Abstract
Background
The role of postoperative chemotherapy in the survival of combined hepatocellular-cholangiocarcinoma (cHCC-CCA) remains undefined. This study investigated the impact of postoperative chemotherapy on patient survival.
Methods
Patients with cHCC-CCA who underwent surgical resection between January 2004 and December 2020 were identified from the Surveillance, Epidemiology, and End Results (SEER) database. Patients were divided into non-chemotherapy (n=138) and postoperative chemotherapy (n=59) groups. Survival analyses were performed using Kaplan-Meier methods, log-rank tests, and Cox proportional hazards models. Propensity score matching (PSM) was used to reduce selection bias.
Results
Among 197 patients, median follow-up was 23 months. Median overall survival (OS) was 32 months [95% confidence interval (CI): 22.7–41.3], with 40 months (95% CI: 26.4–53.7) in the non-chemotherapy group versus 26 months (95% CI: 18.6–33.4) in the chemotherapy group; median cancer-specific survival (CSS) was 54 months (95% CI: 32.3–75.7) in the non-chemotherapy group versus 28 months (95% CI: 19.1–36.9) in the chemotherapy group. The 1-, 3-, and 5-year OS were 73.5%, 46.8%, and 37.4%, and CSS were 77.8%, 51.8%, and 43.8%, respectively. Postoperative chemotherapy did not significantly improve OS [hazard ratio (HR) =1.290, 95% CI: 0.850–1.956, P=0.20] or CSS (HR =1.420, 95% CI: 0.892–2.259, P=0.11) compared to no chemotherapy. Older age (51–74 vs. ≤50 years: HR =2.974, 95% CI: 1.243–7.118, P=0.01; ≥75 vs. ≤50 years: HR =4.097, 95% CI: 1.411–11.892, P=0.009) and higher T stage (T2 vs. T1: HR =1.972, 95% CI: 1.179–3.297, P=0.01; T3 vs. T1: HR =3.010, 95% CI: 1.586–5.713, P=0.001, T4 vs. T1: HR =3.628, 95% CI: 1.659–7.934, P=0.001) were associated with worse OS. Chemotherapy did not yield a survival benefit in any age or T stage subgroup (P>0.05), but subgroup analyses were limited by small sample sizes. In the multivariate analysis of 3-year OS, T stage was an independent factor, whereas postoperative chemotherapy showed no significant benefit. After PSM, 52 patients in each group were matched (n=104 total). There was still no significant difference in OS (HR =1.063, 95% CI: 0.646–1.749, P=0.81) or CSS (HR =1.148, 95% CI: 0.663–1.988, P=0.62) between the two groups.
Conclusions
Our study did not find a significant association between postoperative chemotherapy and improved prognosis in cHCC-CCA patients. Older age and higher T stage were associated with worse prognosis. Prospective studies evaluating postoperative chemotherapy and other adjuvant strategies are needed to improve outcomes for this rare tumor.
Keywords: Combined hepatocellular-cholangiocarcinoma (cHCC-CCA), postoperative chemotherapy, overall survival (OS), prognosis
Highlight box.
Key findings
• In this Surveillance, Epidemiology, and End Results (SEER)-based study, postoperative chemotherapy was not associated with a significant survival benefit in patients with resected combined hepatocellular-cholangiocarcinoma (cHCC-CCA).
• Older age and higher T stage were associated with worse overall survival.
What is known and what is new?
• The role of postoperative chemotherapy in resected cHCC-CCA remains unclear.
• This study provides insights into the impact of postoperative chemotherapy on cHCC-CCA patient prognosis using a large population-based database.
What is the implication, and what should change now?
• Individualized treatment approaches based on patient and tumor characteristics should be explored.
• Prospective randomized trials are needed to clarify the role of postoperative chemotherapy and identify optimal adjuvant strategies for cHCC-CCA.
Introduction
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA), a rare primary liver malignancy, comprises 1–14.2% of hepatic neoplasms (1,2). It is defined by the coexistence of hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) components within a single tumor (3). Compared to HCC and ICC, cHCC-CCA demonstrates a poorer prognosis, with a median overall survival (OS) of only 32 months following surgical resection (4). Despite radical resection, recurrence risk remains elevated, resulting in poor long-term outcomes (5). The 5-year OS post-resection varies from 23.6% to 36.4% (6,7).
Postoperative chemotherapy has shown survival benefits in several neoplasms, such as gastric cancer, breast cancer, and ICC (8-11). However, the role of postoperative chemotherapy in cHCC-CCA remains elusive. Some studies suggest that chemotherapy can improve the prognosis of unresectable cHCC-CCA patients (12,13). However, research specifically addressing its role in resected cHCC-CCA is scarce. Moreover, the optimal chemotherapy regimen for cHCC-CCA has not been determined.
cHCC-CCA is a molecularly heterogeneous tumor, with varying predominance of HCC-like and ICC-like components (14). This heterogeneity likely impacts sensitivity to chemotherapy agents typically used for HCC vs. ICC, and may necessitate individualized treatment approaches based on the dominant component (15). Additionally, the aggressive biology and high recurrence rate of cHCC-CCA may limit the benefit of adjuvant chemotherapy alone (16). Combination strategies incorporating other adjuvant modalities like targeted therapy, immunotherapy, and radiotherapy may be needed to meaningfully improve survival, as has been explored in HCC and ICC (17,18). However, prospective trials evaluating postoperative chemotherapy or combination adjuvant strategies in cHCC-CCA are currently lacking.
Given the scarcity of cHCC-CCA cases and the heterogeneity of previous study findings, we conducted this retrospective study utilizing information from the Surveillance, Epidemiology, and End Results (SEER) registry to assess the impact of postoperative chemotherapy on cHCC-CCA patient prognosis. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-1022/rc).
Methods
Data source and patient selection
The present study utilized information from the publicly accessible SEER registry, which encompasses approximately 34.6% of the U.S. population. This database assembles information on cancer incidence, management, and outcomes from 18 registries in the United States (19). All relevant protocols and regulations concerning research methods and data handling were strictly followed. As the study solely used publicly available data without any personally identifiable information, institutional ethical review board approval was deemed unnecessary. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Patients diagnosed with cHCC-CCA who received surgical treatment between January 1, 2004 and December 31, 2020 were identified. Inclusion criteria encompassed the following: (I) histologically verified cHCC-CCA; (II) International Classification of Diseases for Oncology, Third Edition. (ICD-O-3) histology code 8180/3, and (III) underwent surgical resection. Exclusion criteria were as follows: (I) age <18 years (n=1), (II) received liver transplantation (n=87), (III) received locoregional treatments [radiofrequency ablation (RFA), cryotherapy, intervention, etc.] (n=48) and (IV) pre-operative chemotherapy treatment or unknown sequence of chemotherapy and surgery (n=23).
A total of 197 cHCC-CCA patients fulfilled the eligibility criteria and were incorporated into the analysis. The cohort was categorized into a non-chemotherapy group (n=138) and a postoperative chemotherapy group (n=59). Specific data on chemotherapy agents and regimens were not available in the SEER database, which is a limitation of this study. The patient selection workflow is shown in Figure 1.
Figure 1.
Patient screening flow chart. RFA, radiofrequency ablation.
Data collection and outcomes
Demographic and clinicopathological data were retrieved from the SEER database, comprising gender, age (categorized as ≤50, 51–74, and ≥75 years), race (classified as White, Black, Hispanic, and Asian), marital status(categorized as unmarried, married, and divorced/separated/widow), tumor stage [T, N, and M categories according to the 6th edition of the American Joint Committee on Cancer (AJCC) staging system], household income (categorized as <$55,000, $55,000–$75,000, and >$75,000), surgical procedure, chemotherapy status, radiotherapy status, follow-up time, and survival status.
The primary outcome measures were OS and cancer-specific survival (CSS). OS was calculated as the interval from diagnosis to death due to any reason. CSS was defined as the duration from diagnosis to cHCC-CCA-related mortality and is determined in the SEER database using cause-of-death information from death certificates. While generally reliable, the use of death certificate data for CSS has an inherent limitation: the potential for misclassification of the cause of death, especially in cases involving comorbidities or complex medical histories.
Statistical analysis
Categorical variables were summarized as frequencies and percentages, and continuous variables were expressed as means and standard deviations. Chi-squared tests were employed to evaluate differences in categorical variables between the non-chemotherapy and chemotherapy groups, while independent samples t-tests were used to compare continuous variables.
Kaplan-Meier estimates were generated for OS and CSS, and log-rank tests were performed to assess survival differences between the two groups. Univariate and multivariate Cox proportional hazards models were constructed to identify prognostic factors for OS and CSS. Variables with P<0.10 in the univariate analysis were included in the multivariate Cox model, along with clinically relevant variables (age, gender, T stage, N stage, M stage, radiotherapy status) regardless of their univariate P value.
To obtain a more balanced comparison, propensity score matching (PSM) was performed using a 1:1 nearest-neighbor algorithm with a 0.2 caliper, matching on age, gender, T stage, N stage, M stage, and radiotherapy status, yielding 52 matched pairs (n=104). After matching, standardized differences were calculated to assess balance between the groups. Survival analyses were repeated in the matched cohort. A P value <0.05 was considered statistically significant. The models provided hazard ratios (HRs) and 95% confidence intervals (CIs). Statistical analyses were carried out using SPSS 26 and GraphPad Prism 10.0 software.
Results
Baseline characteristics
A total of 197 patients met the inclusion criteria. The chemotherapy group consisted of 59 (29.9%) patients, while the non-chemotherapy group had 138 (70.1%) patients. Table 1 summarizes and compares the baseline characteristics of the two groups.
Table 1. Comparison of baseline characteristics between the postoperative chemotherapy group and the non-chemotherapy group after surgery for combined hepatocellular-cholangiocarcinoma.
| Variable | Total (n=197) | Non-chemotherapy (n=138) | Chemotherapy (n=59) | P |
|---|---|---|---|---|
| Gender, n (%) | 0.43 | |||
| Female | 59 (29.9) | 39 (28.3) | 20 (33.9) | |
| Male | 138 (70.1) | 99 (71.7) | 39 (66.1) | |
| Age (years), n (%) | 0.39 | |||
| ≤50 | 21 (10.7) | 13 (9.4) | 8 (13.6) | |
| 51–74 | 155 (78.7) | 108 (78.3) | 47 (79.7) | |
| ≥75 | 21 (10.7) | 17 (12.3) | 4 (6.8) | |
| Race | 0.65 | |||
| White | 101 (51.3) | 68 (49.3) | 33 (55.9) | |
| Black | 20 (10.2) | 14 (10.1) | 6 (10.2) | |
| Hispanic | 30 (15.2) | 21 (15.2) | 9 (15.3) | |
| Asian | 45 (22.8) | 35 (25.4) | 10 (16.9) | |
| Marriage, n (%) | 0.23 | |||
| Unmarried | 32 (16.2) | 23 (16.7) | 9 (15.3) | |
| Married | 134 (68.0) | 90 (65.2) | 44 (74.6) | |
| Divorced/separated/widowed | 25 (12.7) | 21 (15.2) | 4 (6.8) | |
| T stage | 0.044 | |||
| T1 | 64 (32.5) | 53 (38.4) | 11 (18.6) | |
| T2 | 42 (21.3) | 29 (21.0) | 13 (22.0) | |
| T3 | 22 (11.2) | 14 (10.1) | 8 (13.6) | |
| T4 | 14 (7.1) | 7 (5.1) | 7 (11.9) | |
| N stage | 0.003 | |||
| N0 | 127 (64.5) | 95 (68.8) | 32 (54.2) | |
| N1 | 10 (5.1) | 3 (2.2) | 7 (11.9) | |
| M stage | 0.08 | |||
| M0 | 131 (66.5) | 96 (69.6) | 35 (59.3) | |
| M1 | 7 (3.6) | 3 (2.2) | 4 (6.8) | |
| Household income (dollar), n (%) | 0.17 | |||
| <55,000 | 27 (13.7) | 23 (16.7) | 4 (6.8) | |
| 55,000–75,000 | 71 (36.0) | 49 (35.5) | 22 (37.3) | |
| >75,000 | 99 (50.3) | 66 (47.8) | 33 (55.9) | |
| Radiotherapy, n (%) | 5 (2.5) | 1 (0.7) | 4 (6.8) | 0.01 |
M, metastasis; N, node; T, tumor.
There were significant differences between the chemotherapy and non-chemotherapy groups in terms of T stage (P=0.044), N stage (P=0.003) and receipt of radiotherapy (P=0.01). The chemotherapy group had a higher proportion of T3–T4 tumors (25.5% vs. 15.2%), node-positive disease (11.9% vs. 2.2%) and radiotherapy treatment (6.8% vs. 0.7%) compared to the non-chemotherapy group. These differences suggest that patients receiving chemotherapy had more advanced disease at baseline, which could confound the relationship between chemotherapy and survival.
The analysis of demographic characteristics revealed no significant differences between the two groups in terms of gender, age, race, marital status, M stage, and household income (all P>0.05).
Survival analysis
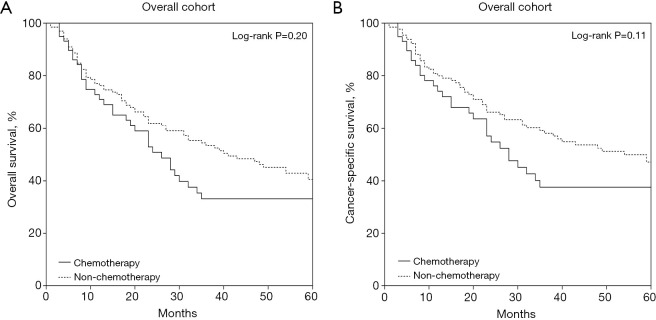
Median follow-up duration for the entire cohort spanned 23 months (range: 0–202 months). The 1-, 3-, and 5-year OS rates for all patients were 73.5%, 46.8%, and 37.4%, respectively, while the corresponding CSS rates were 77.8%, 51.8%, and 43.8%. Kaplan-Meier curves comparing OS and CSS between the chemotherapy and non-chemotherapy groups are shown in Figure 2.
Figure 2.
Patient survival curves based on postoperative chemotherapy subgroups: (A) overall survival; (B) cancer-specific survival.
In the non-chemotherapy group, the 1-, 3-, and 5-year CSS rates were 79.4%, 57.7%, and 46.8%, respectively, compared to 74.1%, 52.3%, and 31.3% in the chemotherapy group. The 1-, 3-, and 5-year OS rates in the non-chemotherapy group were 74.5%, 57.9%, and 39.6%, respectively, while the corresponding rates in the chemotherapy group were 70.9%, 33.1%, and 27.6%. No significant differences were observed between the chemotherapy and non-chemotherapy groups in terms of CSS (HR =1.420, 95% CI: 0.892–2.259, P=0.11) and OS (HR =1.290, 95% CI: 0.850–1.956, P=0.20).
The median OS was 32 months (95% CI: 22.7–41.3) for the entire cohort. The median OS was 40 months (95% CI: 26.4–53.7) in the non-chemotherapy group and 26 months (95% CI: 18.6–33.4) in the chemotherapy group. The median CSS was 40 months (95% CI: 22.0–58.0) for the entire cohort. The median CSS was 54 months (95% CI: 32.3–75.7) in the non-chemotherapy group and 28 months (95% CI: 19.1–36.9) in the chemotherapy group.
Prognostic factors for OS
Univariate analysis identified age, T stage, and node-positive status as significant predictors of OS. Multivariate analysis confirmed age and T stage as independent prognostic factors (Table 2).
Table 2. Univariate and multivariate cox regression analyses of factors affecting overall patient survival.
| Variable | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | ||
| Chemotherapy | 0.29 | 1.239 | 0.835, 1.838 | 0.20 | 1.368 | 0.848, 2.204 | |
| Gender, vs. female | |||||||
| Male | 0.54 | 1.13 | 0.763, 1.673 | 0.90 | 1.031 | 0.630, 1.687 | |
| Age (years) | |||||||
| ≤50 | 0.02 | 0.03 | |||||
| 51–74 | 0.03 | 2.277 | 1.095, 4.736 | 0.01 | 2.974 | 1.243, 7.118 | |
| ≥75 | 0.006 | 3.366 | 1.415, 8.005 | 0.009 | 4.097 | 1.411, 11.892 | |
| Race | |||||||
| White | 0.48 | ||||||
| Black | 0.99 | 0.997 | 0.538, 1.85 | ||||
| Hispanic | 0.59 | 0.866 | 0.512, 1.463 | ||||
| Asian | 0.13 | 0.685 | 0.423, 1.11 | ||||
| Marriage | |||||||
| Unmarried | 0.22 | ||||||
| Married | 0.56 | 1.187 | 0.672, 2.097 | ||||
| Divorced/separated/widowed | 0.11 | 1.755 | 0.879, 3.501 | ||||
| T stage | |||||||
| T1 | <0.001 | 0.001 | |||||
| T2 | 0.001 | 2.236 | 1.375, 3.635 | 0.010 | 1.972 | 1.179, 3.297 | |
| T3 | <0.001 | 3.563 | 2.036, 6.234 | 0.001 | 3.010 | 1.586, 5.713 | |
| T4 | <0.001 | 3.662 | 1.871, 7.168 | 0.001 | 3.628 | 1.659, 7.934 | |
| N stage, vs. N0 | |||||||
| N1 | 0.02 | 2.25 | 1.127, 4.489 | 0.18 | 1.784 | 0.772, 4.120 | |
| M stage, vs. M0 | |||||||
| M1 | 0.059 | 2.115 | 0.971, 4.606 | 0.94 | 1.041 | 0.386, 2.808 | |
| Household income (dollar) | |||||||
| <55,000 | 0.46 | ||||||
| 55,000–75,000 | 0.65 | 0.881 | 0.513, 1.513 | ||||
| >75,000 | 0.26 | 0.733 | 0.426, 1.259 | ||||
| Radiotherapy | 0.18 | 0.047 | 0.001, 4.119 | 0.97 | <0.001 | <0.001, >999 | |
CI, confidence interval; HR, hazard ratio; M, metastasis; N, node; T, tumor.
Patients aged 51–74 years (HR =2.974, 95% CI: 1.243–7.118, P=0.01) and ≥75 years (HR =4.097, 95% CI: 1.411–11.892, P=0.009) had a higher mortality risk compared to those aged ≤50 years. Similarly, patients with T2 (HR =1.972, 95% CI: 1.179–3.297, P=0.01), T3 (HR =3.010, 95% CI: 1.586–5.713, P=0.001), and T4 stages (HR =3.628, 95% CI: 1.659–7.934, P=0.001) had an increased mortality risk compared to those with T1 stage. Chemotherapy did not significantly influence OS (HR =1.368, 95% CI: 0.848–2.204, P=0.20) in multivariate analysis.
Subgroup analysis by age and T stage
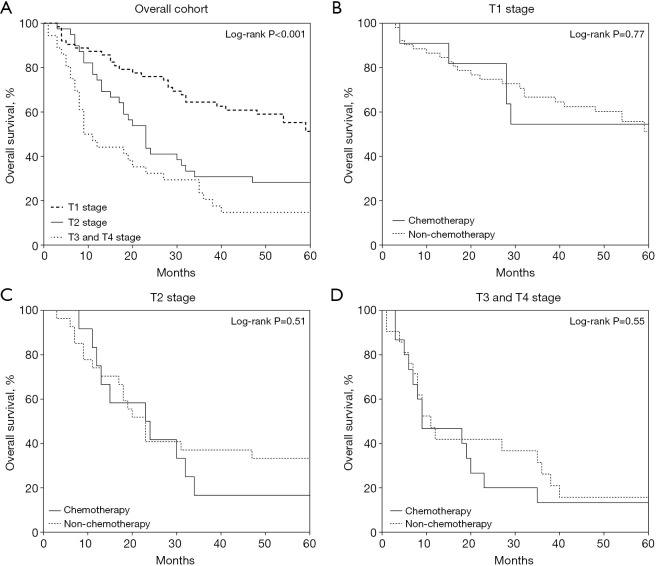
A stratified analysis was conducted to investigate the effect of postoperative chemotherapy on OS in different patient subgroups. When stratified by age, patients ≤50 years old exhibited the best OS, followed by those aged 51–74 and ≥75 years (P=0.03) (Figure 3A). Chemotherapy did not provide a significant survival benefit in any age subgroup (P=0.98 for age ≤50 years, P=0.09 for 51–74 years, P=0.19 for ≥75 years) (Figure 3B-3D). However, these subgroup analyses were limited by the small sample sizes in each age category.
Figure 3.
Stratified analysis of overall patient survival by age: (A) age ≤50, 51–74, and ≥75 years; (B) chemotherapy stratification in patients aged ≤50 years; (C) chemotherapy stratification in patients 51–74 years; (D) chemotherapy stratification in patients aged ≥75 years.
When stratified by T stage, T3 and T4 stages were combined into a single group due to the limited number of patients. The T1 group demonstrated the highest survival rate, followed by the T2 group and the combined T3/T4 group (P<0.001) (Figure 4A). Chemotherapy did not significantly improve survival in the T1 (P=0.77), T2 (P=0.51), or T3/T4 subgroups (P=0.55) (Figure 4B-4D). Again, conclusions from these subgroup analyses are constrained by the small patient numbers in each T stage category.
Figure 4.
Stratified analysis of overall patient survival by T stage: (A) T1, T2, and T3/T4; (B) chemotherapy stratification in T1 patients; (C) chemotherapy stratification in T2 patients; (D) chemotherapy stratification in T3/T4 patients.
Prognostic factors for 3-year OS
Given that the survival curves plateaued after 3 years, we specifically analyzed prognostic factors for 3-year OS to identify determinants of early mortality. Cox regression analyses were performed to identify factors influencing 3-year OS (Table 3). Univariate analysis revealed that T stage and node-positive status were associated with 3-year OS.
Table 3. Univariate and multivariate cox regression analyses of factors affecting overall patient survival of 3-year.
| Variable | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | ||
| Chemotherapy | 0.07 | 1.473 | 0.967, 2.246 | 0.12 | 1.511 | 0.903, 2.528 | |
| Gender, vs. female | |||||||
| Male | 0.14 | 1.415 | 0.895, 2.239 | 0.26 | 1.394 | 0.778, 2.498 | |
| Age (years) | |||||||
| ≤50 | 0.27 | 0.38 | |||||
| 51–74 | 0.20 | 1.658 | 0.763, 3.601 | 0.22 | 1.725 | 0.716, 4.152 | |
| ≥75 | 0.11 | 2.14 | 0.853, 5.371 | 0.18 | 2.127 | 0.709, 6.384 | |
| Race | |||||||
| White | 0.81 | ||||||
| Black | 0.79 | 0.909 | 0.447, 1.848 | ||||
| Hispanic | 0.92 | 1.028 | 0.585, 1.805 | ||||
| Asian | 0.37 | 0.783 | 0.462, 1.329 | ||||
| Marriage | |||||||
| Unmarried | 0.60 | ||||||
| Married | 0.71 | 1.125 | 0.607, 2.085 | ||||
| Divorced/separated/widowed | 0.34 | 1.451 | 0.671, 3.138 | ||||
| T stage | |||||||
| T1 | <0.001 | 0.004 | |||||
| T2 | 0.001 | 2.597 | 1.489, 4.532 | 0.01 | 2.117 | 1.174, 3.819 | |
| T3 | <0.001 | 3.487 | 1.846, 6.588 | 0.002 | 3.173 | 1.528, 6.587 | |
| T4 | <0.001 | 4.157 | 2.006, 8.614 | 0.007 | 3.262 | 1.389, 7.662 | |
| N stage, vs. N0 | |||||||
| N1 | 0.02 | 2.361 | 1.132, 4.928 | 0.22 | 1.738 | 0.715, 4.228 | |
| M stage, vs. M0 | |||||||
| M1 | 0.17 | 1.797 | 0.78, 4.141 | 0.57 | 0.735 | 0.257, 2.106 | |
| Household income (dollar) | |||||||
| <55,000 | 0.73 | ||||||
| 55,000–75,000 | 0.99 | 0.996 | 0.533, 1.863 | ||||
| >75,000 | 0.60 | 0.847 | 0.454, 1.58 | ||||
| Radiotherapy | 0.28 | 0.048 | <0.001, 12.304 | 0.97 | <0.001 | <0.001, >999 | |
CI, confidence interval; HR, hazard ratio; M, metastasis; N, node; T, tumor.
Multivariate analysis demonstrated that T stage was the independent predictor of 3-year OS. Higher T stages were associated with an increased risk of death at 3 years (T2 vs. T1: HR =2.117, 95% CI: 1.174–3.819, P=0.01; T3 vs. T1: HR =3.173, 95% CI: 1.528–6.587, P=0.002; T4 vs. T1: HR =3.262, 95% CI: 1.389–7.662, P=0.007). Furthermore, chemotherapy did not significantly influence 3-year OS (HR =1.511, 95% CI: 0.903–2.528, P=0.12) in multivariate analysis.
PSM
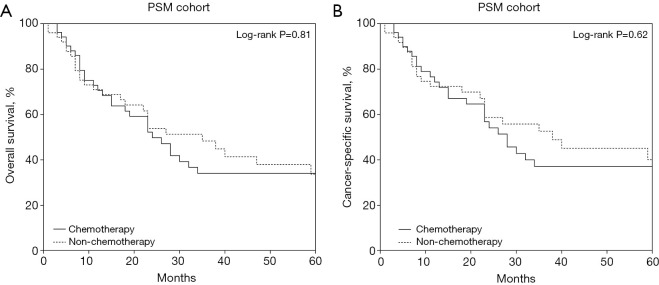
After PSM, 52 patients in the chemotherapy group were successfully matched with 52 patients in the non-chemotherapy group (Table S1). The multivariate Cox survival analysis of the PSM cohort showed no significant benefit of chemotherapy (HR =1.500, 95% CI: 0.783–2.873, P=0.22), consistent with the results before PSM (Table S2). Kaplan-Meier analysis in the PSM cohort still showed no significant difference in OS (HR =1.063, 95% CI: 0.646–1.749, P=0.81) or CSS (HR =1.148, 95% CI: 0.663–1.988, P=0.62) between the two groups (Figure 5).
Figure 5.
PSM cohort patient survival curves based on postoperative chemotherapy subgroups: (A) overall survival; (B) cancer-specific survival. PSM, propensity score matching.
Discussion
Utilizing data from the SEER database, the present study sought to evaluate the influence of postoperative chemotherapy on cHCC-CCA patient prognosis. Our results showed that older age, advanced T stage, and node-positive disease were associated with worse prognosis. However, chemotherapy did not confer a survival advantage compared to non-chemotherapy in postoperative cHCC-CCA patients.
The proportion of cHCC-CCA patients receiving postoperative chemotherapy was low (<30%), possibly reflecting the paucity of robust evidence and consensus guidelines advocating its routine use. Chemotherapy has shown potential to improve prognosis in unresectable cHCC-CCA patients. A European multicenter retrospective study demonstrated a significantly prolonged median OS in chemotherapy-treated patients compared to the untreated cohort (15.5 vs. 5.3 months) (20). Trikalinos et al. observed that gemcitabine-based regimens, particularly when combined with platinum agents, often yielded superior outcomes for cHCC-CCA patients undergoing systemic therapy alone (13).
Few studies have investigated the impact of postoperative chemotherapy on survival outcomes for resected cHCC-CCA patients. Chun et al. analyzed 80 cHCC-CCA patients, of which only 8 (10%) received postoperative chemotherapy (21). Ma et al. studied 42 postoperative cHCC-CCA patients, 13 of whom received adjuvant therapy (encompassing transarterial chemotherapy, radioembolization, systemic chemotherapy, radiotherapy, targeted therapy, or a combination thereof), and found no improvement in prognosis with postoperative adjuvant therapy (22). These limited findings align with our study’s suggestion of minimal clinical benefit for adjuvant chemotherapy in resected cHCC-CCA. Notably, both prior studies were limited by their small sample sizes (n=8 and n=13 for adjuvant therapy, respectively); our retrospective study addresses this limitation by analyzing a larger population-based cohort.
Several factors may contribute to the apparent lack of efficacy of postoperative adjuvant therapy in cHCC-CCA patients. First, cHCC-CCA is a molecularly heterogeneous tumor, with varying predominance of HCC- or ICC-like components (14). This heterogeneity, as highlighted in previous studies, likely impacts sensitivity to chemotherapy agents, and a one-size-fits-all adjuvant approach may not be appropriate (16,23). SEER lacks detailed histopathological or molecular subtype data beyond ICD-O-3 code 8180/3, limiting analysis of HCC- vs. ICC-predominant components, which could inform tailored regimens and merits further study. Second, the aggressive biology and high recurrence rate of cHCC-CCA may limit the benefit of adjuvant chemotherapy alone (16,24). Combination strategies incorporating other adjuvant modalities like targeted therapy, immunotherapy, and radiotherapy may be needed to meaningfully improve survival, as has been explored in HCC and ICC (17,25,26). For instance, cabozantinib plus atezolizumab has shown efficacy in HCC (17), and durvalumab with gemcitabine-cisplatin in biliary cancers (25), suggesting potential for cHCC-CCA, though prospective trials are lacking.
Our analysis identified several prognostic factors. Older age (>50 years) and advanced T stage correlated with heightened mortality risk, aligning with previous reports (16,27). Node-positive disease also predicted worse survival, particularly for the 3-year endpoint, although it was not a significant factor after adjusting for age and T stage, likely due to the limited number of node-positive cases in the present cohort. Previous research has identified lymph node involvement as a poor prognostic indicator in other studies (16).
While our initial results showed similar survival rates between the chemotherapy and non-chemotherapy groups, despite the former having more advanced disease, we must emphasize that this does not definitively demonstrate a benefit of chemotherapy. Multiple limitations preclude such a conclusion. First, unmeasured confounders, such as liver function and performance status, could significantly impact both treatment decisions and outcomes. Second, the SEER database lacks details on chemotherapy regimens, doses, and adherence, making it impossible to assess the adequacy of treatment. Third, the relatively small sample size, particularly in the chemotherapy group, limits the statistical power to detect a true difference. Finally, although we performed PSM to reduce bias, residual confounding may still be present.
Another critical limitation is the lack of data on disease progression, recurrence, and subsequent treatments. SEER does not capture recurrence rates or post-recurrence therapies, which could confound results if non-chemotherapy patients received effective second-line treatments after relapse. Recurrence, reported at >50% within 2 years (24,28), is a major determinant of survival, yet we cannot assess whether chemotherapy delayed progression or altered recurrence patterns. The small chemotherapy group (n=59) further limits statistical power to detect subtle effects.
The median follow-up of 23 months is a limitation, particularly in cHCC-CCA where late recurrences are common, as it may not be sufficient to fully assess long-term survival trends. While a 36-month landmark analysis demonstrated no benefit of chemotherapy, future research requires longer follow-up to evaluate the durability of treatment and provide a more complete picture of survival patterns.
Our study focused solely on comparing postoperative chemotherapy to no chemotherapy, and we did not evaluate other potential adjuvant strategies like transarterial chemoembolization (TACE), targeted therapy, or immunotherapy due to data limitations in the SEER database (17,25). These modalities are increasingly being explored in liver cancers and may play a role in the adjuvant treatment of cHCC-CCA. Future research should investigate the effectiveness of these therapies, alone or in combination with chemotherapy, to improve outcomes for cHCC-CCA patients.
There are several limitations in this study. As a retrospective analysis of a population-based registry, it is susceptible to potential selection bias and confounding. The SEER database lacks detailed information on performance status, liver function (e.g., Child-Pugh score), resection margin status, recurrence, and chemotherapy regimens, which could impact survival outcomes. The SEER database does not distinguish between different histological subtypes of cHCC-CCA, preventing subgroup analyses based on HCC-like or ICC-like components. Furthermore, the rare incidence of cHCC-CCA and the strict inclusion criteria used led to a limited sample size, potentially reducing the statistical power to identify significant associations.
Conclusions
In conclusion, this study found no significant survival benefit (OS or CSS) with the addition of postoperative chemotherapy compared to surgery alone for patients with resected cHCC-CCA. Older age and advanced tumor stage involvement were identified as adverse prognostic factors. Our findings suggest that the routine use of postoperative chemotherapy in cHCC-CCA patients should be carefully considered, and individualized treatment approaches tailored to patient and tumor characteristics warrant further exploration. Future prospective randomized trials are needed to elucidate the role of postoperative chemotherapy and other adjuvant strategies like targeted therapy and immunotherapy in cHCC-CCA and identify the optimal adjuvant therapy regimen for this challenging tumor.
Supplementary
The article’s supplementary files as
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. As the study solely used publicly available data without any personally identifiable information, institutional ethical review board approval was deemed unnecessary.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-1022/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-1022/coif). The authors have no conflicts of interest to declare.
References
- 1.Wang J, Li E, Yang H, et al. Combined hepatocellular-cholangiocarcinoma: a population level analysis of incidence and mortality trends. World J Surg Oncol 2019;17:43. 10.1186/s12957-019-1586-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stavraka C, Rush H, Ross P. Combined hepatocellular cholangiocarcinoma (cHCC-CC): an update of genetics, molecular biology, and therapeutic interventions. J Hepatocell Carcinoma 2019;6:11-21. 10.2147/JHC.S159805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunt E, Aishima S, Clavien PA, et al. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology 2018;68:113-26. 10.1002/hep.29789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarnagin WR, Weber S, Tickoo SK, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer 2002;94:2040-6. 10.1002/cncr.10392 [DOI] [PubMed] [Google Scholar]
- 5.Wakizaka K, Yokoo H, Kamiyama T, et al. Clinical and pathological features of combined hepatocellular-cholangiocarcinoma compared with other liver cancers. J Gastroenterol Hepatol 2019;34:1074-80. 10.1111/jgh.14547 [DOI] [PubMed] [Google Scholar]
- 6.Yin X, Zhang BH, Qiu SJ, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann Surg Oncol 2012;19:2869-76. 10.1245/s10434-012-2328-0 [DOI] [PubMed] [Google Scholar]
- 7.Zhang G, Chen BW, Yang XB, et al. Prognostic analysis of patients with combined hepatocellular-cholangiocarcinoma after radical resection: A retrospective multicenter cohort study. World J Gastroenterol 2022;28:5968-81. 10.3748/wjg.v28.i41.5968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:1389-96. 10.1016/S1470-2045(14)70473-5 [DOI] [PubMed] [Google Scholar]
- 9.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) . Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet 2019;393:1440-52. 10.1016/S0140-6736(18)33137-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo C, Hyung J, Chan SL. Recent Advances in Systemic Therapy for Advanced Intrahepatic Cholangiocarcinoma. Liver Cancer 2024;13:119-35. 10.1159/000531458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma KW, Cheung TT, Leung B, et al. Adjuvant chemotherapy improves oncological outcomes of resectable intrahepatic cholangiocarcinoma: A meta-analysis. Medicine (Baltimore) 2019;98:e14013. 10.1097/MD.0000000000014013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi S, Terashima T, Shiba S, et al. Multicenter retrospective analysis of systemic chemotherapy for unresectable combined hepatocellular and cholangiocarcinoma. Cancer Sci 2018;109:2549-57. 10.1111/cas.13656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trikalinos NA, Zhou A, Doyle MBM, et al. Systemic Therapy for Combined Hepatocellular-Cholangiocarcinoma: A Single-Institution Experience. J Natl Compr Canc Netw 2018;16:1193-9. 10.6004/jnccn.2018.7053 [DOI] [PubMed] [Google Scholar]
- 14.Joseph NM, Tsokos CG, Umetsu SE, et al. Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. J Pathol 2019;248:164-78. 10.1002/path.5243 [DOI] [PubMed] [Google Scholar]
- 15.Goodwin B, Lou J, Butchy M, et al. Hepatocellular-Cholangiocarcinoma Collision Tumors: An Update of Current Management Practices. Am Surg 2023;89:2685-92. 10.1177/00031348221124323 [DOI] [PubMed] [Google Scholar]
- 16.Schizas D, Mastoraki A, Routsi E, et al. Combined hepatocellular-cholangiocarcinoma: An update on epidemiology, classification, diagnosis and management. Hepatobiliary Pancreat Dis Int 2020;19:515-23. 10.1016/j.hbpd.2020.07.004 [DOI] [PubMed] [Google Scholar]
- 17.Kelley RK, Rimassa L, Cheng AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2022;23:995-1008. 10.1016/S1470-2045(22)00326-6 [DOI] [PubMed] [Google Scholar]
- 18.Oh DY, Ruth He A, Qin S, et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid 2022;1:EVIDoa2200015. [DOI] [PubMed]
- 19.Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute. 2024. Available online: https://seer.cancer.gov/. Accessed 12/05 2024.
- 20.Pomej K, Balcar L, Shmanko K, et al. Clinical characteristics and outcome of patients with combined hepatocellular-cholangiocarcinoma-a European multicenter cohort. ESMO Open 2023;8:100783. 10.1016/j.esmoop.2023.100783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chun SJ, Jung YJ, Choi Y, et al. Prognostic Evaluation and Survival Prediction for Combined Hepatocellular-Cholangiocarcinoma Following Hepatectomy. Cancer Res Treat 2025;57:229-39. 10.4143/crt.2024.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma KW, Chok KSH. Importance of surgical margin in the outcomes of hepatocholangiocarcinoma. World J Hepatol 2017;9:635-41. 10.4254/wjh.v9.i13.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye L, Schneider JS, Ben Khaled N, et al. Combined Hepatocellular-Cholangiocarcinoma: Biology, Diagnosis, and Management. Liver Cancer 2024;13:6-28. 10.1159/000530700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penzkofer L, Gröger LK, Hoppe-Lotichius M, et al. Mixed Hepatocellular Cholangiocarcinoma: A Comparison of Survival between Mixed Tumors, Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma from a Single Center. Cancers (Basel) 2023;15:639. 10.3390/cancers15030639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein O, Kee D, Nagrial A, et al. Evaluation of Combination Nivolumab and Ipilimumab Immunotherapy in Patients With Advanced Biliary Tract Cancers: Subgroup Analysis of a Phase 2 Nonrandomized Clinical Trial. JAMA Oncol 2020;6:1405-9. 10.1001/jamaoncol.2020.2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Wei M, Shen S, et al. The Combination of Radiation Therapy and Immunotherapy Is Effective and Well-Tolerated for Unresectable Biliary Tract Cancer. Int J Radiat Oncol Biol Phys 2022;113:816-24. 10.1016/j.ijrobp.2022.03.019 [DOI] [PubMed] [Google Scholar]
- 27.He C, Zhang Y, Cai Z, et al. Competing risk analyses of overall survival and cancer-specific survival in patients with combined hepatocellular cholangiocarcinoma after surgery. BMC Cancer 2019;19:178. 10.1186/s12885-019-5398-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamashita YI, Aishima S, Nakao Y, et al. Clinicopathological characteristics of combined hepatocellular cholangiocarcinoma from the viewpoint of patient prognosis after hepatic resection: High rate of early recurrence and its predictors. Hepatol Res 2020;50:863-70. 10.1111/hepr.13507 [DOI] [PubMed] [Google Scholar]