Abstract
Previous studies showed that antagonists of bombesin (BN)/gastrin-releasing peptide (GRP) inhibit the growth of various cancers by interfering with the growth-stimulatory effects of BN-like peptides and down-regulating epidermal growth factor receptors on tumors. Because the overexpression of the human epidermal growth factor receptor-2 (ErbB-2/HER-2/neu) oncogene plays a role in the progression of many breast cancers, we investigated whether BN/GRP antagonists can affect HER-2 in mammary tumors. Female nude mice bearing orthotopic xenografts of MDA-MB-435 human estrogen-independent breast cancers were treated daily with BN/GRP antagonists RC-3095 (20 μg) or RC-3940-II (10 μg) for 6 weeks. The expression of BN/GRP receptors on tumors was analyzed by reverse transcription–PCR and immunoblotting. We also evaluated whether the mRNA expression for the c-jun and c-fos oncogenes is affected by the therapy. Both BN/GRP antagonists significantly inhibited growth of MDA-MB-435 cancers; RC-3095 reduced tumor volume by 40% and RC-3940-II by 65%. The GRP receptors (subtype 1) were detected in MDA-MB-435 tumors, showing that they mediate the inhibitory effect of the antagonists. Tumor inhibition was associated with a substantial reduction in the expression of mRNA and protein levels of the ErbB/HER receptor family as well as with a decrease in the expression of c-jun and c-fos oncogenes. BN/GRP antagonists RC-3940-II and RC-3095 could be considered for endocrine therapy of estrogen-independent breast cancers that express members of the ErbB/HER receptor family and the c-jun and c-fos oncogenes.
Breast carcinoma is the most frequently diagnosed malignancy among women in the Western world and the second leading cause of cancer-related deaths in women (1). Recent progress in diagnosis and therapy increased the survival of women with estrogen-dependent breast cancer, but estrogen-independent tumors have a poor prognosis, because the treatment options available are not completely satisfactory. New therapeutic approaches must be explored (2). The involvement of bombesin (BN)/gastrin-releasing peptide (GRP) and epidermal growth factor (EGF) in breast cancer development and progression suggests that new therapies aimed at interference with these growth factors or their receptors could be developed.
Neuropeptides including BN and GRP act as mitogens or morphogens for some normal and neoplastic tissues including breast (3) and bind to cell surface receptors, which belong to the G protein-coupled receptor superfamily. EGF receptor (EGFR) and human EGFR (HER)-2, -3, and -4, also known as ErbB-2, -3, and -4, are members of the ErbB/HER type I tyrosine kinase receptor family, which mediates the proliferation and differentiation of normal epithelial cells. An inappropriate overexpression of these receptors as a result of oncogene amplification may contribute to tumor growth and invasion (4). An overexpression of HER-2, encoded by the neu protooncogene, was found in 25–30% of invasive breast cancers (5) and is associated with chemoresistance and poor prognosis (6). Because the overexpression of HER-2 is a relatively common phenomenon in breast cancers and its presence is associated with poor survival, HER-2 seems to be an excellent target for therapeutic strategies (5–7). A humanized monoclonal antibody against HER-2 was developed and now is in clinical trials in women with metastatic breast cancer and HER-2 overexpression (5).
In view of the involvement of BN-like peptides in the pathogenesis of a wide range of human tumors, numerous BN/GRP receptor (GRPR) antagonists have been developed (reviewed in refs. 2 and 8). BN/GRP antagonists RC-3095 and RC-3940-II, synthesized in our laboratory (9), inhibit growth of various experimental tumors including small cell lung carcinoma (SCLC) and breast cancers xenografted into nude mice (2, 8, 10, 11). Tumor growth inhibition was invariably associated with a significant down-regulation of EGFRs in tumors (11–14). In previous studies, we evaluated the effects of BN/GRP antagonists RC-3095 and RC-3940-II on MDA-MB-231 and MDA-MB-468 estrogen-independent human breast cancers (11, 14). However, the effects of BN antagonists on HER-2 expression in cancers have not been investigated.
The current preclinical study was carried out on the MDA-MB-435 breast carcinoma cell line, which is derived from a metastatic ductal breast cancer of a 31-year-old female (15). This tumor line is negative for estrogen and progesterone receptors and expresses all ErbB/HER receptor family members (16). The MDA-MB-435 cells were injected orthotopically into the mammary fat pad of nude mice, and the effects of BN antagonists RC-3095 and RC-3940-II on tumor growth were compared. We also evaluated whether these BN antagonists can affect HER-2, -3 and -4 in addition to EGFR. In addition, we investigated the expression of c-jun and c-fos oncogenes after treatment with both BN antagonists.
Materials and Methods
Peptides.
The BN/GRP antagonist [D-Tpi6,Leu13ψ(CH2NH)Leu14]BN(6–14) (RC-3095), originally synthesized in our laboratory (2, 8, 17), was manufactured by ZENTARIS AG (Frankfurt am Main, Germany) in the form of acetate salt as D22213. The more modern BN/GRP antagonist [Hca6,Leu13ψ(CH2N)Tac14]BN(6–14) (RC-3940-II) was synthesized and purified in our laboratory (refs. 2, 8, 18, and 19; Hca is desaminophenylalanine, Tac is thiazolidine-4-carboxylic acid, and Tpi is 2,3,4,9-tetrahydro-H-pyrido[3,4-b]indol-3-carboxylic acid). The peptides were dissolved in DMSO (final concentration 0.1%) and diluted with 0.15 M sodium chloride in water for injection.
Cell Culture and Animals.
The MDA-MB-435 estrogen-independent breast carcinoma cell line was obtained from the American Type Culture Collection. MDA-MB-435 cells were cultured as described (20). All culture medium components were purchased from GIBCO. Female athymic nude mice (Ncr nu/nu), 5–6 weeks old on arrival, were obtained from the Frederick Cancer Research Facility of the National Cancer Institute. The mice were housed and fed as described (11). All animal studies were conducted in accordance with institutional guidelines for the care and use of experimental animals.
Experimental Protocol.
The MDA-MB-435 human breast carcinoma cell line was injected orthotopically into the mammary fat pad of nude mice as described previously (21). The mammary fat pad was exposed under methoxyflurane (Metofane, Pittman–Moore, Mundelein, IL) anesthesia and an inoculum of 2 × 106 cells per 30 μl was injected into the tissue through a 27-gauge needle. The skin incisions were closed with wound clips, which were removed 1 week later. When tumors reached a volume of ≈100 mm3, the nude mice were divided randomly into three experimental groups of 10 animals each and received the following treatment as s.c. injections: group 1 (control), vehicle solution; group 2, BN/GRP antagonist RC-3095 at a dose of 20 μg/day per animal; group 3, and BN/GRP antagonist RC-3940-II at a dose of 10 μg/day per animal. The treatment was continued for 42 days. The tumors were measured once a week with microcalipers, and the tumor volume was calculated as length × width × height × 0.5236. Body weight of the animals was recorded. At the end of the experiment, the mice were anesthetized with methoxyflurane and decapitated. The trunk blood was collected, and the serum was separated and analyzed by RIA. The tumors were dissected, weighed, and snap-frozen for molecular biology analyses. Liver, heart, lungs, kidneys, uterus, and ovaries were removed carefully, cleaned, and weighed.
RNA Extraction and Reverse Transcription (RT)-PCR.
Total RNA was extracted from frozen tissue samples and cells by using RNAzolB (Tel-Test, Friendswood, TX) as described (14). Three micrograms of total RNA were reverse-transcribed into cDNA by using Moloney murine leukemia virus reverse transcriptase according to manufacturer instructions (Perkin–Elmer). For amplification of cDNA transcripts, gene-specific primers for human GRPR, neuromedin B receptor (NMBR), BN receptor subtype (BRS)-3, EGFR, HER-2, -3 and -4, c-Jun, c-Fos, and β-actin were used as described in detail (10, 14, 22–26). The number of cycles was determined in preliminary experiments to be within the exponential range of PCR amplification. Negative controls were run in parallel to exclude genomic DNA contamination. PCR products were subjected to electrophoresis on a 2% agarose gel, then stained with ethidium bromide, and visualized under UV light. Bands of PCR products then were scanned and analyzed semiquantitatively by using an imaging densitometer (GS-700, Bio-Rad). All experiments were repeated at least twice, and mRNA levels for each gene were normalized vs. the corresponding levels of mRNA for β-actin.
Preparation of Membranes.
Tumor tissue was homogenized by using a Polytron in homogenization buffer (50 mM Tris⋅HCl, pH 7.5/0.25 M sucrose/0.5 mM EDTA/0.1 mM phenylmethylsulfonyl fluoride/5 μg/ml leupeptin/0.5 mg/ml bacitracin). The homogenate was centrifuged at 500 × g for 5 min at 4°C. The supernatant was centrifuged at 45,000 × g for 60 min at 4°C. The final pellet was diluted in washing buffer (homogenization buffer without sucrose) and then frozen at −70°C until use. Protein concentration was determined with a Bio-Rad protein assay kit using BSA as a standard.
Immunodetection of EGFR and HER-2, -3, and -4.
Membrane proteins (20–40 μg) were solubilized in 20 mM Tris⋅HCl buffer (pH 8.0) containing 10% (vol/vol) glycerol, 1% (wt/vol) SDS, 1 mM EDTA, and 1 mM DTT and heated for 15 min. Proteins were resolved on a 6% (for EGFR and HER-2, -3, and -4) or 10% (for β-actin) SDS/PAGE and then transferred to nitrocellulose sheets (Hoeffer). The nitrocellulose sheet was soaked in Tris-buffered saline (10 mM Tris⋅HCl, pH 7.5/50 mM NaCl) containing 0.1% Tween-20 (TBST buffer). Excess protein-binding sites were saturated with TBST buffer containing 5% nonfat dried milk. The blotted membranes were incubated with rabbit polyclonal anti-human EGFR, HER-2, and HER-3 or goat polyclonal anti-human HER-4 and anti-β-actin antibodies (all from Santa Cruz Biotechnology) diluted 1:2,000–1:3,000 in the TBST buffer for 1 h at room temperature. The immunoreactive proteins were developed with peroxidase-conjugated anti-rabbit and anti-goat IgG antibodies (Santa Cruz Biotechnology) and visualized by a chemiluminescent detection system (Pierce). The bands were analyzed with the imaging densitometer specified above, and the relative protein levels were normalized vs. the corresponding levels of actin.
Histological Methods.
Samples of tumor tissue were fixed in 10% buffered formalin. The specimens were embedded in Paraplast (Oxford Labware, St. Louis, MO). Six-micrometer-thick sections were cut and stained with hematoxylin/eosin. Mitotic and apoptotic cells were counted in 10 standard high-power microscopic fields containing, on the average, 400 cells, and their numbers per 1,000 cells were accepted as the mitotic and apoptotic indices, respectively. For demonstration of the nucleolar organizer region in tumor cell nuclei, the silver nucleolar organizer region method was used (27). The silver-stained black dots in 50 cells of each tumor were counted, and the silver nucleolar organizer region number per cell was calculated. For the detection of metastases, the entire lungs and three sections of different lobes of the livers were analyzed histologically.
RIAs for Gastrin and EGF.
Serum gastrin levels were measured by double-antibody RIA using a kit provided by ICN. EGF was extracted from the mouse sera by a modified acid-ethanol extraction method and measured by RIA as described (11).
Statistical Methods.
Data are expressed as mean ± SE. Statistical analyses of the tumor data were performed by using the Student's t test. All P values are based on two-sided hypothesis testing. Histological data were evaluated by one way ANOVA, and the treated groups were compared with the control by Dunnett's test. P < 0.05 was considered significant.
Results
Effect of BN/GRP Antagonists RC-3095 and RC-3940-II on the Growth of MDA-MB-435 Human Breast Cancers in Nude Mice.
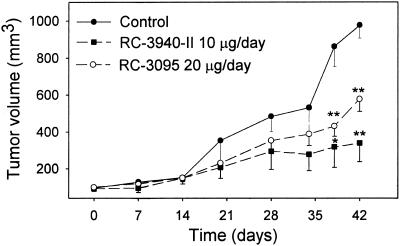
Both antagonists suppressed growth of MDA-MB-435 tumors in nude mice (Fig. 1). Therapy with antagonist RC-3940-II resulted in a greater inhibition of tumor burden and volume. After 42 days of treatment with RC-3095, the final tumor volume was decreased by 40% (P < 0.01), whereas RC-3940-II produced a 65% inhibition (P < 0.01) compared with controls. At the end of the experiment, there were no significant differences in body weights among the groups. Tumor burden was also reduced significantly by RC-3095 and RC-3940-II to 26.6 ± 1.9 (P < 0.05) and 24.5 ± 4.9 (P < 0.05) mg/g of body weight, respectively, compared with 44.5 ± 5.6 mg/g of body weight for control. No significant differences in the weights of various organs such as liver, kidney, lung, uterus, ovaries, and heart were observed between the control and treated animals (data not shown).
Figure 1.
Changes in tumor volume of MDA-MB-435 human estrogen-independent breast carcinomas, implanted orthotopically into athymic female nude mice, during treatment with BN/GRP antagonists RC-3095 or RC-3940-II. The vertical bars show SE values. *, P < 0.05 vs. control; **, P < 0.01 vs. control.
Serum Gastrin and EGF Levels.
Serum gastrin and EGF levels in controls and animals treated with RC-3095 or RC-3940-II were measured. There were no significant changes in serum gastrin levels after chronic treatment with RC-3095 (121.1 ± 13.4 pg/ml) or RC-3940-II (115.5 ± 10.3 pg/ml) compared with controls (122.4 ± 6.7 pg/ml). Serum EGF levels also showed no significant differences after treatment with RC-3095 (3.1 ± 0.4 ng/ml) or RC-3940-II (2.5 ± 0.2 ng/ml) compared with those of controls (4.1 ± 0.9 ng/ml).
Histology.
MDA-MD-435 mammary cancers were undifferentiated tumors consisting of polygonal epithelial cells without any special arrangement. The nuclei of cells were round or oval, relatively pale, and contained prominent nucleoli. Scattered giant multinuclear tumor cells were found. The amount of stroma was minimal. The quantitatively measured parameters (the number of mitotic cells, the frequency of apoptosis, or the number of silver nucleolar organizer regions) showed no significant changes after treatment with BN antagonists compared with control values (data not shown). There were no macroscopically detectable metastases in the lungs or livers. Histologically, no metastases were found in the livers, but microscopic metastatic nodules were seen in the lungs of some animals. The occurrence of metastasis and number of metastatic nodules were not significantly different among the groups (data not shown).
Expression of mRNA for BN/GRPRs in MDA-MB-435 Human Breast Carcinoma.
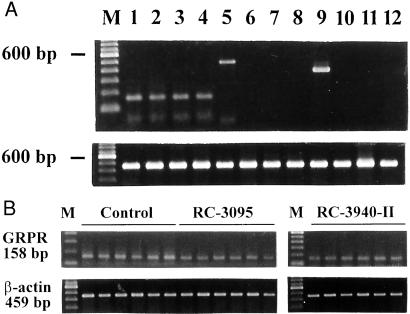
RT-PCR analyses demonstrated that MDA-MB-435 tumors of control animals expressed mRNA for BRS-1 (GRPR) but not NMBR or BRS-3 (Fig. 2A). PC-3 human prostatic, MDA-MB-468 human breast, and H-69 human SCLC cell lines were used as positive controls for GRPR, NMBR, and BRS-3 receptors, respectively (10, 14, 22).
Figure 2.
(A) Expression of mRNA for BRSs in MDA-MB-435 human breast carcinoma. Lanes 2–4, GRPR; lanes 6–8, NMBR; lanes 10–12, BRS-3; lanes 1, 5, and 9, positive controls for GRPR (PC3 human prostatic cancer cell line), NMBR (MDA-MB-468 human breast cancer cell line), and BRS-3 (H-69 human SCLC cell line), respectively (Upper). All preparations were normalized according to the expression of mRNA for β-actin (Lower). PCRs yielded products of the expected size of 158, 484, 375 and 459 bp for the GRPR, NMBR, BRS-3, and β-actin, respectively. (B) RT-PCR analysis of the expression of mRNA for GRPR and β-actin in MDA-MB-435 human estrogen-independent breast cancers. The lanes represent control tumors and those treated with 20 μg/day of RC-3095 or 10 μg/day of RC-3940-II as indicated. The sizes of the expected products are shown. Lane M, 100-bp molecular DNA marker.
RT-PCR analyses of mRNA expression for GRPR yielded a product of the expected size of 158 bp for GRPR in samples from the control as well as the RC-3095- and RC-3940-II-treated groups (Fig. 2B). Amplification with the primers specific for β-actin produced a single PCR product of 459 bp (Fig. 2B). Densitometric analyses of RT-PCR products after normalization of the expression of GRPR mRNA according to the levels of mRNA for β-actin revealed that treatment with RC-3095 decreased the expression of mRNA for GRPRs in tumors by 38% (P < 0.05) and with RC-3940-II by 54% (P < 0.01) as compared with the controls.
Effect of BN/GRP Antagonists RC-3095 and RC-3940-II on the Expression of mRNA for the Members of the ErbB/HER Type I Tyrosine Kinase Receptor Family in MDA-MB-435 Human Breast Cancers.
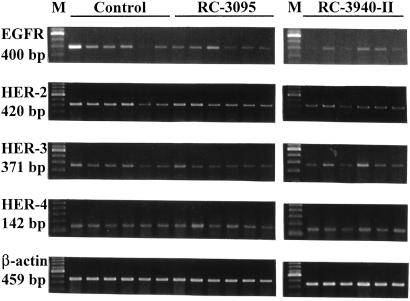
To investigate whether the treatment with BN/GRP antagonists had an effect on the expression of receptors for the ErbB/HER family, RT-PCR analyses were performed. PCR products of 400, 420, 371, and 142 bp, corresponding to the EGFR and HER-2, -3, and -4, respectively, were detected after ethidium-bromide staining (Fig. 3). No PCR products were amplified from the negative controls, ruling out the possibility of genomic DNA contamination. Amplification with β-actin-specific primers produced a single product of 459 bp from all samples, confirming that there was no degradation of the RNA preparations. Densitometric analyses of the RT-PCR products shown in Table 1 after normalization of the expression of mRNA for HER members family vs. the corresponding β-actin mRNA levels demonstrated that RC-3095 diminished mRNA for HER-2 and HER-3 by 30% (P < 0.05) and 15% (P < 0.05), respectively. In addition, there was a decrease (not significant) in the expression levels of mRNA for EGFR in the RC-3095-treated group when compared with the control, whereas no effect was observed on the HER-4 mRNA levels (Table 1). The RT-PCR analyses also revealed an even greater decrease in the expression of mRNA for EGFR and HER-2, -3, and -4, amounting to 35% (P < 0.05), 31% (P < 0.05), 38% (P < 0.005), and 28% (P < 0.005), respectively, in MDA-MB-435 tumors from animals treated with RC-3940-II (Table 1).
Figure 3.
Expression of mRNA for EGFR and HER-2, -3, and -4 in MDA-MB-435 human estrogen-independent breast cancers grown in nude mice after treatment with RC-3095 (20 μg/day) or RC-3940-II (10 μg/day). Total RNA (3 μg) was reverse-transcribed, and cDNA was amplified with specific primers. The PCR products were resolved on a 2% agarose ethidium-bromide gel. mRNA expression for β-actin was used as internal control. M, 100-bp molecular DNA marker. The sizes of the expected products are shown. mRNA levels were standardized according to β-actin mRNA levels and are expressed as a percentage of control value shown in Table 1. Results are the mean of at least two independent experiments.
Table 1.
Effect of treatment with RC-3095 and RC-3940-II on mRNA expression and protein levels of ErbB/HER family members in MDA-MB-435 human breast carcinomas
| mRNA, % of control
|
Protein, % of control
|
|||||
|---|---|---|---|---|---|---|
| Control | RC-3095 | RC-3940-II | Control | RC-3095 | RC-3940-II | |
| EGFR | 100.0 ± 13.6 | 72.4 ± 6.3 | 65.1 ± 5.6* | 100.0 ± 5.9 | 86.1 ± 20.9 | 73.7 ± 8.6* |
| HER-2 | 100.0 ± 5.9 | 72.3 ± 7.1* | 68.7 ± 8.0* | 100.0 ± 5.7 | 59.0 ± 4.1† | 21.5 ± 7.2* |
| HER-3 | 100.0 ± 2.7 | 85.5 ± 4.8* | 61.9 ± 3.7† | 100.0 ± 10.6 | 40.2 ± 10.8† | 30.3 ± 3.5† |
| HER-4 | 100.0 ± 3.6 | 106.0 ± 4.5 | 72.5 ± 4.8† | 100.0 ± 18.8 | 195.7 ± 14.3† | 71.7 ± 7.9* |
Values are mean ± SE. Analyses were quantified by densitometric analysis, and the data were normalized to β-actin values.
, P < 0.05 vs. control.
, P < 0.005 vs. control.
Effect of BN/GRP Antagonists RC-3095 and RC-3940-II on the Expression of ErbB/HER Family Receptor Proteins in MDA-MB-435 Human Breast Cancers.
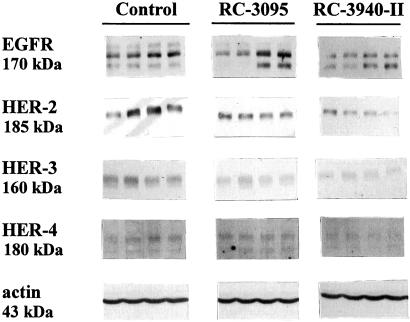
All members of the ErbB/HER receptor family were investigated by Western blotting with specific antibodies in breast tumors treated with RC-3095 or RC-3940-II. Bands at 170, 185, 160, and 180 kDa correspond to the EGFR and HER-2, -3, and -4 proteins, respectively (Fig. 4). Densitometric analyses of the bands representing the ErbB/HER receptor family members after normalization to β-actin levels demonstrated that the protein concentration was correlated with the mRNA expression of the same receptor. EGFR and HER-2, -3, and -4 protein levels in MDA-MB-435 cancers from animals treated with RC-3940-II showed a 26% (P < 0.05), 78% (P < 0.05), 70% (P < 0.005), and 28% (P < 0.05) decrease, respectively (Table 1). In contrast, RC-3095 reduced only HER-2 protein levels by 41% (P < 0.005) and HER-3 by 60% (P < 0.005) and caused a slight and insignificant decrease in EGFR and an increase in HER-4 protein (Table 1).
Figure 4.
Effect of BN antagonists RC-3095 (20 μg/day) and RC-3940-II (10 μg/day) on protein levels of EGFR and HER-2, -3, and -4 in MDA-MB-435 human estrogen-independent breast cancer grown in nude mice. Membranes were resolved and immunoblotted by using specific antisera (see Materials and Methods). Actin was used as internal control. The molecular masses of the proteins are indicated. Four representative samples from each group are shown. Protein levels were normalized to actin protein and are expressed as percentages of control values shown in Table 1. The data are the mean of at least three independent experiments.
Effect of BN/GRP Antagonists RC-3095 and RC-3940-II on the Expression of mRNA for c-jun and c-fos Oncogenes in MDA-MB-435 Human Breast Cancers.
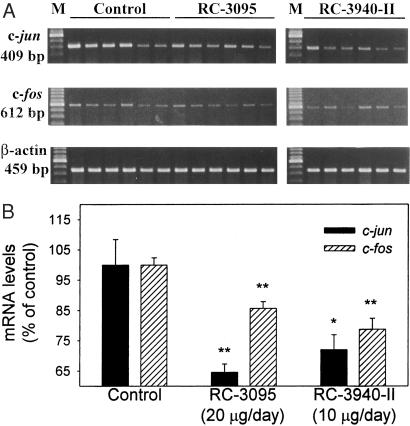
In an attempt to elucidate further the mechanism of antitumor action of BN/GRP antagonists, the expression of c-jun and c-fos oncogenes was investigated by RT-PCR followed by densitometric analyses. Treatment with RC-3095 resulted in a 35% (P < 0.005) decrease in the mRNA levels for the c-jun oncogene in MDA-MD-435 tumors, whereas RC-3940-II caused a 28% (P < 0.05) reduction compared with controls (Fig. 5 A and B). A significant decrease in the mRNA levels for the c-fos oncogene was observed also, 14% (P < 0.005) in the group treated with RC-3095 and 21% (P < 0.005) in animals receiving RC-3940-II (Fig. 5 A and B).
Figure 5.
Expression of mRNA for c-jun and c-fos oncogenes in MDA-MB-435 human estrogen-independent breast cancers grown in nude mice. (A) PCR products after electrophoresis in 2% agarose gel for control and groups treated with RC-3095 and RC-3940-II. The expression of β-actin was used as internal control. M, 100-bp molecular DNA marker. The size of the expected products is also shown. (B) the levels of c-jun and c-fos mRNA were standardized according to the levels of β-actin mRNA and are expressed as percentages of control values. The results are the mean of at least two independent experiments. The vertical bars show ± SE. *, P < 0.05 vs. control; **, P < 0.005 vs. control.
Discussion
In accord with our previous findings in MDA-MB-231 and MDA-MB-468 human mammary tumors and MXT mouse mammary cancers (11, 12, 14), the treatment with our BN/GRP antagonists RC-3095 and 3940-II results in a marked reduction in growth of MDA-MB-435 human mammary cancers in nude mice. RC-3940-II at doses of 10 μg/day proved to be more powerful than RC-3095 at 20 μg/day. This result could be due to the fact that RC-3940-II shows a higher binding affinity to GRPRs than RC-3095 (19). Greater responses to RC-3940-II than to RC-3095 have been obtained also in human H-69 SCLCs, OV-1063 ovarian cancers, and MDA-MB-468 and MDA-MB-231 breast cancers (11, 13, 14, 26).
Various previous studies reported the presence of BN-like peptides in breast carcinomas, and it was demonstrated that they acted as autocrine/paracrine growth factors in stimulating tumor growth through the BN/GRPRs (2, 8, 28). To define the receptors that mediate the effects of the BN/GRP antagonists, we studied the expression pattern for BN/GRPRs subtypes in MDA-MB-435 tumors. To date, four structurally distinct receptors for BN-like peptides have been cloned and characterized: GRPR (GRPR/BRS-1), NMBR (NMBR/BRS-2), BRS-3, and BRS-4 (8). Three of the four receptor subtypes (GRPR, NMBR, and BRS-3) are known to exist in mammals, whereas at present, BRS-4 has been identified only in amphibians. GRP and neuromedin B bind to GRPR and NMBR, respectively, with high affinity, whereas BRS-3 responds only to relatively high concentrations of known BN-like peptides, its natural ligand being still unknown. Our study revealed the expression of only BRS-1 (GRPR) in MDA-MB-435 breast cancers. The same GRPR subtype 1 was found by others in malignant breast tissue (3). The inhibition of tumor growth by BN/GRP antagonists was accompanied by a marked down-regulation of GRPRs as shown by a major fall in the mRNA levels for this receptor. Similar findings in lung cancers and ovarian tumors have been reported (13, 29).
Various investigations demonstrate that GRP and BN regulate cell proliferation through multiple cell-specific signal transduction pathways (30, 31). Briefly, the signaling cascade includes phospholipase C-β (32, 33), protein kinase C (34), and finally mitogen-activated protein kinase (Elk-1; refs. 35 and 36), which after phosphorylation is translocated into the nucleus and increases immediate-early expression of oncogenes such as c-fos and c-jun (37, 38). Both c-fos and c-jun produced by BN may form heterodimers and activate AP-1 sites, stimulating gene expression. In this regard, one of the genes stimulated may be the GRP gene that has a 5′-upstream DNA sequence (TGAGTCAG; refs. 39–41).
Numerous studies have shown that HER-2 is overexpressed in 25–30% of human breast cancers, and its overexpression identifies patients with estrogen receptor-negative tumors who have a poor prognosis (42), women with estrogen receptor-positive tumors likely to be resistant to chemotherapy (43), or those who fail to respond to endocrine therapy on relapse (44). Recent evidence indicates that by forming heterodimers, HER-2 amplifies the signals generated by other receptors of the ErbB/HER family (45). These receptors are inactive in their monomeric forms and must undergo dimerization to become activated. Heterodimers have a stronger stimulatory action on the growth of normal and tumor cells than the corresponding homodimers (46), and HER-2/HER-3 is the most representative heterodimer in breast tumors (47). Heterodimerization leads to intracellular autophosphorylation at multiple tyrosine residues and provides docking sites for enzymes and adaptor molecules (48), which transfer the activation signal to the mitogen-activated protein kinases, leading to cell growth and transformation (49, 50). In addition to the mechanism of a ligand-induced signal generation, a number of reports suggest the possibility of a ligand-independent EGFR and HER-2 transactivation (51, 52). Thus, it has been demonstrated recently that Gq-coupled receptors can phosphorylate EGFRs on tyrosine by a process termed transactivation (53). In this regard, BN also has been shown to activate the extracellular response kinase pathway, leading to the stimulation of immediate-early genes and proliferation. Considering these findings, it seems that the reduction in mitogenic effects generated through the HER system might be a promising approach to tumor therapy.
Our results show that the treatment of MDA-MB-435 estrogen-independent breast cancers with RC-3940-II significantly decreased the expression of mRNA for all HER family members, whereas RC-3095 reduced only mRNA for HER-2 and HER-3. Accordingly, in addition to decreasing the levels of EGFR, as it was reported in previous studies (11–14), BN/GRP antagonists also induce down-regulation of other members of the ErbB/HER family. The expression of mRNA for EGFR and HER-2, -3, and -4 in general was well correlated with receptor protein levels, although Western blot analyses revealed a somewhat greater decrease in HER-2 and HER-3 protein levels after treatment with BN/GRP antagonists. This shows that BN/GRP antagonists also may affect the posttransductional regulation of such ErbB/HER family members.
Previous studies showed that BN/GRP-like peptides bind to cell surface receptors, which are predominantly coupled to Gq, activate protein kinase C, and stimulate the release of Ca2+ (34), leading finally to an increase in expression of immediate-early genes such as c-fos and c-jun (38). Both c-fos and c-jun produced by BN can form heterodimers that regulate the expression of genes with AP-1 activation elements. The gene that encodes for the GRP ligand belongs to this group (40). Because transcription factors, the nuclear proteins that control DNA transcription and gene expression, are the most distal components of diverse converging mitogenic signal transduction pathways, we evaluated the c-jun and c-fos oncogenes in MDA-MB-435 tumors. Our studies revealed that the levels of mRNA for both oncogenes decreased significantly after treatment with RC-3095 and RC-3940-II. It has been demonstrated that BN-like peptides increase the mRNA levels for these oncogenes in SCLC, glioblastoma, and ovarian cancer, and treatment with different BN/GRP antagonists blocks c-fos and c-jun gene expression (23, 26, 39). It was shown also that a mutant form of c-jun, in which the transactivation domain has been deleted, inhibited AP-1 transcriptional activity in different breast cancer cell lines (54). Consequently, the down-regulation of c-jun and c-fos oncogenes and a possible subsequent AP-1 inactivation may contribute to the antitumor activity of BN/GRP antagonists.
In conclusion, the present study sheds more light on the inhibitory action of BN/GRP antagonists RC-3940-II and RC-3095 on the growth of MDA-MB-435 estrogen-independent human mammary cancers. The tumor-inhibitory effect of BN/GRP antagonists seems to involve complex mechanisms. After binding, BN/GRP antagonists down-regulate the subtype-1 BN/GRPRs on cancer cells and block the stimulatory effects of autocrine/paracrine BN-like peptides on tumors. Therefore, a possible transactivation of the ErbB/HER receptor family members by mitogenic signals is prevented also. Our most important finding is that BN antagonists can produce a reduction in the expression of mRNA and protein levels of all four members of the ErbB/HER receptor family as well as a decrease in the expression of c-jun and c-fos oncogenes. The mechanisms responsible for these effects need to be clarified fully. Liebow et al. (55) showed that BN-like peptides can up-regulate EGFRs in various cancers, and BN antagonists block this action. Consequently, BN/GRP antagonists seem to inhibit MDA-MB-435 human estrogen-independent breast carcinoma by blocking the effects of BN-like peptides and inducing a down-regulation of the HER system.
BN antagonist RC-3095 is already in clinical phase I/II trials in patients with EGF-dependent tumors. Herceptin, a monoclonal antibody that targets HER-2, is used presently for the treatment of women with HER-2-overexpressing metastatic breast cancers (56, 57). Our findings suggest the merit of therapeutic trials with BN antagonists in the management of HER-2-positive breast carcinomas. The use of BN/GRP antagonists could provide another approach to breast cancer therapy, but further studies are needed to fully elucidate the mechanism of action of these peptide analogs.
Acknowledgments
We thank Harold Valerio and Brady Wagner for excellent technical assistance. The work described in this paper was supported by the Medical Research Service of the Veterans Affairs Department and a grant from ZENTARIS AG (Frankfurt am Main, Germany) to Tulane University (all to A.V.S.). Tulane University holds patents on the BN/GRP antagonists RC-3095 and RC-3940-II, and A.V.S. is the coinventor on those patents.
Abbreviations
- BN
bombesin
- GRP
gastrin-releasing peptide
- EGF
epidermal growth factor
- EGFR
EGF receptor
- ErbB/HER
human EGFR
- GRPR
GRP receptor
- SCLC
small cell lung carcinoma
- RT
reverse transcription
- NMBR
neuromedin B receptor
- BRS
BN receptor subtype
References
- 1.Greenle R T, Hill-Harmon M B, Thun M. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Schally A V, Comaru-Schally A M. In: Cancer Medicine. 5th Ed. Holland J F, Frei E, II, Bast RC Jr, Kufe D W, Pollock R E, Weichselbaum R R, editors. Hamilton, ON: Dekker; 2000. pp. 715–729. [Google Scholar]
- 3.Gugger M, Reubi J C. Am J Pathol. 1999;155:2067–2076. doi: 10.1016/S0002-9440(10)65525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker R A. J Pathol. 1998;185:234–235. doi: 10.1002/(SICI)1096-9896(199807)185:3<234::AID-PATH128>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Pereira M B, Dias A J, Reis C A, Schmitt F C. J Clin Pathol. 2001;54:210–213. doi: 10.1136/jcp.54.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung M C, Lau Y K. Semin Oncol. 1999;26:51–59. [PubMed] [Google Scholar]
- 7.Harari D, Yarden Y. Oncogene. 2000;19:6012–6014. doi: 10.1038/sj.onc.1203973. [DOI] [PubMed] [Google Scholar]
- 8.Schally A V, Comaru-Schally A M, Nagy A, Kovacs M, Szepeshazi K, Plonowski A, Varga J L, Halmos G. Front Neuroendocrinol. 2001;22:248–291. doi: 10.1006/frne.2001.0217. [DOI] [PubMed] [Google Scholar]
- 9.Cai R Z, Qin Y, Ertl T, Schally A V. Int J Oncol. 1995;6:1165–1172. doi: 10.3892/ijo.6.6.1165. [DOI] [PubMed] [Google Scholar]
- 10.Kiaris H, Schally A V, Nagy A, Sun B, Armatis P, Szepeshazi K. Br J Cancer. 1999;81:966–971. doi: 10.1038/sj.bjc.6690794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyazaki M, Lamharzi N, Schally A V, Halmos G, Szepeshazi K, Groot K, Cai R Z. Eur J Cancer. 1998;34:710–717. doi: 10.1016/s0959-8049(97)10123-x. [DOI] [PubMed] [Google Scholar]
- 12.Szepeshazi K, Schally A V, Halmos G, Lamharzi N, Groot K, Horvath J E. Proc Natl Acad Sci USA. 1997;94:10913–10918. doi: 10.1073/pnas.94.20.10913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koppan M, Halmos G, Arencibia J M, Lamharzi N, Schally A V. Cancer (Philadelphia) 1998;83:1335–1343. doi: 10.1002/(sici)1097-0142(19981001)83:7<1335::aid-cncr10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Kahan Z, Sun B, Schally A V, Arencibia J M, Cai R, Groot K, Halmos G. Cancer (Philadelphia) 2000;88:1384–1392. doi: 10.1002/(sici)1097-0142(20000315)88:6<1384::aid-cncr16>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Cailleau R, Olive M, Cruciger Q V. In Vitro. 1978;14:911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- 16.Adelsman M A, McCarthy J B, Shimizu Y. Mol Biol Cell. 1999;10:2861–2878. doi: 10.1091/mbc.10.9.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radulovic S, Cai R Z, Serfoso P, Groot K, Redding T W, Pinski J, Schally A V. Int J Pept Protein Res. 1991;38:593–600. doi: 10.1111/j.1399-3011.1991.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 18.Cai R Z, Reile H, Armatis P, Schally A V. Proc Natl Acad Sci USA. 1994;91:12664–12668. doi: 10.1073/pnas.91.26.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reile H, Cai R-Z, Armatis P, Schally A V. Int J Oncol. 1995;7:749–754. doi: 10.3892/ijo.7.4.749. [DOI] [PubMed] [Google Scholar]
- 20.Chatzistamou I, Schally A V, Nagy A, Armatis P, Szepeshazi K, Halmos G. Clin Cancer Res. 2000;6:4158–4165. [PubMed] [Google Scholar]
- 21.Price J E, Polyxos A, Zhang R D, Daniels L M. Cancer Res. 1990;50:717–721. [PubMed] [Google Scholar]
- 22.Plonowski A, Nagy A, Schally A V, Sun B, Groot K, Halmos G. Int J Cancer. 2000;88:652–657. doi: 10.1002/1097-0215(20001115)88:4<652::aid-ijc21>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Kiaris H, Schally A V, Sun B, Armatis P, Groot K. Oncogene. 1999;18:7168–7173. doi: 10.1038/sj.onc.1203213. [DOI] [PubMed] [Google Scholar]
- 24.Arencibia J M, Schally A V, Krupa M, Bajo A M, Nagy A, Szepeshazi K, Plonowski A. Int J Oncol. 2001;19:571–577. doi: 10.3892/ijo.19.3.571. [DOI] [PubMed] [Google Scholar]
- 25.Gilbertson R J, Clifford S C, MacMeekin W, Wright C, Perry R H, Kelly P, Pearson A D J, Lunec J. Cancer Res. 1998;58:3932–3941. [PubMed] [Google Scholar]
- 26.Chatzistamou I, Schally A V, Sun B, Armatis P, Szepeshazi K. Br J Cancer. 2000;83:906–913. doi: 10.1054/bjoc.2000.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szepeshazi K, Korkut E, Schally A V. Am J Pathol. 1991;138:1273–1277. [PMC free article] [PubMed] [Google Scholar]
- 28.Pagani A, Papotti M, Sanfilippo B, Bussolati G. Int J Cancer. 1991;47:371–375. doi: 10.1002/ijc.2910470310. [DOI] [PubMed] [Google Scholar]
- 29.Chatzistamou I, Schally A V, Szepeshazi K, Groot K, Hebert F, Arencibia J M. Cancer Lett (Shannon, Irel) 2001;171:37–45. doi: 10.1016/s0304-3835(01)00543-2. [DOI] [PubMed] [Google Scholar]
- 30.Rozengurt E. Eur J Clin Invest. 1991;21:123–134. doi: 10.1111/j.1365-2362.1991.tb01801.x. [DOI] [PubMed] [Google Scholar]
- 31.Benya R V, Fathi Z, Kusui T, Pradhan T, Battey J F, Jansen R T. Mol Pharmacol. 1994;46:235–245. [PubMed] [Google Scholar]
- 32.Charlesworth A, Broad S, Rozengurt E. Oncogene. 1996;12:1337–1345. [PubMed] [Google Scholar]
- 33.Jian X, Sainz E, Clark W A, Jensen R T, Battey J K, Northup J K. J Biol Chem. 1999;274:11573–11581. doi: 10.1074/jbc.274.17.11573. [DOI] [PubMed] [Google Scholar]
- 34.Mayer EA. In: Biochemistry and Physiology. Walsh J H, Dockray G J, editors. New York: Raven; 1994. pp. 33–74. [Google Scholar]
- 35.Chen R, Sarnecki C, Blenis J. Mol Cell Biol. 1992;12:1261–1264. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Draoui M, Moody T W, Fathi Z, Battey J. Cell Growth Differ. 1993;4:723–729. [PubMed] [Google Scholar]
- 37.Hipskind R A, Baccarini M, Nordheim A. Mol Cell Biol. 1994;14:6219–6231. doi: 10.1128/mcb.14.9.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beno D W, Brady L M, Bissonnette M, Davis B H. J Biol Chem. 1995;270:3642–3647. doi: 10.1074/jbc.270.8.3642. [DOI] [PubMed] [Google Scholar]
- 39.Draoui M, Chung P, Park M, Birrer M, Jakowlew S, Moody W. Peptides (Tarrytown, NY) 1995;16:289–292. doi: 10.1016/0196-9781(94)00173-1. [DOI] [PubMed] [Google Scholar]
- 40.Kim H J, Evers B M, Litvak D A, Hellmich M R, Townsend C M., Jr Am J Physiol. 2000;279:C326–C334. doi: 10.1152/ajpcell.2000.279.2.C326. [DOI] [PubMed] [Google Scholar]
- 41.Weber H C, Walters J, Leyton J, Casibang M, Purdom S, Jensen R T, Coy D H, Ellis C, Clark G, Moody T W. Eur J Pharmacol. 2001;412:13–20. doi: 10.1016/s0014-2999(00)00941-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berns E M J J, Klijn J G M, Vanstaveren I L, Potengen H, Noordegraaf E, Foekens J A. Eur J Cancer. 1992;28:697–700. doi: 10.1016/s0959-8049(05)80129-7. [DOI] [PubMed] [Google Scholar]
- 43.Allred D C, Clark G M, Tandon A K, Molina R, Tormey D C, Osborne C K, Gilchrist K W, Mansour E G, Abeloff M, Eudey L, McGuire W L. J Clin Oncol. 1992;10:599–605. doi: 10.1200/JCO.1992.10.4.599. [DOI] [PubMed] [Google Scholar]
- 44.Wright C, Nicholson S, Angus B, Sainsbury J R, Farndon J, Cairns J, Harris A L, Horne C H. Br J Cancer. 1992;65:118–121. doi: 10.1038/bjc.1992.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klapper L N, Kirschbaum M H, Sela M, Yarden Y. Adv Cancer Res. 2000;77:25–79. [PubMed] [Google Scholar]
- 46.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin B J, Yarden Y. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menard S, Tagliabue E, Campiglio M, Pupa S M. J Cell Physiol. 2000;182:150–162. doi: 10.1002/(SICI)1097-4652(200002)182:2<150::AID-JCP3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 48.Ricci A, Lanfrancone K, Chiari R, Belardo G, Pertica C, Natali P G, Pelicci P G, Segatto O. Oncogene. 1995;11:1519–1529. [PubMed] [Google Scholar]
- 49.Robinson M J, Cobb M H. Curr Opin Cell Biol. 1997;9:181–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 50.Tzadar E, Yarden Y. Biochim Biophys Acta. 1998;1377:M25–M37. doi: 10.1016/s0304-419x(97)00032-2. [DOI] [PubMed] [Google Scholar]
- 51.Carpenter G. J Cell Biol. 1999;146:697–702. doi: 10.1083/jcb.146.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luttrell L M, Daaka Y, Lefkowitz R J. Curr Opin Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 53.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. Nature (London) 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 54.Ludes-Meyers J H, Liu Y, Munoz-Medellin D, Hilsenbeck S G, Brown P H. Oncogene. 2001;20:2771–2780. doi: 10.1038/sj.onc.1204377. [DOI] [PubMed] [Google Scholar]
- 55.Liebow C, Crean D H, Lee M T, Kamer A R, Mang T S, Schally A V. Proc Natl Acad Sci USA. 1994;91:3804–3808. doi: 10.1073/pnas.91.9.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pegram M, Slamon D. Semin Oncol. 2000;27:13–19. [PubMed] [Google Scholar]
- 57.Molina M A, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Cancer Res. 2001;61:4744–4749. [PubMed] [Google Scholar]