 could be the molecular signal linking these two transcription factors. Sry may antagonize Dax1 via Wnt-4, preventing its “antitestis” role, which, in turn, would result in male development. In addition, we hypothesize that Sry could antagonize Wnt-4 either by direct transcriptional regulation of Wnt-4 expression or by interfering with the Wnt signaling cascade. The former mechanism is consistent with the serial expression pattern of Sry (peak at 11.5 dpc) (Koopman et al. 1990; Hacker et al. 1995), Wnt-4 (down-regulated in males, at 11.5 dpc) (Vainio et al. 1999), and Dax1 (down-regulated in males, at 12.5 dpc) (Swain et al. 1996, 1998). The latter mechanism is consistent with recent experimental evidence of direct inhibition of Wnt signaling molecules by Xenopus Sox3 (Zorn et al. 1999), the closest homologue to Sry (Graves 1998).
We have localized WNT-4 to cytogenetic band 1p35, a segment syntenic to the distal end of mouse chromosome 4q. Interestingly, this region of mouse chromosome 4q has already been implicated in XY sex reversal in mice, since it is thought to contain tda-1, an autosomal locus involved in the XY sex-reversal phenotype observed in C57BL/6J mice (musculus strain) carrying a Y chromosome of Poschiavinus (domesticus strain) origin (Eicher et al. 1996). Linkage analysis of the XY sex-reversed mice from this congenic strain revealed that a gene at this tda-1 locus prevented normal male development and may also have served to promote ovarian development (Eicher et al. 1996). Several markers on mouse chromosome 4q (from D4Mit31 to Tel4q) exhibited significant evidence for linkage with the tda-1 locus, with peak χ2 values near D4Smh6b. Although the Wnt-4 locus is not located at the absolute peak, our investigation suggests that it maps within this range of significant χ2 values. Therefore, because of its chromosomal localization and its proposed function as a pro-ovary gene, Wnt-4 is a strong candidate for tda-1 in this mouse model.
In this study, we have identified an XY female patient in whom WNT-4 is overexpressed, suggesting that WNT-4 is a sex-determining gene in humans. In normal testicular cells, Wnt-4 up-regulates the expression of Dax1, and it is therefore likely that overexpression of WNT-4 in an XY female results in an up-regulation of DAX1 in the testicular tissue. Furthermore, Wnt-4 up-regulation mimics the phenotype observed in XY females with duplication of DSS, a 160-kb locus on Xp21.3 containing DAX1 (Bardoni et al. 1994). These findings lead us to conclude that XY sex reversal associated with overexpression of WNT-4 is a new example of sex reversal associated with the dosage sensitivity of a sex-determining gene. Both DAX1 duplication observed in XY females (Bardoni et al. 1994) and SOX9 duplication described in an XX male (Huang et al. 1999) suggest that gene dosage is critical at most steps of the sex-determining pathway. However, the relatively low frequency (1/20,000) reported for sex reversal in humans (McElreavey et al. 1995) indicates that gene dosage may be tightly controlled in human sex determination.
Acknowledgments
We thank A. McMahon for the Wnt-4 construct, D. H. Cohn for the radiation-hybrid panel, and E. R. B. McCabe for discussion and critical comments. We are also grateful to Susan and Eric Smidt for their generous gift. This work was supported by grants from the National Institutes of Health (to E.V., P.D., and X.-N.C.) and from the Medical Investigation of Neurodevelopmental Disorders Institute (to B.K.J.).
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank Overview, http://www.ncbi.nlm.nih.gov/Genbank/GenbankOverview.html (for WNT-4 cDNA [accession number AF316543], Wnt-4 cDNA [accession number M89797], and BAC containing WNT-4 [accession number AL031281])
- Human-Mouse Homology Map, http://www.ncbi.nlm.nih.gov/Homology/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for DSS [MIM 300018], SF1 [MIM 184757], SOX9 [MIM 114290], SRY [MIM 480000], and WNT-4 [MIM 603490])
References
- Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL (1999) A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet 22:125–126 [DOI] [PubMed] [Google Scholar]
- Bardoni B, Zanaria E, Guioli S, Floridia G, Worley KC, Tonini G, Ferrante E, Chiumello G, McCabe ERB, Fraccaro M, Zuffardi O, Camerino G (1994) A dosage sensitive locus at chromosome Xp21 is involved in male to female sex reversal. Nat Genet 7:497–501 [DOI] [PubMed] [Google Scholar]
- Cousineau AJ, Higgins JV, Hackel E, Waterman DF, Toriello H, Carlile PA, Cook PJ (1981) Cytogenetic recognition of chromosomal duplication [dup(1)(p31.4→p22.1)] and the detection of three different alleles at the PGM1 locus. Ann Hum Genet 45:337–340 [DOI] [PubMed] [Google Scholar]
- Dale TC (1998) Signal transduction by the Wnt family of ligands. Biochem J 329:209–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher EM, Washburn LL, Schork NJ, Lee BK, Shown EP, Xu X, Dredge RD, Pringle MJ, Page DC (1996) Sex-determining genes on mouse autosomes identified by linkage analysis of C57BL/6J-YPOS sex reversal. Nat Genet 14:206–209 [DOI] [PubMed] [Google Scholar]
- Elejalde BR, Opitz JM, de Elejalde MM, Gilbert EF, Abellera M, Meisner L, Lebel RR, Hartigan JM (1984) Tandem dup (1p) within the short arm of chromosome 1 in a child with ambiguous genitalia and multiple congenital anomalies. Am J Med Genet 17:723–730 [DOI] [PubMed] [Google Scholar]
- Goodfellow PN, Camerino G (1999) DAX-1, an “antitestis” gene. Cell Mol Life Sci 55:857–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves JAM (1998) Evolution of the mammalian Y chromosome and sex-determining genes. J Exp Zool 281:472–481 [PubMed] [Google Scholar]
- Hacker A, Capel B, Goodfellow P, Lovell-Badge R (1995) Expression of Sry, the mouse sex determining gene. Development 121:1603–1614 [DOI] [PubMed] [Google Scholar]
- Huang B, Wang S, Ning Y, Lamb AN, Bartley J (1999) Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet 87:349–353 [DOI] [PubMed] [Google Scholar]
- Kawabe K, Shikayama T, Tsuboi H, Oka S, Oba K, Yanase T, Nawata H, Morohashi K (1999) Dax-1 as one of the target genes of Ad4BP/SF-1. Mol Endocrinol 13:1267–1284 [DOI] [PubMed] [Google Scholar]
- Koopman P (1999) Sry and Sox9: mammalian testis-determining genes. Cell Mol Life Sci 55:839–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P, Münsterberg A, Capel B, Vivian N, Lovell-Badge R (1990) Expression of a candidate sex-determining gene during mouse testis differentiation. Nature 348:450–452 [DOI] [PubMed] [Google Scholar]
- Korenberg JR, Chen X-N, Sun Z, Shi ZY, Ma S, Vataru E, Yimlamai D, Weissenbach JS, Shizuya H, Simon MI (1999) Human genome anatomy: BACs integrating the genetic and cytogenetic maps for bridging genome and biomedicine. Genome Res 9:994–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl M, Sheldahl LC, Park M, Miller JR, Moon RT (2000) The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet 16:279–283 [DOI] [PubMed] [Google Scholar]
- McElreavey K, Barbaux S, Ion A, Fellous M (1995) The genetic basis of murine and human sex determination: a review. Heredity 75:599–611 [DOI] [PubMed] [Google Scholar]
- Mohammed FM, Farag TI, Gunawardana SS, al-Digashim DD, al-Awadi SA, al-Othman SA, Sundareshan TS (1989) Direct duplication of chromosome 1, dir dup(1)(p21.2→p32) in a Bedouin boy with multiple congenital anomalies. Am J Med Genet 32:353–355 [DOI] [PubMed] [Google Scholar]
- Swain A, Lovell-Badge R (1999) Mammalian sex determination: a molecular drama. Genes Dev 13:755–767 [DOI] [PubMed] [Google Scholar]
- Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R (1998) Dax1 antagonizes Sry action in mammalian sex determination. Nature 391:761–767 [DOI] [PubMed] [Google Scholar]
- Swain A, Zanaria E, Hacker A, Lovell-Badge R, Camerino G (1996) Mouse Dax1 expression is consistent with a role in sex determination as well as in adrenal and hypothalamus function. Nat Genet 12:404–409 [DOI] [PubMed] [Google Scholar]
- Tamai KT, Monaco L, Alastalo TP, Lalli E, Parvinen M, Sassone-Corsi P (1996) Hormonal and developmental regulation of DAX-1 expression in Sertoli cells. Mol Endocrinol 10:1561–1569 [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkilä M, Kispert A, Chin N, McMahon AP (1999) Female development in mammals is regulated by Wnt-4 signalling. Nature 397:405–409 [DOI] [PubMed] [Google Scholar]
- Vilain E, Guo W, Zhang YH, McCabe ERB (1997) DAX1 gene expression upregulated by steroidogenic factor 1 in an adrenocortical carcinoma cell line. Biochem Mol Med 61:1–8 [DOI] [PubMed] [Google Scholar]
- Vilain E, McCabe ERB (1998) Mammalian sex determination: from gonads to brain. Mol Genet Metab 65:74–84 [DOI] [PubMed] [Google Scholar]
- Wieacker P, Missbach D, Jakubiczka S, Borgmann S, Albers N (1996) Sex reversal in a child with the karyotype 46,XY, dup (1) (p22.3p32.3). Clin Genet 49:271–273 [DOI] [PubMed] [Google Scholar]
- Yu RN, Ito M, Saunders TL, Camper SA, Jameson JL (1998) Role of Ahch in gonadal development and gametogenesis. Nat Genet 20:353–357 [DOI] [PubMed] [Google Scholar]
- Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, Moser C, Walker AP, McCabe ERB, Meitinger T, Monaco AP, Sassone-Corsi P, Camerino G (1994) An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 372:635–641 [DOI] [PubMed] [Google Scholar]
- Zorn AM, Barish GD, Williams BO, Lavender P, Klymkowsky MW, Varmus HE (1999) Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol Cell 4:487–498 [DOI] [PubMed] [Google Scholar]