Abstract
The German cockroach, , is a widespread indoor pest and a vector of enteric human pathogens, including serovar Typhimurium ( Typhimurium). Insecticidal baits are the most commonly used tools to control these cockroaches in built environments. Sublethal exposure to insecticidal baits has been a major driver of adaptive evolution, leading to physiological resistance to insecticides and behavioral aversion to glucose in some cockroach populations. Here, we conducted the first study investigating the effects of sublethal bait exposure on human pathogen biology in . Our results show that a sublethal exposure to bait containing the common insecticide indoxacarb can increase susceptibility to subsequent infection by ingested Typhimurium in surviving cockroaches within the same generation. Interestingly, increased susceptibility to infection after sublethal bait exposure was cockroach strain dependent and did not increase the rate of shedding of the pathogen in excreta. These findings establish for the first time a potential link between a common anthropogenic intervention used to control this prevalent indoor pest and its capacity to maintain pathogens. In doing so, our work reveals a possible unintended consequence of failed pest control efforts. That is, some cockroach populations may become inadvertently more adept at maintaining pathogens due to sublethal exposure to baits stemming from existing insecticide resistance. Additional studies should further investigate this phenomenon to determine its extent and impact.
Keywords: cockroach, salmonella, infection, bait, sublethal, resistance


The German cockroach, , is a globally distributed invasive pest that has evolved to live exclusively indoors. Exploiting their diet and foraging behavior, insecticidal gel baits have become the most commonly used tools to control these cockroaches in domestic settings. Baits are comprised of an active ingredient (insecticide) incorporated into an inert, palatable matrix consisting of a variety of phagostimulatory food products. Exposure to insecticidal baits has been a major evolutionary driver in cockroaches. This anthropogenic challenge has not only selected for physiological resistance to some insecticidal compounds ,, but has also led to the development of behavioral resistance mediated by aversion to the taste of glucose present in baits. , The discovery of the basis of bait aversion represented a major contribution to the field of evolutionary entomology, and this phenomenon is regarded as a prime example of neuronal reorganization and the evolution of adaptive behavior in insects. −
Due to their frequent interaction with microbially diverse environments, German cockroaches are suspected of contributing to the spread of enteric human pathogens, including hepatitis viruses, spp., and , among others. ,,, Of the various bacterial pathogens associated with cockroaches, serovar Typhimurium ( Typhimurium) is a leading cause of gastroenteritis worldwide. Typhimurium transmission by cockroach vectors has traditionally been described as mechanical. However, recent studies have challenged this paradigm, demonstrating replication in the gut of as well as active bacterial mechanisms involved in gut colonization and fecal transmission. − Despite this recent work, relatively little is still known about the dynamics of pathogen transmission by cockroach vectors and how endogenous and exogenous factors influence this process. Of notable interest, the effects of environmental selection pressures such as insecticide exposure, which is pervasive among cockroaches, have not been investigated. The impact of insecticide exposure on pathogen infection in insect vectors remains poorly resolved in general.
Both behavioral and physiological resistance are growing problems that have hindered the effectiveness of baits against German cockroaches in the field. ,, Increased physiological resistance to the insecticides used in baits subsequently leads to more sublethal exposures to baits among cockroach populations. Therefore, it is essential to understand what effects, if any, such failed control efforts may have on the ability of cockroaches to acquire and transmit pathogens. The ingredients present in cockroach baits, including insecticides, antimicrobial preservatives and various sugars, proteins, and fats, all have the potential to alter the microenvironment of the cockroach gut. In German cockroaches, dietary macronutrient intake influences the composition of the gut microbiota, ,, which can in turn affect colonization of the gut by ingested and Typhimurium through both positive and negative mechanisms. ,, Further, links between insecticide metabolism and immunity are known in some insect systems, and the existence of similar links in cockroaches could drive changes in susceptibility to pathogen infection after insecticide exposure. Indeed, one transcriptomic study reported that experimental selection for indoxacarb resistance in by oral exposure over six generations resulted in a significant reduction in transcripts derived from commensal microbes, suggesting that insecticide exposure and the processes driving the evolution of resistance also directly or indirectly affect microbial dynamics.
Given this evidence, we hypothesized that sublethal exposure to baits could lead to changes in susceptibility to infection by human pathogenic bacteria, such as Typhimurium, ingested by surviving cockroaches. To address this hypothesis, here we exposed two different strains of to a commercial bait product containing indoxacarb (Advion Evolution, 0.6% indoxacarb, Syngenta Corporation), one of the most commonly used insecticides against cockroaches. We then assessed the susceptibility of surviving cockroaches to oral infection by Typhimurium as well as subsequent shedding of the pathogen in excreta and compared these to matched control cockroaches that were not exposed to baits.
Results
Susceptibility to Advion Evolution Gel Bait in Cockroach Strains
The Gilpin and Hopewell field strains of , along with the Orlando laboratory reference strain, were all visually observed to readily consume Advion Evolution bait within the first day of exposure. Observations included cockroaches directly feeding on the bait, signs of consumption over time in bait aliquots, and bait-colored fecal streaking in the arenas. No mortality occurred in Orlando strain cockroaches that were not exposed to bait. However, after 3 days of exposure, the Orlando strain had an average mortality of 75%, indicating an expected high susceptibility to indoxacarb in this long-term laboratory strain (Figure ). In contrast, the Gilpin and Hopewell strains both had much lower average mortality. After 3 days, mortality was only 15% in the Gilpin strain and 30% in the Hopewell strain, indicating reduced susceptibility to indoxacarb. Though this assay was not meant to quantitatively assess insecticide resistance levels nor product efficacy, the results importantly confirm the general presence of resistance and the potential for sublethal exposure to bait in both field infestations and our laboratory experiments due to reduced susceptibility in some populations.
1.
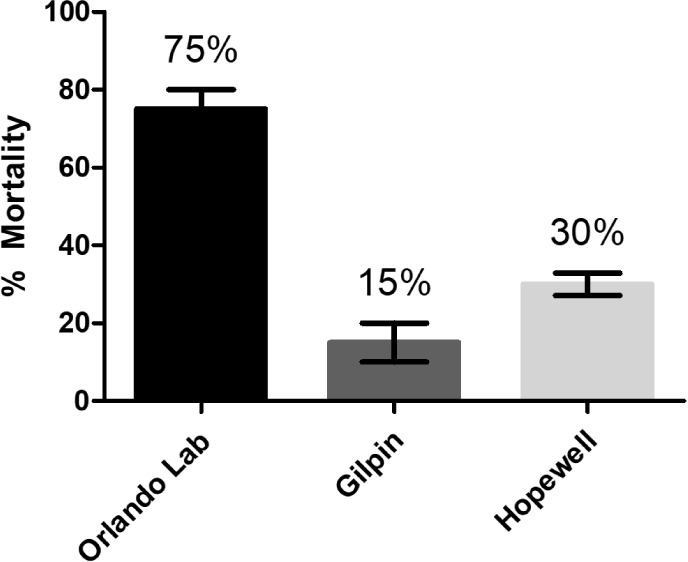
Susceptibility to Advion Evolution (0.6% indoxacarb) gel bait in three German cockroach strains. Cockroaches were exposed to bait and mortality including moribund cockroaches was assessed after 3 days. The exposure was replicated independently three times for each strain using 20 nymphs per replicate and average mortality with standard error (SEM) is shown.
Sublethal Exposure to Bait Does Not Impact Subsequent Consumption of Bacteria by Cockroaches
Sublethal exposure to Advion Evolution bait resulted in noticeable defects in movement in surviving cockroaches (e.g., twitching, erratic walking), as expected given the neurotoxic effects of indoxacarb. As experimental infection of cockroaches with Typhimurium is administered via free feeding in liquid media to mimic natural acquisition, we first sought to ensure that sublethal bait exposure did not affect the ability of the surviving cockroaches to consume the bacterial media, nor alter the volume ingested relative to control cockroaches not exposed to bait, as such a shift could confound infection experiments (Figure ). Despite some defects in movement, cockroaches that had received a sublethal bait exposure readily fed on bacterial media when it was offered. When feeding on bacterial media containing blue #1 dye was quantified using an established spectrophotometric assay, , no significant difference in volume of feeding was observed in either the Gilpin strain (t-test, t = 1.18, df = 42, p = 0.2418) or Hopewell strain (t-test, t = 0.51, df = 33, p = 0.6162) when comparing groups of cockroaches that received a sublethal bait exposure to matched controls.
2.

Sublethal exposure to bait does not impact subsequent consumption of bacteria by cockroaches. Consumption of LB media used for growth of Typhimurium was assessed in (A) Gilpin strain and (B) Hopewell strain cockroaches with or without sublethal exposure to bait. Groups of cockroaches were provided media spiked with blue #1 dye and allowed to feed for 30 min. Immediately following the feeding period individual cockroaches were mechanically homogenized in PBS and the concentration of blue dye in each sample was determined by measuring absorbance at 629 nm (A629 nm) as proxy for the volume of media consumed. , Dots represent individual cockroach samples and the bars show the mean and standard error (SEM) of absorbance for each group. T-tests were used to test for differences between the means within each strain.
Susceptibility to Infection Is Increased after Sublethal Bait Exposure
When cockroaches from the Gilpin strain received an oral inoculum of Typhimurium (Figure A), the prevalence of detectable infection 24 h later was 47% in controls not exposed to bait. When the same strain received a sublethal exposure to Advion Evolution prior to the Typhimurium inoculum, infection prevalence was 77%, which was a statistically significant increase (Fisher’s exact test, p = 0.044). Further, in Gilpin cockroaches that were infected without bait exposure the average load of Typhimurium 24 h post-infection was 5211 CFUs/insect, whereas in those that received a sublethal bait exposure, the average load was 8221 CFU/insect, a 1.58-fold increase (t-test, t = 0.97, df = 29, p = 0.338). In the Hopewell strain, the prevalence of infection at 24 h was 73% in controls and 93% after sublethal bait exposure (Fisher’s exact test, p = 0.083). Meanwhile the average Typhimurium load in infected Hopewell cockroaches was 3159 CFU/insect for controls and 3138 CFU/insect for those with a prior sublethal bait exposure, which was nearly identical (t-test, t = 0.13, df = 45, p = 0.899). Although the increase in infection load in infected Gilpin cockroaches after sublethal exposure was not statistically significant, it is likely of biological significance for transmission potential considering the magnitude of the increase coupled with the substantial increase in infection prevalence for this strain. Supporting this interpretation, comparison of CFU loads including insects that cleared the infection (CFU = 0) rather than only those with detectable CFUs yielded statistical significance for the Gilpin strain (Mann–Whitney test, p = 0.015) but not the Hopewell strain (Mann-Whitney test, p = 0.268). Taken together these results indicate that sublethal bait exposure can increase susceptibility to Typhimurium infection in a manner that is cockroach strain dependent.
3.
Sublethal exposure to bait increases susceptibility to Typhimurium infection. Groups of (A,B) Gilpin strain and (C,D) Hopewell strain cockroaches with or without sublethal bait exposure were orally infected with Typhimurium. One-day post-infection, whole insects were homogenized to assess infection status by selective plating. (A,C) The prevalence of infection (proportion of insects harboring detectable colony forming units (CFU)) and (B,D) load of infection (CFU/insect in infected insects) were both examined. Statistical analysis consisted of Fisher’s exact test for prevalence data and t-test on log transformed CFU values for load of infection. Bars show the mean with standard error (SEM) and dots represent individual cockroaches. Three independent replicates were performed, including a total of 22–30 cockroaches per group.
Shedding of Is Not Significantly Altered in Cockroaches That Become More Susceptible to Infection after Sublethal Bait Exposure
Given that increases in both infection prevalence and infection load were observed in the Gilpin strain following sublethal bait exposure, we next investigated whether this also resulted in increased shedding of Typhimurium in excreta (Figure ). When German cockroaches are infected with Typhimurium, they will begin to excrete the bacteria within the first 24 h of infection, with excretion levels being cockroach strain dependent. Following an inoculum of Typhimurium, 73% of Gilpin cockroaches not exposed to bait excreted detectable CFUs, whereas 47% of those that experienced a sublethal bait exposure excreted viable CFUs (Fisher’s exact test, p = 0.264). Similarly, cockroaches that excreted without bait exposure excreted an average of 2600 CFU/insect/24 h, whereas cockroaches that excreted following a sublethal bait exposure excreted an average of 1571 CFU/insect/24 h (t-test, t = 0.51, df = 16, p = 0.614). Thus, sublethal bait exposure does not appear to increase, and may possibly decrease, Typhimurium shedding in excreta despite increasing susceptibility to infection.
4.
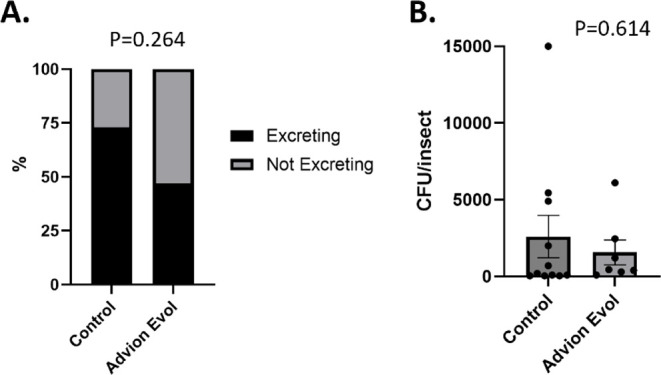
Shedding of Typhimurium is not affected by sublethal bait exposure. Groups of Gilpin strain cockroaches with or without sublethal bait exposure were orally infected with Typhimurium. Immediately after infection, individual insects were placed into individual wells of a 12-well tissue culture plate lined with paper for collection of excreta. After 1 day, excreta were extracted from the filter papers and Typhimurium was quantified by selective plating. (A) The prevalence of excretion (proportion of insects excreting detectable colony forming units (CFU)) and (B) load of excretion (CFU excreted per insect) were both examined. Statistical analysis consisted of Fisher’s exact test for prevalence data and t-test on log transformed CFU values for load of excretion. Bars show the mean with standard error (SEM) and dots represent individual cockroaches. Three independent replicates were performed, including a total of 15 cockroaches per group.
Discussion
Here, our work identifies a novel aspect of cockroach physiology that can be significantly altered by exposure to the anthropogenic challenge of baits, in the form of enhancement of infection by ingested bacteria. Specifically, we show that sublethal bait exposure can, in some cockroach strains, result in increased susceptibility to infection by ingested Typhimurium within the same generation. These findings are of high priority because they establish for the first time a possible link between a common anthropogenic insecticidal intervention used to control this prevalent structural pest and its ability to carry human pathogens.
Interestingly, the effects of sublethal bait exposure on infection were more apparent in one cockroach strain than another, indicating some influence of genotypic or microbiome variation on the mechanism(s) linking bait exposure to infection biology. For example, the effects may be driven by shifts in specific microbial taxa present only in some cockroach strains, or may depend on specific resistance alleles. We also observed a lack of effect of the sublethal bait exposure on pathogen shedding in the excreta of the Gilpin strain even though internal pathogen loads were increased. The latter result was not entirely surprising and confirms our previous finding that internal Typhimurium loads and excreted loads are decoupled and regulated by different bacterial and host mechanisms. , However, it is critical to still recognize the potential importance of internal loads for pathogen maintenance and transmission by cockroaches. For example, significant horizontal transmission of Typhimurium among German cockroaches sharing harborage sites can take place via necrophagy, and cockroaches that are more highly infected may contribute to this process more efficiently. In addition, highly infected cockroaches may shed the pathogen into the environment for longer periods or over larger distances despite not excreting at a higher rate.
The mechanism(s) underlying the effects of bait exposure on Typhimurium infection, and whether this effect is heritable and increases over multiple generations, remain unknown. Additional controlled studies are needed to address these questions. It is possible that microbial community shifts resulting from consumption of the macronutrients that comprise the bulk of bait, or perhaps preservative components with antimicrobial activity, are responsible. The gut microbiota of cockroaches is uniquely diverse among insects , and its composition and structure are influenced by numerous variables including development and diet. ,, The cockroach gut microbiota in turn regulates important physiological processes. Relevant to the findings reported here, recent work demonstrated that commensal bacteria in the gut of influence orally acquired and Typhimurium infections via multiple mechanisms, ,, in line with numerous studies demonstrating that the microbiota plays a key role in modulating pathogen resistance in other vector arthropods. , Prior studies have also found differences between the microbial communities of insecticide resistant and susceptible strains of ,,, suggesting that insecticides, rather than inactive bait ingredients, could exert some selection pressure on the microbiome directly. Notably, boric acid intake by was found to alter the relative abundance of some commensal bacteria in the gut while also increasing susceptibility to a fungal entomopathogen. Though demonstrating a similar concept to our work, the effects described by Yang et al. appear likely related to the physical damage to the gut caused by boric acid. On the other hand, the impact of bait consumption on pathogen infection may not be linked to shifts in the microbiota at all. It is also possible that bait consumption has direct effects on the host response to infection by leading to physiological and ultimately evolutionary trade-offs between xenobiotic detoxification and immunity, which should be further explored using as a model.
Regardless of the mechanism(s) involved, our findings newly reveal a possible unintended consequence of failed pest control efforts that requires further consideration. That is, we show proof-of-principle that in instances where baits fail on account of existing insecticide resistance, sublethal exposure of cockroaches may result in some localized populations becoming more adept at maintaining human pathogens. Given this possibility, resistance management through strategies such as bait rotation should continue to be regarded of importance to public health. However, additional research is required to determine the extent of this possible concern.
Some limitations of our study include that it only involved two cockroach strains and one bait product. Our results may or may not hold true for other products or in other cockroach strains. Indeed, we observed enhancement of infection in one cockroach strain but not another. We also attempted similar experiments with a different bait product (MaxForce FC Magnum, 0.05% fipronil), but all cockroach strains that we tested were highly susceptible to this bait, so we were unable to extrapolate to another active ingredient. The phenomenon that we report here should be further investigated not only in the lab with different bait and residual spray products to understand its generalizability and the mechanism(s) at play, but also under field conditions to determine its ultimate ecological significance. The phenomenon should also be explored in other insect pests that are controlled using baits. For example, similar effects could potentially be conserved in flies, which acquire, maintain, and disseminate enteric pathogens in a manner similar to cockroaches. ,
Materials and Methods
Cockroach Strains and Rearing
This study included three different strains of . Infections with Typhimurium were carried out in two recently collected field strains (Gilpin, Hopewell). The Gilpin strain was collected from public housing in Richmond, VA in February 2023, while the Hopewell strain was collected from public housing in Hopewell, VA in February 2023. Both were maintained in the lab for multiple generations prior to the experiments reported here. The American Cyanamid Orlando normal strain, which has been maintained in laboratory culture for decades, was also included as a control in bait susceptibility assays. Rearing of all strains took place in plastic arenas containing egg carton harborages, dog chow (Purina, St. Louis, MO, USA), and tap water, at 25 ± 1°C and ∼40–45% relative humidity with a 12:12 (L:D) hour photoperiod.
Determination of Cockroach Susceptibility to Bait
Prior to infection experiments, we examined the general level of susceptibility to a gel bait containing the active ingredient indoxacarb (Advion Evolution, 0.6% indoxacarb, Syngenta Corporation, Wilmington, DE) in the two recently collected field strains and in the Orlando laboratory strain as a reference. The purpose of this assay was not to quantitatively assess insecticide resistance levels nor product efficacy. Rather, this was done to confirm the general presence of resistance and ensure that experimental sublethal exposures would be feasible in these field strains (i.e., they were not completely susceptible to the bait). Briefly, 20 medium sized nymphs from each colony were transferred to experimental plastic arenas containing a harborage and a water source but no food. Approximately 0.7 g of bait was immediately added to each arena without an acclimation period. After the first day of bait exposure, dog chow was added to each arena along with the bait. Mortality was measured daily for a total of 3 days and dead cockroaches were removed from the arenas each day. Cumulative mortality at the end of the three-day period included moribund cockroaches that were unable to right themselves when probed. Replication consisted of three independent biological replicates (60 total cockroaches per strain). Mortality was also assessed in the Orlando strain subjected to the same process without bait exposure (dog chow only) as a control for baseline mortality in each replicate.
Sublethal Bait Exposure
To determine the effects of sublethal exposure to indoxacarb bait on infection, cockroaches underwent a sublethal exposure prior to being infected with Typhimurium. The sublethal exposure was identical to that describe above in section 2.2, but using adult males, to remain consistent with prior work on Typhimurium infection in . , Arenas were also visually monitored to confirm consumption of the bait. Cockroaches that survived at the end of the three-day bait exposure were transferred to new arenas for additional experiments as described below, with matched controls subjected to the same process and handling but without bait exposure (dog chow only).
Quantitation of Cockroach Consumption of Bacteria
Prior to carrying out oral infections via free feeding of Typhimurium cultures as in our previous work, ,− we sought to ensure that sublethal exposure to bait did not alter cockroach consumption of the liquid media in which the bacteria are administered. We therefore performed an experiment to compare the amount of bacterial media that cockroaches would consume in our bacterial feeding protocol with or without sublethal bait exposure. The experiment was conducted in both the Gilpin and Hopewell strain using a modified version of a protocol to quantify feeding in that we previously successfully adapted to detect feeding differences in cockroaches. To summarize, groups of adult male cockroaches underwent sublethal bait exposure or a matched control treatment as described in the section above (Sublethal Bait Exposure). At the end of the three-day bait exposure, survivors were starved of food and water for 3 days, as in our infection protocol. Subsequently, cockroaches were provided a shallow Petri dish containing LB media spiked with blue #1 dye and allowed to feed freely for 30 min. Immediately following the feeding period, individual cockroaches were placed into tubes containing 500 μL of sterile PBS and mechanically homogenized to release the ingested blue dye from the gut into solution. The homogenates were then centrifuged at high speed to pellet tissue and cellular debris, and aliquots of the supernatants containing the dye were transferred to individual wells of a 96 well plate in triplicate for each cockroach sample. Absorbance at 629 nm (A629 nm) was measured on a spectrophotometer for relative quantitation of the concentration of blue dye in each sample, which served as a proxy for the volume of blue dye ingested, and triplicate values were averaged. For each treatment group, 15–24 individual cockroaches were assayed after feeding. A t-test was used to assess differences in the mean absorbance between samples from cockroaches with or without sublethal bait exposure within each strain.
Culture
serovar Typhimurium ( Typhimurium) strain 14028s strain was used in this study. The strain expresses both kanamycin resistance and GFP markers that enable quantitation by selective plating. Cultures were grown in liquid LB media containing 100 μg/mL of kanamycin at 37°C on a shaker.
Infection and Quantitation of Susceptibility to Infection
Typhimurium infection was administered orally to mimic natural acquisition, as described in our previous work. ,− Adult male cockroaches from the Gilpin and Hopewell strains were used after sublethal exposure to bait or a matched control treatment without bait exposure. Prior to infection, the cockroaches were starved of food and water for 3 days, which promotes consistent experimental feeding. The cockroaches were then offered a shallow petri dish containing a stationary-phase culture of Typhimurium standardized to a concentration of OD600 = 1, which results in a physiologically relevant inoculum upon consumption (3.58 × 106 CFU/insect). Blue #1 dye was added to the culture to enable tracking of fed cockroaches. After 30 min, the culture and any unfed cockroaches without visible blue dye in the gut were removed from arenas, and dog chow and water were provided. Internal infection status was assayed in individual cockroaches after 24 h by selective plating. This timepoint represents an important bottleneck during infection of the cockroach gut identified in our previous studies. To quantify internal Typhimurium, individual cockroaches were cold anesthetized, and surface sterilized by successive rinses in 10% bleach, 70% ethanol, and water. The cockroaches were then mechanically homogenized in sterile PBS using a handheld electronic tissue homogenizer. The resulting solution was serially diluted and plated on selective LB agar plates containing 100 μg/mL of kanamycin for detection of Typhimurium. The plates were incubated overnight at 37°C and colony forming units (CFUs) of Typhimurium were counted the next day. The prevalence of infection was calculated as the proportion of cockroaches that harbored any detectable CFUs. Raw CFU counts were also used to calculate infection load (CFUs/insect) in those cockroaches that harbored detectable CFUs, based on dilution factor and plating volume. The theoretical limit of detection of this assay was 50 CFUs. Plates which yielded CFUs too numerous to count were conservatively set to a count of 300 (15,000 CFU) in calculations. Replication consisted of three biological replicates (independent infections), totaling 22–30 insects per group. Prevalence data were analyzed using Fisher’s exact test and load data (CFU/insect) were analyzed by t-test on log transformed CFU values or by Mann-Whitney test. Negative controls consisting of uninfected insects from each strain were plated in each replicate and showed no growth on kanamycin plates.
Quantitation of Shedding
For quantitation of Typhimurium shedding, oral infections were performed in males as described above in section 2.6, with the exception that sublethal bait exposure was only done for 1 day instead of three. After feeding on Typhimurium, single cockroaches were transferred using soft forceps into individual wells of a 12-well tissue culture plate lined with filter paper. The cockroaches were allowed to excrete on the filter papers for 24 h and the filter papers were then collected into 2 mL tubes containing 500 μL of sterile PBS. The filter papers were soaked for 30 min with periodic mixing on a vortex mixer every 10 min to dissolve the excreta. The PBS solution was then diluted and plated on selective LB agar plates containing 100 μg/mL of kanamycin for detection of Typhimurium. The plates were incubated overnight at 37°C and colony forming units (CFU) of Typhimurium were counted the next day. The prevalence of excretion was calculated as the proportion of cockroaches that excreted any detectable CFUs. Raw CFU counts were also used to calculate the load excreted (CFUs/insect/24 h) by those cockroaches that excreted detectable CFUs, based on dilution factor and plating volume. The theoretical limit of detection of this assay was 50 CFUs. Plates which yielded CFUs too numerous to count were conservatively set to a count of 300 (15,000 CFU) in calculations. Three biological replicates (independent infections) were conducted, and data were collected from a total of 15 insects per group. Prevalence data were analyzed using Fisher’s exact test and load data (CFU/insect) were analyzed by t-test on log transformed CFU values. Negative controls of excreta from uninfected cockroaches showed no growth on kanamycin plates.
Acknowledgments
This study was funded by the National Institutes of Health, National Institute of Allergy and Infectious Diseases grant R01AI171014 to JEP and by the OW Rollins/Orkin endowment at Purdue University held by JEP. We thank Morgan Wilson and Dini Miller at Virginia Tech University for providing some of the cockroach strains used. We also thank Matthew Turner for assistance with cockroach colony maintenance.
The authors declare no competing financial interest.
References
- Carrasco P., van de Pol C., Baixeras J., Moya A., Latorre A., Latorre A.. Succession of the gut microbiota in the cockroach Blattella germanica. Int. Microbiol. 2014;17:99–109. doi: 10.2436/20.1501.01.212. [DOI] [PubMed] [Google Scholar]
- Dennison N. J., Jupatanakul N., Dimopoulos G.. The mosquito microbiota influences vector competence for human pathogens. Curr. Opin. Insect Sci. 2014;3:6–13. doi: 10.1016/j.cois.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez R., Pérez-Cobas A. E., Cuti P., Pérez-Brocal V., García-Ferris C., Moya A., Latorre A., Gil R.. Interkingdom gut microbiome and resistome of the cockroach Blattella germanica. mSystems. 2021;6(3):10–128. doi: 10.1128/mSystems.01213-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardisi M., Gondhalekar A. D., Ashbrook A. R., Scharf M. E.. Rapid evolutionary responses to insecticide resistance management interventions by the German cockroach (Blattella germanica L.) Sci. Rep. 2019;9(1):8292. doi: 10.1038/s41598-019-44296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondhalekar A. D., Appel A. G., Thomas G. M., Romero A.. A review of alternative management tactics employed for the control of various cockroach species (Order: Blattodea) in the USA. Insects. 2021;12(6):550. doi: 10.3390/insects12060550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel G., Simone M.. Le rôle des Blattes dans la transmission des salmonelloses. Ann. Inst. Pasteur Paris. 1950;79(5):654–660. [PubMed] [Google Scholar]
- Ismael B., Wilson M., Miller D., Pietri J. E.. Differences in Typhimurium infection and excretion among laboratory and field strains of the German cockroach suggest a genomic basis for vector competence. Infect., Genet. Evol. 2024;123:105624. doi: 10.1016/j.meegid.2024.105624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R. R., Xu J.. Mechanisms by which pesticides affect insect immunity. J. Invertebr. Pathol. 2012;109(2):175–182. doi: 10.1016/j.jip.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Juache-Villagrana A. E., Pando-Robles V., Garcia-Luna S. M., Ponce-Garcia G., Fernandez-Salas I., Lopez-Monroy B., Rodriguez-Sanchez I. P., Flores A. E.. Assessing the impact of insecticide resistance on vector competence: A review. Insects. 2022;13(4):377. doi: 10.3390/insects13040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakumanu M. L., Maritz J. M., Carlton J. M., Schal C.. Overlapping community compositions of gut and fecal microbiomes in lab-reared and field-collected German cockroaches. Curr. Microbiol. 2018;84(17):e01037–18. doi: 10.1128/AEM.01037-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko A. E., Bieman D. N., Schal C., Silverman J.. Insecticide resistance and diminished secondary kill performance of bait formulations against German cockroaches (Dictyoptera: Blattellidae) Pest Manage. Sci. 2016;72(9):1778–1784. doi: 10.1002/ps.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Choe D. H., Rust M. K., Lee C. Y.. Reduced susceptibility towards commercial bait insecticides in field German cockroach (Blattodea: Ectobiidae) populations from California. J. Econ. Entomol. 2022;115(1):259–265. doi: 10.1093/jee/toab244. [DOI] [PubMed] [Google Scholar]
- Liang D., McGill J., Pietri J. E.. Unidirectional cross-resistance in German cockroach (Blattodea: Blattellidae) populations under exposure to insecticidal baits. J. Econ. Entomol. 2017;110(4):1713–1718. doi: 10.1093/jee/tox144. [DOI] [PubMed] [Google Scholar]
- Mond M., Pietri J. E.. Horizontal transmission of Typhimurium among German cockroaches and its possible mechanisms. Ecol. Evol. 2023;13(5):e10070. doi: 10.1002/ece3.10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasirian H.. Contamination of cockroaches (Insecta: Blattaria) by medically important bacteriae: a systematic review and meta-analysis. J. Med. Entomol. 2019;56(6):1534–1554. doi: 10.1093/jme/tjz095. [DOI] [PubMed] [Google Scholar]
- Nayduch D., Burrus R. G.. Flourishing in filth: House fly-microbe interactions across life history. Ann. Entomol. Soc. Am. 2017;110(1):6–18. doi: 10.1093/aesa/saw083. [DOI] [Google Scholar]
- Pérez-Cobas A. E., Maiques E., Angelova A., Carrasco P., Moya A., Latorre A.. Diet shapes the gut microbiota of the omnivorous cockroach Blattella germanica. FEMS Microbiol. Ecol. 2015;91(4):fiv022. doi: 10.1093/femsec/fiv022. [DOI] [PubMed] [Google Scholar]
- Pietri J. E., Tiffany C., Liang D.. Disruption of the microbiota affects physiological and evolutionary aspects of insecticide resistance in the German cockroach, an important urban pest. PLoS One. 2018;13(12):e0207985. doi: 10.1371/journal.pone.0207985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R., Potts R., Pietri J. E.. The persistence of infection in German cockroaches (Blattodea: Blattellidae) varies between host developmental stages and is influenced by the gut microbiota. J. Med. Entomol. 2020;57(6):1964–1971. doi: 10.1093/jme/tjaa108. [DOI] [PubMed] [Google Scholar]
- Scharf M. E., Wolfe Z. M., Raje K. R., Fardisi M., Thimmapuram J., Bhide K., Gondhalekar A. D.. Transcriptome responses to defined insecticide selection pressures in the German cockroach (Blattella germanica L.) Front. Physiol. 2022;12:816675. doi: 10.3389/fphys.2021.816675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J., Bieman D. M.. Glucose aversion in the German cockroach, Blattella germanica. J. Insect Physiol. 1993;39(11):925–933. doi: 10.1016/0022-1910(93)90002-9. [DOI] [Google Scholar]
- Silverman J., Ross M. H.. Behavioral resistance of field-collected German cockroaches (Blattodea: Blattellidae) to baits containing glucose. Environ. Entomol. 1994;23(2):425–430. doi: 10.1093/ee/23.2.425. [DOI] [Google Scholar]
- Tang Q., Vargo E. L., Ahmad I., Jiang H., Varadínová Z. K., Dovih P., Kim D., Bourguignon T., Booth W., Schal C.. et al. Solving the 250-year-old mystery of the origin and global spread of the German cockroach, Blattella germanica. Proc. Natl. Acad. Sci. U. S. A. 2024;121(22):e2401185121. doi: 10.1073/pnas.2401185121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarshis I. B.. The cockroacha new suspect in the spread of infectious hepatitis. Am. J. Trop. Med. Hyg. 1962;11:705–711. doi: 10.4269/ajtmh.1962.11.705. [DOI] [PubMed] [Google Scholar]
- Turner M., Peta V., Pietri J. E.. New insight into the relationship between Typhimurium and the German cockroach suggests active mechanisms of vector-borne transmission. Res. Microbiol. 2022;173(3):103920. doi: 10.1016/j.resmic.2021.103920. [DOI] [PubMed] [Google Scholar]
- Turner M., Van Hulzen L., Pietri J. E.. The gut microbiota induces melanin deposits that act as substrates for fimA-mediated aggregation of Typhimurium and enhance infection of the German cockroach vector. Microbiol. Spectrum. 2023;11(5):e0211923. doi: 10.1128/spectrum.02119-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M., Van Hulzen L., Guse K., Agany D., Pietri J. E.. The gut microbiota confers resistance against Typhimurium in cockroaches by modulating innate immunity. iScience. 2024;27(12):111293. doi: 10.1016/j.isci.2024.111293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada-Katsumata A., Silverman J., Schal C.. Changes in taste neurons support the emergence of an adaptive behavior in cockroaches. Science. 2013;340(6135):972–975. doi: 10.1126/science.1234854. [DOI] [PubMed] [Google Scholar]
- Wada-Katsumata A., Hatano E., McPherson S., Silverman J., Schal C.. Rapid evolution of an adaptive taste polymorphism disrupts courtship behavior. Commun. Biol. 2022;5(1):450. doi: 10.1038/s42003-022-03415-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada-Katsumata A., Schal C.. Glucose aversion: a behavioral resistance mechanism in the German cockroach. Curr. Opin. Insect Sci. 2024;63:101182. doi: 10.1016/j.cois.2024.101182. [DOI] [PubMed] [Google Scholar]
- Wang C., Scharf M. E., Bennett G. W.. Behavioral and physiological resistance of the German cockroach to gel baits (Blattodea: Blattellidae) J. Econ. Entomol. 2004;97(6):2067–2072. doi: 10.1093/jee/97.6.2067. [DOI] [PubMed] [Google Scholar]
- Wolfe Z. M., Scharf M. E.. Differential microbial responses to antibiotic treatments by insecticide-resistant and susceptible cockroach strains (Blattella germanica L.) Sci. Rep. 2021;11(1):24196. doi: 10.1038/s41598-021-03695-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R., Piper M. D., Wertheim B., Partridge L.. Quantification of food intake in Drosophila. PLoS One. 2009;4(6):e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Salmonella (non-typhoidal) fact sheet. 2018. https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal).
- Wu-Chuang A., Hodžić A., Mateos-Hernández L., Estrada-Peña A., Obregon D., Cabezas-Cruz A.. Current debates and advances in tick microbiome research. Curr. Res. Parasitol. Vector Borne Dis. 2021;1:100036. doi: 10.1016/j.crpvbd.2021.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z., Zhang J., Zhang R., Huang Z., Wan Q., Zhang Z.. Comparative analysis of gut bacterial communities in housefly larvae fed different diets using a high-throughput sequencing approach. FEMS Microbiol. Lett. 2019;366(11):fnz126. doi: 10.1093/femsle/fnz126. [DOI] [PubMed] [Google Scholar]
- Yang R., Zhang M., Schal C., Jiang M., Cai T., Zhang F.. Boric acid enhances Metarhizium anisopliae virulence in Blattella germanica (L.) by disrupting the gut and altering its microbial community. Biol. Control. 2021;152:104430. doi: 10.1016/j.biocontrol.2020.104430. [DOI] [Google Scholar]
- Zhang F., Yang R.. Life history and functional capacity of the microbiome are altered in beta-cypermethrin-resistant cockroaches. Int. J. Parasitol. 2019;49(9):715–723. doi: 10.1016/j.ijpara.2019.04.006. [DOI] [PubMed] [Google Scholar]
- Zhu J., Wu Y., Lin F., Liao G., Wang J., Wei J., Xu H.. Diet influences the gut microbial diversity and olfactory preference of the German cockroach Blattella germanica. Curr. Microbiol. 2022;80(1):23. doi: 10.1007/s00284-022-03123-w. [DOI] [PubMed] [Google Scholar]
- Zurek L., Schal C.. Evaluation of the German cockroach (Blattella germanica) as a vector for verotoxigenic F18 in confined swine production. Vet. Microbiol. 2004;101(4):263–267. doi: 10.1016/j.vetmic.2004.04.011. [DOI] [PubMed] [Google Scholar]



