Abstract
Patients with chronic lymphocytic leukemia (CLL) treated with adenovirus (Ad)-CD154 (CD40L) gene therapy experience reductions in leukemia cell counts and lymph node size associated with induction of the death receptor Fas (CD95). CD4 T cell lines can induce apoptosis of CD40-activated CLL cells via a CD95 ligand (CD95-L)-dependent mechanism. To examine whether CD95-L was sufficient to induce cytolysis of CD40-activated CLL cells, we used Chinese hamster ovary cells transfected with CD95-L as cytotoxic effector cells. CD40-activated CLL cells were initially resistant to CD95-mediated apoptosis despite high-level expression of CD95. However, after 72 h, CLL cells from seven of seven patients became increasingly sensitive to CD95-mediated apoptosis. This sensitivity correlated with a progressive decline in Flice-inhibitory protein (FLIP), which was induced within 24 h of CD40 ligation. Down-regulation of FLIP with an antisense oligonucleotide or a pharmacologic agent, however, was not sufficient to render CLL cells sensitive to CD95-mediated apoptosis in the 24–72 h after CD40 activation. Although the levels of pro-Caspase-8 appeared sufficient, inadequate levels of Fas-associated death domain protein (FADD) and DAP3 may preclude assembly of the death-inducing signaling complex. Seventy-two hours after CD40 ligation, sensitivity to CD95 and a progressive increase in FADD and DAP3 were associated with the acquired ability of FADD and FLIP to coimmunoprecipitate with the death-inducing signaling complex after CD95 ligation. Collectively, these studies reveal that CD40 ligation on CLL B cells induces a programmed series of events in which the cells initially are protected and then sensitized to CD95-mediated apoptosis through shifts in the balance of the anti- and proapoptotic proteins FLIP and FADD.
Chronic lymphocytic leukemia (CLL) is a malignancy of mature B cells. The neoplastic B cells are relatively resistant to apoptosis and consequently progressively accumulate in the blood, marrow, and secondary lymphoid tissues of affected individuals (1). Currently, there is no established cure for this disease, and new therapeutic treatments are under investigation.
Encouraging results were reported in a recent phase I clinical trial of gene therapy for patients with CLL (2). Within 1–4 weeks of infusion with autologous leukemia cells transduced to express high levels of recombinant CD40 ligand (CD154), patients experienced increases in leukemia-specific and absolute T cell counts (2). Also, circulating bystander noninfected CLL cells were induced to express CD95 (Fas), a member of the tumor necrosis factor death receptor family (3, 4). Such biologic effects were associated with reductions in leukemia cell counts and lymph node size. Although the mechanism(s) responsible for the noted reductions in tumor cell burden were not resolved, it is conceivable that the sustained expression of CD95 induced on the entire leukemia cell population rendered it susceptible to CD95-mediated apoptosis by cells bearing CD95 ligand (CD95-L), such as activated natural killer (5) cells or T cells (6).
To investigate this issue, we generated CD4 T cell lines from the blood T cells of patients with CLL. CD4 cytotoxic T lymphocytes (CTL) could mediate CD95-L-dependent apoptosis of CD40-activated, but not resting, autologous or allogeneic CLL cells. This observation was surprising in view of the previously noted resistance of CD40-activated CLL cells to CD95-mediated apoptosis (7–9). To investigate whether expression of CD95-L was sufficient to induce cytolysis of CD40-activated CLL cells, we used Chinese hamster ovary (CHO) cells transfected with CD95-L as cytotoxic effector cells, which also allowed us to explore the kinetics of acquired sensitivity to CD95-mediated apoptosis in CLL after CD40 activation.
Materials and Methods
Reagents.
3,3′ Dihexyloxacarbocyanine iodide (DiOC6) was purchased from Molecular Probes. z-VAD-fmk was purchased from Kamiya Biochemical (Seattle, WA). PKH26-GL was purchased from Sigma. Anti-CD95 mAb CH-11 was purchased from Panvera (Madison, WI). Anti-CD95-L mAb NOK-2 (10), and fluorochrome-conjugated mAbs specific for CD3, CD4, CD8, CD95, and relevant isotype controls were purchased from PharMingen. Anti-Flice-inhibitory protein (FLIP) mAb (DAVE-3) and anti-CD95 mAb APO-1 were purchased from Alexis (San Diego, CA), anti-Fas-associated death domain protein (FADD) mAb, anti-DAP3 mAb and anti-xIAP mAb were purchased from BD Transduction Laboratories (Lexington, KY). Anti-cIAP1 (sc-7943) and cIAP2 (sc-7944) were purchased from Santa Cruz Biotechnology. Antibodies against pro-Caspase-8 (clone 1856–10), Bcl-xL, Bcl-2, and Bax have been described (11–14). FLIP antisense oligonucleotide and a control scrambled oligonucleotide were kindly provided by Isis (Carlsbad, CA).
Cells.
After informed consent, blood was obtained from patients satisfying diagnostic criteria for B cell CLL (1, 15). Peripheral blood mononuclear cells (PBMC) were isolated by density centrifugation over Histopaque 1077 (Sigma). These cells typically were >95% positive for CD19 and CD5, as assessed by flow cytometry. CLL cells were used fresh or viably frozen in FBS containing 10% DMSO for storage in liquid nitrogen. BJAB and Jurkat cell lines were maintained in RPMI medium 1640 containing 10% FBS. CHO-CD95-L stable transfectants were kindly provided by Mark Cantwell (Tragen, San Diego, CA). HeLa-CD154 (CD40L) cells were previously described (16). CD4+ CTL were generated by coculturing PBMC from CLL patients with CD40-activated autologous CLL cells in the presence of 1,500 units/ml of IL-1β/250 units/ml of IL-2, 20 units/ml of IL-4/150 units/ml of IL-6 (PharMingen) in serum-free AIM-V (GIBCO/BRL) (CTL media). Each week, the T cells were restimulated with CD40-activated autologous CLL cells in fresh CTL media.
Cytotoxicity Assay.
We used a flow cytometric assay to monitor for CTL activity against CLL B cells (17). Effector cells were labeled with PKH26 as previously described (16). After two washes, PKH26-labeled effector cells were suspended in 1 ml of RPMI-10, stained with 40 nM DiOC6 for 15 min at 37°C, and washed. One hundred microliters of PKH26/DiOC6-stained effector cells (2 × 106/ml) was then incubated in polypropylene tubes at 37°C for 20 min with or without 10 μg/ml of NOK-2 anti-CD95-L. CD40-activated cells prepared by coculture on mitomycin C- (Sigma) treated adherent HeLa or HeLa-CD154 cells for 24 h, separated from coculture, and cultured again in media only for 5 days. In some cases, target cells were pretreated with 50 μM Z-VAD-fmk (ZVAD) for 30 min at 37°C. CD40-activated cells were treated with 1 μM 2-cyano-3,12-dioxoolean-1,9-dien-28-oic-acid (CDDO) in some experiments. For antisense experiments, CLL cells were electroporated with 300 nM PBS, FLIP antisense, or control scrambled oligonucleotide. For the cytotoxicity assay, DiOC6-labeled target cells were washed once, resuspended at 2 × 105/ml, and 100 μl of cells were mixed together with PKH26/DiOC6-labeled effector cells. The cells were incubated at 37°C for 8 h and then subjected to flow cytometric analysis. In some cases, 1 μg/ml of CD95 CH-11 (Panvera) mAb was added to target cells alone.
Immunoblot Analyses.
Cell lysates were prepared in RIPA buffer (150 mM NaCl/25 mM Tris, pH 7.4/1% Triton X-100/0.5% SDS/5 mM EDTA) by incubation at 4°C for 15 min and then cleared by centrifugation at 14,000 × g for 20 min at 4°C. Protein concentrations of cleared lysates were measured by the Bradford assay. Lysate (12.5 μg) was then subjected to SDS/PAGE and transferred to Immobilon-P polyvinylidene fluoride (PVDF) transfer membrane (Millipore). Membranes were blocked with 5% nonfat dry milk in immunoblot buffer (10 mM Tris/100 ml of NaCl/0.1% Tween-20) for 1 h at room temperature and then incubated with primary mAb overnight with rocking at 4°C. Membranes were washed five times in immunoblot buffer and incubated with the appropriate horseradish peroxidase (HRP)-conjugated antibody for 1 h at room temperature. After five washes, membrane-bound HRP antibody was detected by using the SuperSignal Chemiluminescent Substrate Kit (Pierce). Scanned immunoblots were analyzed with NIH image software.
Immunoprecipitation Assays.
The CD95 death-inducing signaling complex (DISC) was immunoprecipitated according to Scaffidi et al. (18). Briefly, 107 CLL or BJAB cells were either left untreated or stimulated for 15 min with 2 μg/ml of APO-1 antibody at 37°C and washed once in ice-cold PBS. Cells were then lysed in lysis buffer, incubated for 20 min on ice, and cleared by centrifugation at 14,000 × g at 4°C for 15 min. Unstimulated cells were lysed and cleared before the addition of APO-1 mAb. Lysates were then incubated with protein-A-Sepharose 4B beads (Sigma) for 2 h rotating at 4°C. Beads were washed four times, boiled in 30 μl of sample buffer, and cleared by centrifugation for 3 min at 600 × g. Western blot analysis was then performed as described.
Results
CD4+ T Cell Lines Induce Apoptosis of CD40-Activated CLL Cells via a CD95-L-Dependent Pathway.
T cells isolated from the blood of patients with CLL were cultured with autologous CD40-activated CLL cells in serum-free medium. Each week, newly stimulated CD40-activated CLL cells were added to the cultures in fresh medium. After 10 weeks, we found that >98% of the CD3 T cells within these cultures expressed CD4, as assessed via flow cytometry (data not shown).
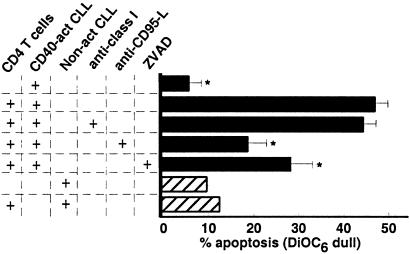
We examined whether the CD4 T cells generated in this fashion could induce apoptosis of CLL cells in vitro. Although noncytotoxic for resting autologous CLL cells (Fig. 1), these cells effected apoptosis of CD40-activated CLL cells at an effector-to-target cell ratio of 10:1. These CD4 T cells also induced apoptosis of CD40-activated, but not resting, allogeneic CLL cells (data not shown). Preincubation of the target cells with W6/32, a mAb specific for MHC class I, did not inhibit CD4 CTL killing. However, T cell-mediated apoptosis of CD40-activated CLL cells was significantly inhibited by anti-CD95-L mAb or the pan-caspase inhibitor ZVAD (P < 0.05) (Fig. 1).
Figure 1.
CD4 CTL induce CD95-dependent apoptosis of CD40-activated CLL cells. CTL activity of CD4 T cells expanded from the blood of patients with CLL was tested against autologous and nonrelated, allogeneic CLL target cells. CLL target cells were mock (hatched bars) or CD40-activated (black bars), and replated for an additional 24 h before use. CD4 effector and CLL target cells were cocultured at an effector-to-target cell ratio of 10:1, incubated for 4 h, and stained with DiOC6 for 15 min at 37°C before flow cytometric analysis. To test for specificity, CD4 effectors or CLL target cells were preincubated with monoclonal antibodies specific for CD95L or MHC class I (W6/32), respectively. Target cells also were preincubated with 50 μM ZVAD to test for caspase dependence.
Temporal Sensitivity of CD40-Activated CLL Cells to CD95-Mediated Apoptosis.
To examine whether expression of CD95-L by these CTL effectors was sufficient for inducing apoptosis of CD40-activated leukemia cells, we used CHO cells transfected with an expression vector encoding the human CD95-L. CHO-CD95-L effector cells, but not nontransfected CHO cells, consistently induced >60% of Jurkat cells to undergo apoptosis within the 8-h cytotoxicity assay (Figs. 5 and 6; data not shown).
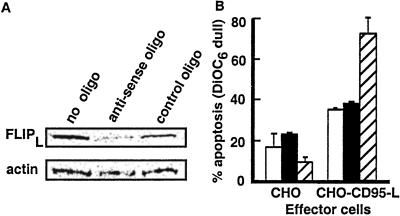
Figure 5.
Down-regulation of FLIP in CLL cells. (A) CLL cells were electroporated with PBS, FLIP antisense, or control scrambled oligo as described. Cells were then CD40 activated for 24 h and harvested for FLIP immunoblot analysis, or (B) CD95 apoptosis assays, as described. Bars indicate the % apoptosis of control-treated CLL cells (open bars), FLIP-antisense-treated CLL cells (black bars), or JURKAT cells (hatched bars) when incubated with CHO cells or CHO cells that express CD95-L (CHO-CD95-L).
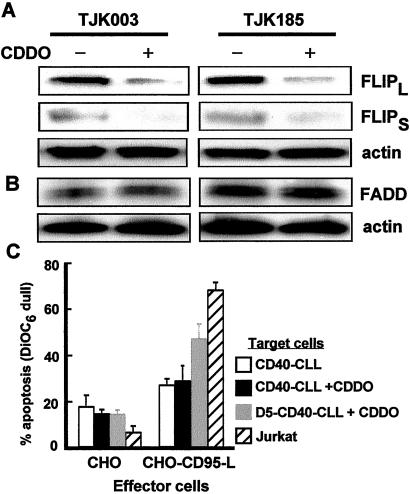
Figure 6.
Down-regulation of FLIP with CDDO. (A) CLL cells were CD40 activated and replated in media alone for an additional 24 h with (−) or without (+) 1 μM CDDO. Cells were then harvested for immunoblot analysis of FLIP and (B) FADD or (C) CD95 apoptosis assays. Data shown are the mean CD95-mediated apoptosis of four patient samples up to 72 h after CD40 activation in the absence (open bar) or presence (black bar) of 1 μM CDDO treatment, ±SD. In one sample (gray bar), sensitivity to CD95-mediated apoptosis was tested at day 5 after CD40 activation to confirm that CDDO-treated CLL cells still acquired sensitivity to CD95. Apoptosis of Jurkat cells demonstrates activity of CHO-FasL (hatched bars).
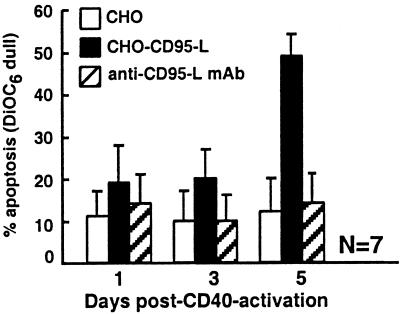
We examined whether CHO-CD95-L could induce apoptosis of leukemia B cells after CD40 activation. CLL cells were cultured for 24 h on cells expressing CD154 (HeLa-CD154) or on control nontransfected HeLa cells. The leukemia cells subsequently were separated from the adherent HeLa cells and then cultured alone at 37°C until examined. Leukemia cells previously cultured on control HeLa cells remained insensitive to CHO-CD95-L-induced apoptosis. Leukemia cells previously cultured on HeLa-CD154 also were resistant to CD95-L-induced apoptosis when examined during the first few days after CD40 activation (Fig. 2). For example, 1 day after CD40 activation, the number of CLL cells induced to undergo apoptosis by coculture with CHO-CD95-L was only 19 ± 9% (mean ± SD, n = 7) (Fig. 2). Spontaneous apoptosis, as measured by coculture of day 1 CD40-activated CLL cells with control CHO cells, was 11 ± 6% (Fig. 2). However, CD40-activated leukemia cells from all seven patient samples tested subsequently acquired sensitivity to CD95-mediated apoptosis on further culture. For example, CHO-CD95-L cells induced 49 ± 5% of the CLL cells to undergo apoptosis when the leukemia cells were examined 5 days after CD40 activation. In contrast, spontaneous apoptosis was only 12 ± 8% (Fig. 2). The significant increase in cell killing by CHO-CD95-L cells over that of CHO cells could be inhibited by anti-CD95-L mAb NOK-2 (Fig. 2). Moreover, CD95-mediated apoptosis of CLL cells activated 5 days earlier could be inhibited by pretreatment of target cells with the pan-caspase inhibitors ZVAD and z-Asp-Glu-Val-Asp-FMK (DEVD) (data not shown).
Figure 2.
CD95-mediated apoptosis of CD40-activated CLL cells. CLL cells were CD40-activated and harvested 1, 3, or 5 days later for use as targets in a DiOC6 apoptosis assay. CHO (open bars), CHO-CD95-L (black bars), or anti-CD95L mAb pretreated CHO-CD95-L (hatched bars) effector cells were cocultured with target cells at an effector-to-target cell ratio of 10:1 for 8 h at 37°C. DiOC6 dull or apoptotic target cells were measured by flow cytometric analysis. Data represent the mean ± SD from experiments testing seven different CLL patient samples.
Temporal Expression of CD95 and FLIP After CD40 Activation.
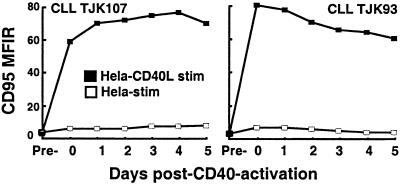
We examined the relative expression of CD95 and FLIP after CD40 ligation. As expected, CLL cells were induced to express CD95 after CD40 activation. Within 2 days, the CLL cells were induced to express high levels of CD95 that were sustained for several days thereafter (Fig. 3). In contrast, CLL cells previously cultured with control HeLa cells were not induced to express CD95 (Fig. 3).
Figure 3.
Time course of CD95 expression on CLL cells after CD40 activation. Flow cytometric analysis of CD95 expression before (pre) and at indicated timepoints after 24-h stimulation on HeLa (open squares) or HeLa-CD154 transfected cells (filled squares). Data shown are the mean fluorescence intensity ratio (MFIR) from two different CLL patient samples stained with phycoerythrin (PE)-labeled anti-CD95 or isotype control mAb.
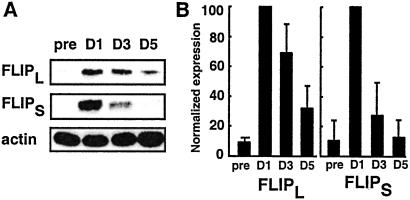
FLIP can block CD95-mediated apoptosis by inhibiting the cleavage and activation of pro-caspase-8 (18, 19). Prior studies indicated that this protein could be induced in germinal center and leukemia B cells by ligation of CD40 (8, 20). Immunoblot analyses confirmed that both isoforms of FLIP, FLIP long (FLIPL), and FLIP-short (FLIPS), were induced after CD40 activation. However, we found that the expression of FLIP peaked on the first day after CD40 ligation (Fig. 4A). Thereafter, we found that expression of both FLIPL and FLIPS decayed rapidly over time. FLIPL protein levels at day 5 were 32 ± 15% (mean ± SD, n = 7 samples) of peak expression levels at day one (Fig. 4B). Similarly, FLIPS levels at day 5 were 12 ± 12% (mean ± SD, n = 7) of peak expression levels noted at day 1 (Fig. 4B).
Figure 4.
Time course of FLIP expression in CLL cells after CD40 activation. (A) Representative immunoblot analysis of FLIPL and FLIPS isoforms. Lysates were prepared from CLL cells harvested before (pre) and at days 1, 3, and 5 (D1, D3, D5) after CD40 activation. For Western analysis, 12.5 μg of protein was subjected to 12% SDS/PAGE and immunoblotted with FLIP mAb. Blots were stripped and reprobed with antiactin mAb to ensure equal protein loading. (B) FLIP immunoblots were scanned and quantitated by using NIH image software. Data were normalized to actin bands and represent the mean ± SD of seven separate experiments.
Down-Regulation of FLIP by Antisense or CDDO Treatment Does Not Increase Sensitivity of CD40-Activated CLL Cells to CD95-Mediated Apoptosis.
To examine whether down-regulation of FLIP was sufficient to sensitize CD40-activated CLL cells to CD95, we electroporated freshly isolated CLL cells with a FLIP antisense oligonucleotide before coculture on HeLa-CD154. Immunoblot analysis confirmed that FLIP antisense, but not a matched scrambled oligonucleotide, down-regulated FLIP protein expression 48 h after CD40 activation (Fig. 5A). However, these cells remained resistant to CD95-mediated apoptosis (Fig. 5B), indicating that factors other than FLIP play a role in the resistance of CLL cells to CD95 in the initial period after CD40 activation. Parallel apoptosis assays performed with Jurkat target cells confirmed the ability of CHO-CD95-L cells to induce CD95-mediated apoptosis.
In separate experiments, we treated cells with the CDDO, a peroxisome proliferator-activated receptor-γ (PPARγ) agonist (21, 22) that was recently shown to specifically down-regulate FLIP protein (Y. Kim, N. Suh, M. Sporn, and J.R.C., unpublished observations). Immunoblot analysis demonstrated that the induced expression of FLIP 1 day after CD40 activation was almost completely abrogated after 24-h treatment with CDDO (Fig. 6A). In contrast, FADD protein expression was not effected in CDDO-treated cells (Fig. 6B). However, when these CDDO-treated cells were used as target cells in the CD95-L apoptosis assay, they were resistant to CD95-mediated apoptosis up to 3 days after CD40 activation (Fig. 6C). Nevertheless, these cells were sensitive to CD95-mediated apoptosis 5 days after CD40 activation, indicating that they still acquired a latent sensitivity to CD95-mediated apoptosis (Fig. 6C).
CD95-Mediated Apoptosis Requires Assembly of DISC.
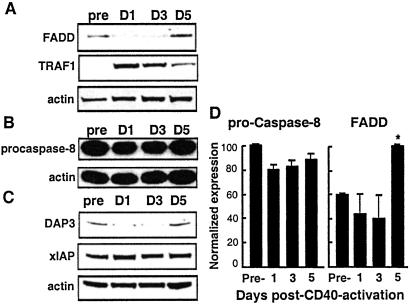
Along with CD95, FADD and pro-caspase-8 are known to be involved in DISC signaling (23). Immunoblot analysis of CD40-activated CLL cells revealed that pro-caspase-8 was expressed and maintained at a similar level up to 5 days after stimulation. As determined by the Bonferroni t test, there was no significant difference when we compared the protein expression of pro-caspase-8 before CD40 activation with the levels observed at days 1, 3, and 5 post-CD40 activation. In contrast, levels of the adapter protein FADD increased significantly by day 5 after CD40-activation (P < 0.05, Bonferroni t test) (Fig. 7 A and D). Interestingly, the same temporal expression pattern was noted for DAP3, a FADD-binding molecule recently noted to facilitate CD95-mediated apoptosis (24, 25) (Fig. 7C).
Figure 7.
Time course analysis of apoptosis regulatory protein expression after CD40 activation of CLL cells. Cells were CD40 activated for 24 h and immunoblot analysis performed for the proteins (A) FADD and tumor necrosis factor–receptor-associated factor 1, (B) pro-caspase-8, and (C) DAP3 and xIAP. (D) Immunoblots for pro-caspase-8 and FADD were scanned, and the mean normalized expression calculated (±SD, n = 3). * indicates significant difference from pre-CD40-activated CLL cells as calculated by the Bonferroni t test.
We also examined the temporal expression of several other antiapoptotic proteins. Tumor necrosis factor-receptor-associated factor 1 (TRAF1) had a temporal expression pattern similar to that of FLIP after CD40 activation. TRAF1 was induced at day 1 but gradually decayed to near preactivation levels by day 5 (Fig. 7A). No significant changes in the antiapoptotic proteins Bcl-2, Bcl-xL, Bax, XIAP, or cIAP-1/2 were observed after CD40 activation (Fig. 7C; data not shown).
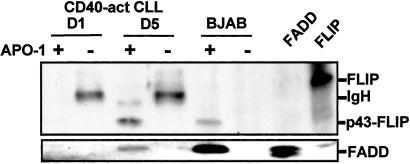
Because newly stimulated CLL cells remained resistant to CD95-mediated killing even in the setting of reduced FLIP expression, we postulated that low expression of FADD in these cells may prevent apoptotic signals through the DISC. FADD is essential for recruiting pro-caspase-8 to the DISC (26). To analyze whether FADD could associate with the activated CD95 receptor in CLL cells 24 h after CD40 activation, we immunoprecipitated CD95 with the agonistic anti-CD95 mAb APO-1. DISC proteins such as FADD, pro-caspase-8, and FLIP coimmunoprecipitate with CD95 after stimulation with APO-1 (18, 27). Control experiments using BJAB cells demonstrated that FADD and FLIP coimmunoprecipitated with the DISC only in APO-1 CD95-stimulated cells (Fig. 8). We found that FADD coimmunoprecipitated with the DISC only in APO-1-stimulated CLL cells activated 5 days earlier with CD40 ligation (Fig. 8 Lower). In contrast, FADD did not coimmunoprecipitate with the DISC in CLL cells stimulated with APO-1 on day 1 after CD40 activation. Similarly, the p43 FLIP isoform that associates with the activated DISC (18, 19) coimmunoprecipitated with the DISC only in APO-1-stimulated CLL cells that had been activated 5 days earlier by CD40 ligation (Fig. 8). Taken together, these findings indicate that CD95 resistance in day 1 CD40-activated CLL cells results from the inability of activated CD95 to assemble the DISC. In contrast, CD95 sensitivity of CLL cells 5 days after CD40 activation is associated with an acquired ability of these cells to form the DISC after CD95 stimulation.
Figure 8.
Immunoprecipitation of DISC. CLL cells were CD40-activated and harvested at days 1 and 5 for DISC immunoprecipitation. BJAB- or CD40-activated CLL cells were either untreated or stimulated with 2 μg/ml of APO-1 mAb for 15 min before the preparation of cell lysates. CD95 DISC was immunoprecipitated by using protein A-Sepharose beads (+). Unstimulated controls were lysed before immunoprecipitation with APO-1 and protein A-Sepharose beads (−). Immunoprecipitates were then subjected to immunoblot analysis with anti-FLIP mAb (Upper) or anti-FADD (Lower). A431- and CD40-activated CLL cell lysates were loaded as controls for the migration position of FADD and FLIP.
Discussion
CLL patients treated with CD154 (CD40L) gene therapy experienced significant reductions in leukemia cell counts and lymph node size that were associated with increases in the numbers of blood T cells that could recognize leukemia cells in vitro (2). In addition, bystander leukemia cells were induced to express CD95 (Fas) in vivo for up to 2 weeks after the infusion of Ad-CD154-transduced cells. The expression of CD95 coincided with the reduction in leukemia cell counts and lymph node size.
Attempting to examine the potential mechanism(s) responsible for clearance of leukemia cells postinfusion, we generated CTL lines from patients with CLL. Repeated stimulation of autologous T cells with CD40-activated leukemia cells stimulated growth of CD4 T cells that had cytolytic activity against leukemia cells activated via CD40-ligation.
These CD4 T cell lines apparently effected cytolysis of CLL cells via a CD95-dependent pathway. First, the CTL were not restricted via class I molecules of the MHC. Rather, these cells could lyse even allogeneic CLL cells provided that they first were activated via CD40-ligation. Second, broad caspase inhibitors such as ZVAD (28) could significantly inhibit apoptosis of leukemia cells (Fig. 1). Such inhibitors can block apoptosis via the CD95-dependent pathway but cannot inhibit the perforin/granzyme-dependent cytolytic activity of CD8 CTL (17, 29). Finally, we found that anti-CD95-L antibodies also could significantly inhibit the CTL activity of these cell lines against CD40-activated CLL cells. Collectively, these data argue that CD4 CTL can effect lysis of CD40-activated CLL cells via a CD95-dependent pathway.
These findings conflict with prior studies that found CD40-activated CLL cells were resistant to CD95-dependent apoptosis (7–9). Conceivably, CD4 CTL expressed some cofactor(s) that rendered CD40-activated CLL cells susceptible to CD95-L mediated lysis. One study, for example, demonstrated treatment of B cells with mAbs to CD150 could sensitize these cells to CD95-mediated killing (30). Although similar treatment failed to sensitize CD40-activated CLL cells to CD95-mediated apoptosis (data not shown), signals derived from CD150 or other accessory molecules still could play a role in sensitizing CD40-activated killing by activated CD4 T cells.
To determine whether CD95 ligation was sufficient to induce apoptosis of CD40-activated CLL cells, we used CHO cells transfected with CD95-L as cytotoxic effector cells. The use of such cells allowed us to compare the apoptosis induced by CHO-CD95-L with that induced by nontransfected CHO cells. In contrast to the CD95-dependent lysis mediated by CD4 CTL, we found that CLL cells were resistant to apoptosis caused by CD95-L-bearing CHO cells during the first 72 h after CD40 activation (Fig. 2). Thereafter, the CLL cells acquired sensitivity to CD95-mediated killing. This latent sensitivity to CD95-mediated apoptosis was noted for the CLL cells from all patients examined (n = 7). These findings contrast with those of previous studies, in which CLL cells only rarely were found to acquire sensitivity to CD95-mediated apoptosis after CD40 ligation (7–9). This difference is likely because of the time interval allowed between CD40 activation and CD95 ligation. In agreement with these previous reports, we also found that CLL cells were resistant to CD95-mediated apoptosis for up to 3 days after CD40 activation. However, all CLL samples tested later acquired a latent sensitivity to CD95-mediated apoptosis after CD40 activation. Similar results were observed by using the IgM anti-CD95 mAb, CH-11 (data not shown).
The latent sensitivity to CD95-mediated killing is similar to that observed for normal B cells (31). As noted in this study, FLIP increased during the 24 h after CD40 activation and then decreased to near baseline levels a few days thereafter. Because high-level expression of FLIP can inhibit CD95-mediated apoptosis (18, 32), these authors reasoned that the induced expression of FLIP was responsible for the noted resistance of CD40-activated B cells to CD95-mediated apoptosis (31). In any case, it appeared that normal B cells also became more sensitive to CD95-mediated killing only a few days after CD40 ligation. Our findings here demonstrate that leukemia B cells retain this capacity to acquire latent sensitivity to CD95-mediated killing after CD40 activation, a physiologic characteristic that may be exploited to develop novel treatment strategies for patients with CLL.
However, our studies also indicate that the up-regulation of FLIP may not be sufficient to account for resistance to CD95-mediated apoptosis in the first few days after CD40 activation. Indeed, we found that treatment of CD40-activated CLL cells with FLIP antisense (Fig. 5A) or CDDO, an insulin-sensitizing drug with antitumor properties (21, 22) (Fig. 6A), could block expression of FLIP induced by CD40 ligation. However, such treatment did not render the CLL B cells susceptible to CD95-mediated killing in the 72-h period after CD40-ligation (Figs. 5B and 6C).
We therefore examined the relative expression levels of other proapoptotic and antiapoptotic proteins after CD40 ligation. We found that TRAF1, a protein that may inhibit apoptotic signaling via members of the tumor necrosis factor (TNF)-receptor family through its effects on NF-κB (33, 34), also was consistently up-regulated after CD40 activation. In addition, we found that FADD, a linker protein that recruits pro-caspase-8 to the DISC (28, 35, 36), is down-regulated 24 h after CD40 activation. In CD95-mediated apoptosis, FADD is essential for the recruitment and activation of pro-caspase-8 at the level of the DISC (26, 37, 38). In vivo studies demonstrated that CD95-mediated apoptosis is completely abrogated in FADD-deficient mice, ruling out the existence of redundant CD95 apoptosis pathways (39). Similar to what we noted for FADD, DAP3 was also expressed at low levels after CD40 ligation. DAP3 is a FADD-associated protein that can increase sensitivity to CD95 and TNF-related apoptosis-reducing ligand-dependent killing (24, 25). Because TRAF1, FADD, and DAP3 each can influence the relative sensitivity of cells to CD95 signaling, changes in the levels of these proteins could contribute to the noted relative early resistance of CLL cells to CD95-mediated killing after CD40 ligation. As such, the mechanism accounting for the initial resistance of CD40-activated CLL cells to CD95 signaling appears more complex than that involving temporal changes in expression of FLIP alone.
Because FADD is required for CD95 apoptosis, we decided to focus our investigation on this linker protein. The transient down-regulation of FADD after CD40 activation was always followed by a 2- to 3-fold increase in the level of FADD by day 5 (n = 5). This observation led us to postulate that the level of FADD in day 1 CD40-activated CLL cells was insufficient for apoptotic signaling through the CD95 DISC. Indeed, immunoprecipitation experiments demonstrated that FADD was associated with the activated CD95 receptor at day 5, but not at day 1, after CD40 activation. Interestingly, in studies that examined the initial resistance of activated T cells to CD95-mediated apoptosis, Scaffidi et al. also concluded that a general defect in DISC assembly, and not FLIP, was responsible for CD95 resistance (18). Our data suggest that insufficient FADD protein prevents productive DISC assembly. In addition, it would be interesting to speculate that the noted up-regulation of DAP3 in CD40-activated CLL cells also might enhance the sensitivity of CLL cells to CD95. In any event, our data support the hypothesis that CD95 resistance in CD40-activated CLL may be in part because of insufficient levels of FADD to form an activated DISC. CD40-activated CLL cells then acquire a latent sensitivity to CD95 when the balance of DISC proteins FLIP and FADD achieves the ratio necessary to induce assembly of and apoptosis via the CD95 DISC.
The latent sensitivity of CD40-activated CLL cells to CD95-mediated apoptosis may account in part for the noted reductions in leukemia cell counts and lymph node size in CLL patients treated with CD154 gene therapy (2). Patients who received autologous CLL cells transfected with Ad-CD154 experienced changes in the bystander nontransfected CLL cells in vivo that were similar to those noted for CLL cells activated via CD40 ligation in vitro. Moreover, the bystander CLL cells of such treated patients were induced to express CD95 after infusion of autologous Ad-CD154-infected CLL cells. Conceivably, the induced expression of CD95 allowed for clearance of leukemia cells by CD95L-bearing cells when they no longer were resistant to CD95-mediated killing. Administration of agents that more rapidly can modify the expression of factors associated with the initial resistance of CD40-activated CLL cells to CD95-mediated apoptosis may enhance the effectiveness of CD154 gene therapy.
Acknowledgments
This work was funded by the National Institutes of Health for the Chronic Lymphocytic Leukemia Research Consortium (Grant NCI-PO1–81534).
Abbreviations
- Ad
adenovirus
- CLL
chronic lymphocytic leukemia
- CDDO
2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid
- CHO
Chinese hamster ovary
- CTL
cytotoxic T lymphocyte
- FADD
Fas-associated death domain protein
- CD95-L
CD95 ligand
- FLIP
Flice-inhibitory protein
- DISC
death-inducing signaling complex
- ZVAD
Z-VAD-fmk
References
- 1.Kipps T J. In: Williams Hematology. Beutler E, Lichtman M A, Coller B S, Kipps T J, Seligsohn U, editors. New York: McGraw–Hill; 2001. pp. 1163–1194. [Google Scholar]
- 2.Wierda W G, Cantwell M J, Woods S J, Rassenti L Z, Prussak C E, Kipps T J. Blood. 2000;96:2917–2924. [PubMed] [Google Scholar]
- 3.Nagata S. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 4.Krammer P H. Nature (London) 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 5.Zamai L, Ahmad M, Bennett I M, Azzoni L, Alnemri E S, Perussia B. J Exp Med. 1998;188:2375–2380. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berke G. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Freeman G J, Levine H, Ritz J, Robertson M J. Br J Haematol. 1997;97:409–417. doi: 10.1046/j.1365-2141.1997.422688.x. [DOI] [PubMed] [Google Scholar]
- 8.Kitada S, Zapata J M, Andreeff M, Reed J C. Br J Haematol. 1999;106:995–1004. doi: 10.1046/j.1365-2141.1999.01642.x. [DOI] [PubMed] [Google Scholar]
- 9.Younes A, Snell V, Consoli U, Clodi K, Zhao S, Palmer J L, Thomas E K, Armitage R J, Andreeff M. Br J Haematol. 1998;100:135–141. doi: 10.1046/j.1365-2141.1998.00522.x. [DOI] [PubMed] [Google Scholar]
- 10.Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H. J Exp Med. 1995;182:1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svingen P A, Karp J E, Krajewski S, Mesner P W, Jr, Gore S D, Burke P J, Reed J C, Lazebnik Y A, Kaufmann S H. Blood. 2000;96:3922–3931. [PubMed] [Google Scholar]
- 12.Krajewski S, Krajewska M, Shabaik A, Wang H G, Irie S, Fong L, Reed J C. Cancer Res. 1994;54:5501–5507. [PubMed] [Google Scholar]
- 13.Krajewski S, Bodrug S, Gascoyne R, Berean K, Krajewska M, Reed J C. Am J Pathol. 1994;145:515–525. [PMC free article] [PubMed] [Google Scholar]
- 14.Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang H G, Reed J C. Am J Pathol. 1994;145:1323–1336. [PMC free article] [PubMed] [Google Scholar]
- 15.Caligaris-Cappio F. Blood. 1996;87:2615–2620. [PubMed] [Google Scholar]
- 16.Chu P, Wierda W G, Kipps T J. Blood. 2000;95:3853–3858. [PubMed] [Google Scholar]
- 17.Hu D, Kipps T J. Cell Immunol. 1999;195:43–52. doi: 10.1006/cimm.1999.1513. [DOI] [PubMed] [Google Scholar]
- 18.Scaffidi C, Schmitz I, Krammer P H, Peter M E. J Biol Chem. 1999;274:1541–1548. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 19.Krueger A, Schmitz I, Baumann S, Krammer P H, Kirchhoff S. J Biol Chem. 2001;276:20633–20640. doi: 10.1074/jbc.M101780200. [DOI] [PubMed] [Google Scholar]
- 20.Hennino A, Berard M, Krammer P H, Defrance T. J Exp Med. 2001;193:447–458. doi: 10.1084/jem.193.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito Y, Pandey P, Place A, Sporn M B, Gribble G W, Honda T, Kharbanda S, Kufe D. Cell Growth Differ. 2000;11:261–267. [PubMed] [Google Scholar]
- 22.Ito Y, Pandey P, Sporn M B, Datta R, Kharbanda S, Kufe D. Mol Pharmacol. 2001;59:1094–1099. doi: 10.1124/mol.59.5.1094. [DOI] [PubMed] [Google Scholar]
- 23.Kischkel F C, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer P H, Peter M E. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kissil J L, Cohen O, Raveh T, Kimchi A. EMBO J. 1999;18:353–362. doi: 10.1093/emboj/18.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki T, Reed J C. Nat Immunol. 2001;2:493–500. doi: 10.1038/88684. [DOI] [PubMed] [Google Scholar]
- 26.Chinnaiyan A M, Tepper C G, Seldin M F, O'Rourke K, Kischkel F C, Hellbardt S, Krammer P H, Peter M E, Dixit V M. J Biol Chem. 1996;271:4961–4495. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- 27.Scaffidi C, Krammer P H, Peter M E. Methods. 1999;17:287–291. doi: 10.1006/meth.1999.0742. [DOI] [PubMed] [Google Scholar]
- 28.Muzio M, Chinnaiyan A M, Kischkel F C, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, et al. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 29.Sarin A, Haddad E K, Henkart P A. J Immunol. 1998;161:2810–2816. [PubMed] [Google Scholar]
- 30.Mikhalap S V, Shlapatska L M, Berdova A G, Law C L, Clark E A, Sidorenko S P. J Immunol. 1999;162:5719–5727. [PubMed] [Google Scholar]
- 31.Hennino A, Berard M, Casamayor-Pallejà M, Krammer P H, Defrance T. J Immunol. 2000;165:3023–3030. doi: 10.4049/jimmunol.165.6.3023. [DOI] [PubMed] [Google Scholar]
- 32.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J L, Schroter M, et al. Nature (London) 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 33.Wang C Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S., Jr Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 34.Leo E, Deveraux Q L, Buchholtz C, Welsh K, Matsuzawa S, Stennicke H R, Salvesen G S, Reed J C. J Biol Chem. 2001;276:8087–8093. doi: 10.1074/jbc.M009450200. [DOI] [PubMed] [Google Scholar]
- 35.Srinivasula S M, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri E S. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 37.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lens S M, den Drijver B F, Potgens A J, Tesselaar K, van Oers M H, van Lier R A. J Immunol. 1998;160:6083–6092. [PubMed] [Google Scholar]
- 39.Zhang J, Cado D, Chen A, Kabra N H, Winoto A. Nature (London) 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]