Abstract
Primary erythermalgia is a rare disorder characterized by recurrent attacks of red, warm, and painful hands and/or feet. The symptoms are generally refractory to treatment and persist throughout life. Five kindreds with multiple cases of primary erythermalgia were identified, and the largest was subjected to a genomewide search. We detected strong evidence for linkage of the primary erythermalgia locus to markers from chromosome 2q. The highest LOD score (Z) was obtained with D2S2330 (Zmax = 6.51). Analysis of recombination events identified D2S2370 and D2S1776 as flanking markers, on chromosome 2q31-32. This defines a critical interval of 7.94 cM that harbors the primary erythermalgia gene. Affected members within the additional families also shared a common haplotype on chromosome 2q31-32, supporting our linkage results. Identification of the primary erythermalgia gene will allow a better clinical classification of this pleomorphic group of disorders.
Primary erythermalgia [MIM 133020] is a rare, hereditary syndrome of red, warm, and burning extremities (Michiels and Van Joost 1988; Drenth and Michiels 1990). A recent literature analysis yielded 26 sporadic cases, but the disorder has also been described in at least seven families displaying an autosomal dominant mode of inheritance (Krebs and Andres 1969; Thompson et al. 1979; Cohen and Samorodin 1982; Kirby 1987; Michiels and Van Joost 1988; Michiels et al. 1989; Gelehrter and Colins 1990; Finley et al. 1992; Drenth et al. 1994; Martin et al. 1994). Primary erythermalgia arises in childhood or adolescence with attacks of bilateral symmetric pain. The symptoms are mainly confined to the feet and lower legs but also can extend to the hands, earlobes, and nose tip. Physical examination reveals only local red-purple discoloration, with minor congestion and increased local temperature. Symptoms can be precipitated by standing, slight exercise such as walking, and exposure to heat, but relief can be obtained by elevating the affected extremities and cooling them. Typically, patients walk barefoot, even in wintertime, and try to alleviate the intense burning by putting their feet in buckets filled with ice. With age, the severity of primary erythermalgia progresses, and symptoms extend over a larger body area (e.g., over the ankles and lower legs) and become constant. The treatment of primary erythermalgia is cumbersome, and most patients have tried a great variety of therapeutic options, with mixed results (Davis et al. 2000). Several authors claim success with continuous epidural infusion of various combinations of bupivacaine and morphine in patients with primary erythermalgia, but a definitive treatment is not yet available (D'Angelo et al. 1992).
The symptoms in primary erythermalgia are reminiscent of two other disorders: erythromelalgia and secondary erythermalgia. Erythromelalgia arises during middle age and is linked to thrombocythemia, which causes obliterative thrombosis of arterioles and digital arteries (Michiels et al. 1985). Patients suffer from unilateral burning distress of toes or fingers, which can be immediately relieved by aspirin. Furthermore, erythermalgia can be associated with underlying disorders or the use of drugs and is then labeled as secondary erythermalgia. These patients respond to treatment of the underlying disorder or to stopping use of the drug responsible (Drenth et al. 1992; Hart 1996).
Thus far, laboratory investigations of patients with primary erythermalgia failed to identify specific biochemical abnormalities. Routine histopathological examination of affected skin lesions show only nonspecific findings (Drenth et al. 1996), but one report suggests a reduced density of skin autonomic nerve plexuses (Uno and Parker 1983). A potential role of vasoactive neuropeptides, such as substance P, was excluded, since we did not recover it in fluid from artificial skin blisters above erythermalgic skin (Drenth et al. 1997). It has been shown that patients with erythermalgia have a higher basal skin vasoconstrictor tone, with a diminished vasoconstrictor response to cold challenge (Littleford et al. 1999). However, this phenomenon was noted in both primary and secondary erythermalgia, making it unlikely that this represents a specific pathophysiological event. Although these observations increase our understanding of primary erythermalgia, they do not elucidate the underlying pathophysiology or the responsible molecule. In an effort to localize the gene for primary erythermalgia, we embarked on a genomewide search that resulted in significant evidence for linkage of the gene responsible for primary erythermalgia on chromosome 2q31-32.
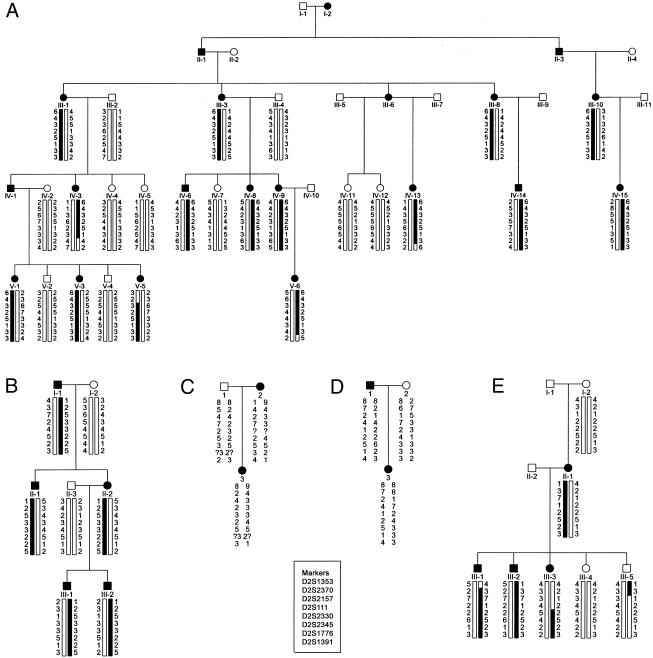
Appropriate informed consent was obtained from all individuals who participated. A Medline search for the period 1966–2000, using the keywords “familial,” “erythermalgia,” and “erythromelalgia,” identified six families with primary erythermalgia, and another large kindred has been published in a textbook of medical genetics (Gelehrter and Colins 1990). One of the authors (P. H. or J. D.) contacted either the first or the senior author of each article, and contact was established with five families (Thompson et al. 1979; Michiels et al. 1989; Finley et al. 1992; Martin et al. 1994; Guillet et al. 1995). Studies of some members of these families have been published elsewhere, and the pedigree structure of those individuals participating in this study from families 1–5 is shown in figure 1A–1E. Family 1 came from Alabama, families 2 and 4 from France, family 3 from the Netherlands, and family 5 from Canada. All patients had a physical examination, and a medical history was recorded. In total, 47 individuals were studied (28 were affected, 9 of whom were male). Autosomal dominant inheritance was confirmed by the presence of male-to-male transmission (family 2). In the present study, the diagnosis of primary erythermalgia was confirmed by the patient’s history and the clinical findings. Specifically, patients had to fulfill the set of criteria for primary erythermalgia (Drenth and Michiels 1994), which included (1) attacks of bilateral or symmetrical burning pain in the hands or feet; (2) initiation or aggravation of attacks by standing, exercise, or exposure to heat; (3) relief by elevation and cold; (4) warmth, flushedness, and congestion of the affected parts during attacks; and (5) the disorder's being refractory to treatment. Affected persons typically manifested symptoms in the first decade of life, and attacks persisted during life. There was no consistent beneficial therapeutic regimen. Some patients from family 2 benefited from pizotifen, an antiseritonergic drug, but its effect wore out after 1 year. Reexamination of family 1 revealed that individuals V:3 and V:5 developed symptoms at the ages of 6 years and 2 years, respectively. Compared to her older sisters, individual V:5 had more-severe symptoms. The first daughter in family 4 died as a result of septic shock, secondary to infected wounds caused by excessive cooling. Individuals I:1 and I:2, from family 5, were first cousins. Individual III:3, from family 5, underwent a bilateral knee disarticulation, because of infected ulcers resulting from excessive cooling. Individual III:2, from family 5, underwent bilateral transfemoral amputations, because of persistent infected ulcers. Individual IV:9, from family 1, who was severely affected, is thought to have died from an overdose of drugs.
Figure 1.
Pedigree of five families (A, family 1; B, family 2; C, family 3; D, family 4; E, family 5, with autosomal dominant–inherited primary erythermalgia. Females are indicated by circles and males by squares. Blackened figures denote affected individuals, and figures with slashes denote deceased individuals. Genotypes for chromosome 2 markers were determined for all living persons shown. Haplotypes for boxed portions of the pedigree are presented.
Genomic DNA was isolated and purified from peripheral blood by means of standard protocols (Miller et al. 1988). For the purpose of the systematic genome screening, we used fluorescently labeled short tandem repeat polymorphisms (STRPs) from the Center for Medical Genetics, Marshfield Medical Research Foundation's screening set 6A. The spacing of the marker set varies from 10.0 cM to 24.2 cM. Additional markers for fine mapping were obtained from the Généthon marker set (Dib et al. 1996). Marker order and intermarker distances were based on the existing Cooperative Human Linkage Center linkage map. Genomic DNA (25 ng) was amplified in a 10-ml PCR reaction, containing 1× GeneAmp PCR Gold Buffer; 1.5 mM MgCl2; 25 ng of fluorescent forward primer; 25 ng unlabeled reverse primer; and 0.4 units of AmpliTaq Gold DNA polymerase. Initial denaturation was 15 s at 95°C, followed by 32 cycles of 30 s of denaturation at 95°C, 30 s of annealing at 55°C, and 90 s of extension at 72°C. Reactions were prepared using a Beckman Biomek 2000 robot system and were performed in 384-well plates. Amplification was done using a dual 384-well–equipped GeneAmp PCR System 9700 (Applied Biosystems). PCR products were pooled and loaded on an ABI 377 automated sequencer (filterset D; 5% denaturing FMC LongRanger acrylamide gel). Data were analyzed by means of ABI GeneScan 3.1 and ABI Genotyper 2.1 software. Binning of the alleles and preparation of prelinkage files were performed using Linkage Designer 1.0 (Van Camp et al. 1997).
Linkage analysis and calculation of the two-point LOD scores between the disease locus and each individual marker was performed using the MLINK and ILINK programs of the LINKAGE package (version 5.1) (Laboratory of Statistical Genetics, Rockefeller University) (Lathrop and Lalouel 1984). Maximum LOD and location scores were calculated for each marker under the assumption that erythermalgia was an autosomal dominant disorder. Because of the early onset of the disease and the severity of the symptoms, we assumed full penetrance. In view of the relative rarity of the disease, the frequency of the abnormal allele was set at .0001. Recombination frequency (θ) was assumed to be equal for males and females. No phenocopies were allowed, and allele frequencies were calculated from all genotyped families. Multipoint analysis was performed using the LINKMAP program by subsequent three-point linkage analysis on all tested markers. Haplotypes were constructed in such a way as to minimize the number of crossovers in each family.
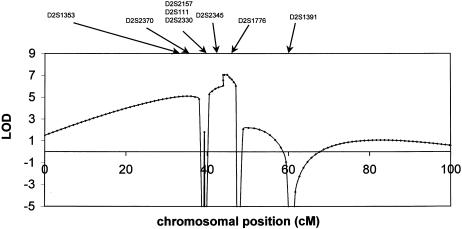
We started to genotype all available individuals from the largest family (family 1), and, using an semiautomated systematic genome scan for this family, we obtained positive LOD scores at θ = .01 for two markers (D2S1353 and D2S1776) on chromosome 2q. Further refinement of this region by saturation with additional markers within it (table 1) confirmed these findings, and two-point linkage analysis yielded a maximum LOD score (Zmax) of 6.51 at θ = 0 for marker D2S2330. Subsequent multipoint analysis yielded a Zmax= 7.05 at θ = 0 for marker D2S2330 (fig. 2). There was no significant linkage with markers from other chromosomal regions. Haplotypes were then constructed by parsimony (fig. 1). Several recombinants that defined the limits of the erythermalgia-susceptibility region were detected. The recombination events in family 1 suggest that the region is limited by marker D2S2370 on the centromeric side (V:5) and by marker D2S1776 on the telomeric side (IV:3). Therefore, the critical region spans 7.94 cM on the sex-averaged linkage map. We then tested the relevant chromosome 2 markers in four other families with primary erythermalgia who, by themselves, were not powerful enough to give significant evidence for linkage, and we constructed haplotypes for all tested markers for these families. This revealed that affected individuals within these primary erythermalgia families share a common allele for markers in the susceptibility region that is not shared by healthy individuals. This suggests that the gene responsible for the disorder in these families is also localized to chromosome 2q. A recombination event for marker D2S2157 in family 5 (individual III:5) would reduce the critical region to 3.59 cM on the sex-averaged linkage map. However, in this report, the haplotypes in this family cannot be determined unambiguously, because several markers in this region are not fully informative. Therefore, a conservative estimate suggests that the maximum region of linkage is defined by a 7.94-cM critical interval, extending from D2S2370 to D2S1776, that must contain the erythermalgia gene.
Table 1.
Two-Point Linkage Analysis Data at Various Recombination Fractions, for the Primary Erythermalgia–Susceptibility Gene and for Markers on Chromosome 2q, in Family 1
| Marker | 0 | .01 | .05 | .1 | .2 | .3 | .4 |
| D2S1353 | − ∞ | 4.33 | 4.6 | 4.35 | 3.49 | 2.41 | 1.2 |
| D2S2370 | − ∞ | 2.24 | 2.64 | 2.55 | 2.04 | 1.37 | .63 |
| D2S2157 | 3.83 | 3.75 | 3.44 | 3.05 | 2.24 | 1.41 | .57 |
| D2S111 | 6.31 | 6.2 | 5.77 | 5.22 | 4.03 | 2.76 | 1.39 |
| D2S2330 | 6.51 | 6.4 | 5.96 | 5.37 | 4.14 | 2.82 | 1.41 |
| D2S2345 | 6.19 | 6.09 | 5.66 | 5.11 | 3.95 | 2.7 | 1.36 |
| D2S1776 | − ∞ | .83 | 1.32 | 1.36 | 1.12 | .73 | .3 |
| D2S1391 | − ∞ | −3.89 | −.74 | .32 | .91 | .85 | .51 |
Figure 2.
Multilocus analysis, with the primary erythermalgia gene, against a fixed genetic map of linked markers for family 1. Order of markers and distances are taken from published sources (Dib et al. 1996). The highest probability for location of the primary erythermalgia gene is in the region between D2S1353 and D2S1391.
Given the rarity of primary erythermalgia, it might be hypothesized that there is a founder effect. The families originated from various parts of Northern Europe, the United States, and Canada, and a common ancestor could not be determined. The haplotype of the five affected families did not show any common region in the disease-bearing chromosomes. This, in combination with the relative small distance of the linked interval, suggests that the mutation is different in each family. Given the phenotypical similarities between the familial and isolated cases of primary erythermalgia, the question arises as to whether they share a molecular defect in the same gene. This would, however, imply some degree of nonpenetrance, since none of the parents of the isolated patients have the disease. The primary erythermalgia in our kindred was fully penetrant—that is, all patients carried the disease-associated haplotype. The identification of the causative gene in primary erythermalgia will be necessary to address this question.
Despite the uniform presentation of primary erythermalgia, the intensity of symptoms did vary even within families. Although, in one family, two siblings needed constant cooling with subsequent severe excoriation of the skin eventually leading to limb amputation, the mother and another brother had much milder symptoms (Kirby 1987). Although primary erythermalgia is not a life-threatening disease, we saw severe and potential lethal complications of the symptoms. Excessive cooling of the skin led in at least three cases to progressive ulcerative inflammation of the extremities, which highly increases the likelihood of a gram-positive septic shock. Indeed, this was the cause of death in one of our patients.
It is suggested that symptoms in erythermalgia, regardless of cause, develop as a result of maldistribution of available perfusion in favor of arteriovenous anastomoses, resulting in skin warming, followed by increased metabolism and subsequent hypoxia (Mork et al. 2000). Indeed, in patients with erythermalgia, there is increased skin blood flow in areas of AV shunts during attacks. A study with skin perfusion indicated that they have a paradoxal increased vascular tone, leading to a vasoconstrictor tendency, perhaps resulting from hypoxic injury to the microvasculature (Littleford et al. 1999). This correlates with the pathological changes observed in primary erythermalgia, in which a perivascular mononuclear infiltrate, thickened blood-vessel basement membranes, abundant perivascular edema, and endothelial cell swelling can be observed (Drenth et al. 1996).
The protein coded by the autosomal dominant gene for primary erythermalgia is unknown, but it might be presumed, given the disturbed microcirculatory flow, to be expressed in endothelial cells and to affect endothelial function. A search of the GeneMap '99 database for the chromosome 2q linkage interval reveals several genes and numerous expressed sequence tags (ESTs) with no known function in most instances. On the basis of available functional information for these ESTs, no obvious candidate gene for primary erythermalgia emerges, but the linkage interval does encompass two possible candidate genes: alpha-6 integrin and interleukin (IL) 17. Patients with an absence of alpha-6 integrin have junctional epidermolysis bullosa with pyloric atresia and esophageal stenosis (MIM 226730). Primary erythermalgia differs phenotypically, and it is difficult to generalize the clinical description to other mutations in the alpha-6 integrin gene. IL17 (MIM 603149) induces the production of IL6, increases the surface expression of intracellular adhesion molecules in cultured fibroblasts, and is probably involved in osteoclastogenesis. It is difficult to foresee whether IL17 will have a role in the pathogenesis of erythermalgia.
Further refinement of critical region is not easy to achieve, given the relatively small genetic distance of the linked interval and the limited number of families with the syndrome that has been described. A positional (candidate)-gene approach that includes extensive analysis of the newly available genomic-sequence data might now allow the identification of the incriminated gene. Pursuit of the causative gene for primary erythermalgia may lead to diagnostic and therapeutic approaches for individuals with this rare disease. Understanding the molecular pathology will not only shed light on pathogenic mechanisms in primary erythermalgia but may also benefit patients with more-common entities related to an impaired skin microcirculation, such as Raynaud’s syndrome.
Acknowledgments
We thank the study participants for their willingness to cooperate with us in this research effort. Joost P. H. Drenth is an investigator of the Royal Dutch Academy of Arts and Sciences. Jan-Paul Drenth is thanked for his invaluable help in the collection of samples from family 2. Saskia van der Velde-Visser provided assistance with the cell cultures. Bianca M. de Graaf and Angela Jacobs are thanked for technical assistance. We thank Professor H. Galjaard for his continuous support.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://research.marshfieldclinic.org/genetics/ (for information regarding marker order and relative genetic distances)
- Cooperative Human Linkage Center, http://lpg.nci.nih.gov/CHLC (for marker order and intermarker distances)
- GeneMap '99, http://www.ncbi.nlm.nih.gov/genemap99 (for identification of candidate genes)
- Généthon, http://www.genethon.fr (for microsatellite markers)
- Linkage Laboratory of Statistical Genetics, Rockefeller University, ftp://linkage.rockefeller.edu/software/linkage (for LINKAGE software)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for primary erythermalgia [MIM 133020], junctional epidermolysis bullosa with pyloric atresia and esophageal stenosis [MIM 226730], and IL17 [MIM 603149])
References
- Cohen IJ, Samorodin CS (1982) Familial erythromelalgia. Arch Dermatol 118:953–954 [PubMed] [Google Scholar]
- D'Angelo R, Cohen IT, Brandom BW (1992) Continuous epidural infusion of bupivacaine and fentanyl for erythromelalgia in an adolescent. Anesth Analg 74:142–144 [PubMed] [Google Scholar]
- Davis MD, O'Fallon WM, Rogers RS, Rooke TW (2000) Natural history of erythromelalgia: presentation and outcome in 168 patients. Arch Dermatol 136:330–336 [DOI] [PubMed] [Google Scholar]
- Dib C, Faure S, Fizames Cl, Samson D, Drouot N, Vignal A, Millasseau P, et al. (1996) A comprehensive genetic map of the human genome based on 5264 microsatellites. Nature 380:152–154 [DOI] [PubMed] [Google Scholar]
- Drenth JP, Michiels JJ (1990) Three types of erythromelalgia. BMJ 301:454–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth JP, Michiels JJ (1994) Erythromelalgia and erythermalgia: diagnostic differentiation. Int J Dermatol 33:393–397 [DOI] [PubMed] [Google Scholar]
- Drenth JP, Michiels JJ, Van Joost T (1994) Primaire en secundaire erythermalgie. Ned Tijdschr Geneeskd 138:2231–2234 [PubMed] [Google Scholar]
- Drenth JP, Michiels JJ, Van Joost T (1997) Substance P is not involved in primary and secondary erythermalgia. Acta Derm Venereol 77:325–326 [DOI] [PubMed] [Google Scholar]
- Drenth JP, Michiels JJ, Van Joost T, Vuzevski VD (1992) Verapamil-induced secondary erythermalgia. Br J Dermatol 127:292–294 [DOI] [PubMed] [Google Scholar]
- Drenth JP, Vuzevski V, Van Joost T, Casteels-Van DM, Vermylen J, Michiels JJ (1996) Cutaneous pathology in primary erythermalgia. Am J Dermatopathol 18:30–34 [DOI] [PubMed] [Google Scholar]
- Finley WH, Lindsey JRJ, Fine JD, Dixon GA, Burbank MK (1992) Autosomal dominant erythromelalgia. Am J Med Genet 42:310–315 [DOI] [PubMed] [Google Scholar]
- Gelehrter TD, Colins FS (1990) Mendelian inheritance. In: Gelehrter TD, Collins FS (eds) Principles of medical genetics. Williams & Wilkins, Baltimore, pp 27–47 [Google Scholar]
- Guillet MH, Le Noach E, Milochau P, Sassolas B, Guillet G (1995) Erythermalgie familiale traitée par pizotifène. Ann Dermatol Venereol 122:777–779 [PubMed] [Google Scholar]
- Hart JJ (1996) Painful swollen and erythematous hands and feet. Arthritis Rheum 39:1761–1762 [DOI] [PubMed] [Google Scholar]
- Kirby RL (1987) Erythromelalgia: not so benign. Arch Phys Med Rehabil 68:389 [PubMed] [Google Scholar]
- Krebs A, Andres HU (1969) Zum Krankheitsbild der Erythromelalgie Familiares Auftreten einer idiopathischen Form bei Muter und Tochter. Schweiz Med Wochenschr 99:344–349 [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM (1984) Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet 36:460–465 [PMC free article] [PubMed] [Google Scholar]
- Littleford RC, Khan F, Belch JJ (1999) Skin perfusion in patients with erythromelalgia. Eur J Clin Invest 29:588–593 [DOI] [PubMed] [Google Scholar]
- Martin JC, Lacombe D, Lefebvre D, Bonafe JL, Taieb A, Maleville J (1994) Erythromélalgie: une observation familiale: discussion sur le rôle du mercure. Ann Dermatol Venereol 121:309–314 [PubMed] [Google Scholar]
- Michiels JJ, Abels J, Steketee J, van Vliet HH, Vuzevski VD (1985) Erythromelalgia caused by platelet-mediated arteriolar inflammation and thrombosis in thrombocythemia. Ann Intern Med 102:466–471 [DOI] [PubMed] [Google Scholar]
- Michiels JJ, Van Joost T (1988) Primary and secondary erythermalgia: a critical review. Neth J Med 33:205–208 [PubMed] [Google Scholar]
- Michiels JJ, Van Joost T, Vuzevski VD (1989) Idiopathic erythermalgia: a congenital disorder. J Am Acad Dermatol 21:1128–1130 [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mork C, Asker CL, Salerud EG, Kvernebo K (2000) Microvascular arteriovenous shunting is a probable pathogenetic mechanism in erythromelalgia. J Invest Dermatol 114:643–646 [DOI] [PubMed] [Google Scholar]
- Thompson GH, Hahn G, Rang M (1979) Erythromelalgia. Clin Orthop 144:249–254 [PubMed] [Google Scholar]
- Uno H, Parker F (1983) Autonomic innervation of the skin in primary erythermalgia. Arch Dermatol 119:65–71 [PubMed] [Google Scholar]
- Van Camp G, Balemans W, Willems PJ (1997) Linkage Designer and Linkage Reporter software for automated gene localization studies. Trends Genet (Technical Tips Online) 13:82535232 [Google Scholar]