Abstract
Signaling via mitogen-activated protein kinases is implicated in heart failure induced by agonists for G protein-coupled receptors that act via the G protein Gαq. However, this assertion relies heavily on pharmacological inhibitors and dominant-interfering proteins and not on gene deletion. Here, we show that endogenous cardiac MAPK/ERK kinase kinase-1 (MEKK1)/(MAP3K1), a mitogen-activated protein kinase kinase kinase, is activated by heart-restricted overexpression of Gαq in mice. In cardiac myocytes derived from embryonic stem cells in culture, homozygous disruption of MEKK1 selectively impaired c-Jun N-terminal kinase activity in the absence or presence of phenlyephrine, a Gαq-dependent agonist. Other terminal mitogen-activated protein kinases were unaffected. In mice, the absence of MEKK1 abolished the increase in cardiac mass, myocyte size, hypertrophy-associated atrial natriuretic factor induction, and c-Jun N-terminal kinase activation by Gαq, and improved ventricular mechanical function. Thus, MEKK1 mediates cardiac hypertrophy induced by Gαq in vivo and is a logical target for drug development in heart disease involving this pathway.
Cardiac muscle hypertrophy entails the increase in cell size that occurs as a nominally adaptive response to increased mechanical load (as with hypertension or structural anomalies) or decreased mechanical performance (as with ischemic damage or intrinsic cardiac muscle disorders; refs. 1–4). Hypertrophy also is triggered, less often, in hereditary cardiomyopathies with seemingly normal performance and load. Characteristics of cardiac enlargement in all of these settings include increased myocyte size, sarcomere formation, and “reprogramming” of cardiac gene expression, including atrial natriuretic factor (ANF) induction as the most generalizeable transcriptional response. In humans, cardiac hypertrophy is a principal risk factor for the development of overt heart failure and subsequent cardiac death.
Among potential mediators of hypertrophy, one upstream signaling protein of especially broad importance is Gαq (Gq), a heterotrimeric G protein to which are coupled the heptahelical, serpentine receptors for multiple cardiac growth factors including angiotensin II, endothelin, serotonin, and α1B-adrenergic agonists (5). Stimulation of signaling pathways via Gq suffices to provoke cardiac hypertrophy in cultured cardiac myocytes and transgenic mouse models (2, 5–7). Conversely, dominant-inhibitory mutations that sequester, deactivate, or compete with Gq indicate that this protein is required at least for α1B-adrenergic signaling (8, 9) and perhaps other forms of hypertrophy (10). Significantly, antagonists of Gq-coupled receptors can promote the regression of left ventricular mass and improve prognosis in heart failure (11, 12). These lines of evidence assert the importance of Gq-dependent cardiac hypertrophy, yet leave unanswered the identity of the essential downstream signaling partner(s).
In cultured cardiac myocytes, activation of G protein-coupled receptors initiates a complex ensemble of mitogen-activated protein kinases (MAPKs; refs. 2, 5, and 13). This superfamily is composed of three overall branches of serine/threonine kinases—extracellular signal-regulated protein kinase (ERK), c-Jun N-terminal kinase (JNK), and p38, along with the upstream activating kinases (MAP2Ks and MAP3Ks)—which collectively mediate signaling for diverse stimuli (14–16). Agonists for Gq-coupled receptors activate all three terminal MAPKs in cultured cardiac muscle cells (2, 5, 13), but it is ambiguous which kinases mediate which components of hypertrophy provoked by Gq, and which upstream kinases, if any, are obligatory in this web. The MAP3K discovered first, apart from Raf, was MAPK/ERK kinase (MEK) kinase-1 (MEKK1; ref. 17), which preferentially activates JNK, more subtly influences ERK, and has little or no effect on p38 (18). Although widespread in expression, MEKK1 is most abundant in heart and spleen (17). By using cardiac myocytes derived from wild-type vs. MEKK1−/− embryonic stem (ES) cells, we previously demonstrated that MEKK1 is essential for activation of JNK by oxidative stress, with little or no contribution to activating other MAPKs in this context (19). MEKK1 also is potentially implicated in the hypertrophic responses of cultured cardiac myocytes (20), especially to agonists for Gq-coupled receptors (21, 22).
The essential downstream effectors for Gq are not known conclusively, however, because the genetic approaches tested thus far are limited to constitutively activated and dominant-negative mutations: both sets of interventions can lack stringent specificity, e.g., by supranormal activation of downstream targets or sequestering a shared protein that is present in limiting amounts. Hence, we have used gene disruption by homologous recombination to provide a more direct and definitive test here for the role of MEKK1. Cardiac myocytes from homozygous-null ES cells were evaluated for initial proof-of-concept studies. In wild-type cardiac muscle, endogenous MEKK1 was activated by heart-specific overexpression of Gq. In MEKK1−/− mice, the absence of this kinase was sufficient to prevent the most cardinal features of hypertrophy induced by Gq—cardiac mass, myocyte enlargement, ANF induction, and JNK activation. Most importantly from a translational perspective, the absence of MEKK1 conferred protection from adverse effects of Gq on cardiac pump function.
Materials and Methods
In Vitro Differentiation of Cardiac Myocytes Derived from Embryonic Stem Cells.
Wild-type and MEKK1−/− ES cells were differentiated into cardiac myocytes and selected as described (19). Experiments were performed 24 h after transfer to serum-free medium.
Mouse Models.
Transgenic FVB/N mice overexpressing wild-type murine Gq in myocardium (αMHC-Gq; 25-copy line; refs. 6 and 7) were mated with MEKK1−/− C57BL/6J mice (23). Southern blot analysis was carried out for MEKK1 (23) and PCR for αMHC-Gq (forward 5′-ATGACAGACAGATCCCTCCTATCTCC-3′, reverse 5′-TCTCGAACCAATTGTGCATG-3′). F1 hemizygous MEKK1 offspring carrying the Gq transgene were bred to MEKK1+/− littermates lacking the transgene to obtain F2 mice with five different genotypes: wild-type, the Gq gain-of-function (αMHC-Gq), the MEKK1 loss-of-function (MEKK1−/−), and the combinatorial genotypes (αMHC-Gq/MEKK1−/−, and αMHC-Gq/MEKK1+/−). Hemizygous MEKK1 mice also result from this mating strategy but were not further analyzed, except where noted. All studies were carried out with age-matched wild-type littermate controls.
Western Blot Analysis.
Total protein was extracted from cultured cells and tissues, 75-μg aliquots were size-fractionated by SDS/PAGE, and proteins were transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH). The membranes were incubated in 5% nonfat milk/0.1% Tween 20/Tris-buffered saline (TBS, 10 mM Tris, pH 7.6/150 mM NaCl) for 1 h at room temperature, followed by primary antibodies in 3% BSA/0.1% Tween 20/TBS overnight at 4°C. Rabbit antibodies to Gq (C-19), MEKK1 (C-22), ERK1 (K-23), or JNK1 (SC-571), and goat antibodies to p38 (SC-S35-G) or sarcomeric plus cytoskeletal actin (SC-1615) were used at dilutions of 1:100–1:1,000 (Santa Cruz Biotechnology). Phosphorylated MAP kinases were detected by using rabbit antibodies against phospho-p44/42 MAP kinase (Thr 202/204), phospho-SAPK/JNK (Thr-183/Tyr-185), or phospho-p38 MAP kinase (Thr-180/Tyr-182) and compared with positive and negative control lysates for each (Cell Signaling Technology, Beverly, MA). Horseradish peroxidase-conjugated, species-specific secondary antibodies were used at 1:2,000 in 3% nonfat milk. Protein expression was visualized with enhanced chemiluminescence reagents (Amersham Pharmacia).
Immune Complex Kinase Assays.
In vitro kinase activity of MEKK1, ERK, JNK, and p38 was measured as described (24, 25), with minor modifications. Cell lysates (400 μg) were immunoprecipitated with primary antibody (2 μg) for 4 h, followed by incubation with Protein G-Sepharose (50% wt/vol, Amersham Pharmacia) for 2 h at 4°C. Gluthatione S-transferase (GST) fusion proteins were used as substrate: GST-SEK1 (Upstate Biotechnology, Lake Placid, NY) for MEKK1, GST-c-Jun (I-79, Santa Cruz Biotechnology) for JNK, GST-activating transcription factor-2 (I-96, Santa Cruz Biotechnology) for p38, and myelin basic protein (amino acids 94–102, SC-3011, Santa Cruz Biotechnology) for ERK. For MEKK1, JNK1, and p38, samples were resolved by SDS/PAGE, and phosphorylated substrates were detected and quantified by using a PhosphorImager (Molecular Probes). For ERK, [32P] incorporation was determined by scintillation counting.
Histology.
Paraffin sections 4–5 μm thick were stained with hematoxylin and eosin. Immunostaining for laminin was performed by using rabbit primary antibody (IMMH-7, Sigma), biotinylated goat anti-rabbit IgG, avidin-conjugated peroxidase, and 3-amino-9-ethylcarbazole in N,N-diethylformamide as the chromagen (Sigma). Myocyte crosssectional area was calculated by using NIH IMAGE v.1.62; ≈100 cells were counted per heart by two blinded observers (n = 3 per genotype).
Echocardiography.
M-mode and Doppler echocardiography were performed as reported (26, 27) (n = 4–7 per genotype).
Gene Expression.
Total RNA was extracted from left ventricular tissue by using RNeasy (Qiagen, Chatsworth, CA), quantified, denatured, and blotted on nitrocellulose filters with a dot-blot filtration manifold (Bio-Rad). RNA dot blots were probed with end-labeled oligonucleotides for mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH), α-MHC, β-MHC, or ANF (6). Hybridization signals were quantified by using a Storm 860 PhosphorImager and IMAGEQUANT software (Molecular Dynamics).
Statistics.
Numerical data are expressed as the means ± SE. Analysis of variance was followed by Scheffé's test, with a significance level of P < 0.05.
Results
Endogenous MEKK1 Is Activated by Gq-Coupled Agonists in ES Cell-Derived Cardiac Myocytes and Is Required for Normal JNK Activity.
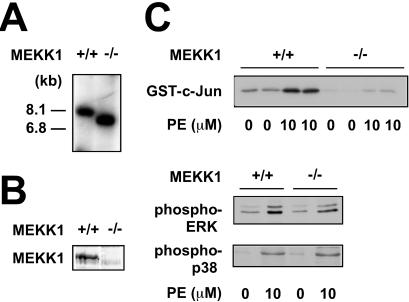
As a first step to exploring the role of MEKK1 in activation of MAPKs via Gq, we compared wild-type and MEKK1−/− ES cell-derived cardiac myocytes, selected by using the α-myosin heavy chain (MHC) promoter to drive the zeocin resistance gene (ref. 19; Fig. 1A). Immunostaining with antibody to sarcomeric MHC and electron microscopy to show sarcomeric structure and intercalated discs confirmed the presence of these properties in cardiac myocytes derived from both backgrounds (19). Wild-type cells expressed MEKK1 protein, whereas MEKK1−/− cells did not (Fig. 1B). Treating wild-type cells for 10 min with the α1-adrenergic agonist phenylephrine (10 μM) caused activation of all three terminal MAPKs, as measured by immune complex kinase assays (JNK) and antibodies specific for the activated kinases (ERK and p38). The absence of MEKK1 markedly blunted JNK activation by phenylephrine, with no effect on ERK or p38 (Fig. 1C). The lack of MEKK1 also impaired JNK activation by angiotensin II, the agonist for a complementary Gq-coupled receptor (not shown). As in other cells lacking MEKK1 (23, 28), baseline JNK activity also was reduced markedly; hence, the residual activation by agonists cannot be estimated with accuracy. Baseline kinase activities are susceptible to multiple stimuli in culture, even in serum-free medium, including endogenous growth factors and changes in cell shape.
Figure 1.
MEKK1 is required in ES cell-derived cardiac myocytes for JNK activation by phenylephrine, a Gq-dependent agonist. (A) Genotype of wild-type and MEKK1−/− ES cell-derived cardiac myocytes by Southern blotting. (B) Western blot analysis confirming the presence of MEKK1 in wild-type ES cell-derived cardiac myocytes and its absence from the MEKK1−/− cells. (C) MEKK1 is required for JNK activation by phenylephrine. In contrast, neither ERK nor p38 activation was affected by the absence of MEKK1.
Endogenous MEKK1 Is Activated by Gq in Mouse Myocardium.
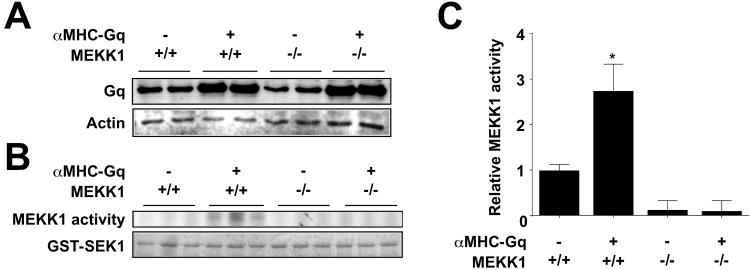
To test whether MEKK1 also would be activated by Gq in the intact heart, we analyzed cardiac-specific Gq mice by Western blot and immune complex kinase assays. As reported (6, 7), Gq protein content increased 4-fold in the myocardium of αMHC-Gq mice (Fig. 2A). The absence of MEKK1 did not affect transgene expression. Cardiac MEKK1 kinase activity increased 3-fold in αMHC-Gq mice compared with littermate controls (Fig. 2B), confirming the prediction that stimulation of the Gq pathway activates endogenous MEKK1 in myocardium. No MEKK1 kinase activity was seen in homozygous-null mice (Fig. 2B).
Figure 2.
Endogenous MEKK1 is activated by Gq in transgenic mouse myocardium. (A) Gq protein expression in myocardium. The increased expression of Gq in αMHC-Gq mice was not affected by deletion of MEKK1 (Gq panel). Total sarcomeric plus cytoskeletal actin is shown for comparison (Actin panel). (B) Cardiac lysates were assessed for MEKK1 activity by an immune complex kinase assay with GST-SEK1 as substrate. Proteins were resolved by SDS/PAGE and phosphorylation was visualized by autoradiography (MEKK1 activity). Total GST-SEK1 in the kinase reactions was shown by Coomassie blue staining (GST-SEK1). Numerical data are compared with wild-type mice as the reference (n = 3 in each group). *, P < 0.05, compared with wild-type mice.
MEKK1 Is Required for Gq-Induced Hypertrophy.
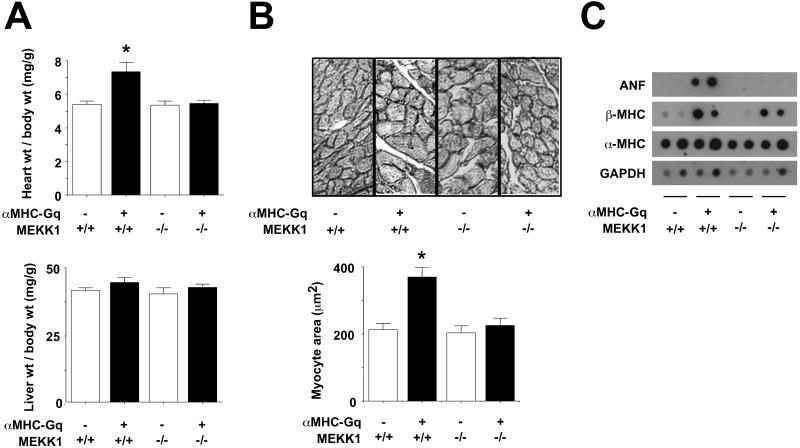
Gq-induced cardiac growth was assessed as both cardiac mass and myocyte size (Fig. 3 A and B). As reported (6), the Gq transgene provoked a 36% increase in the heart weight/body weight ratio at age 12 weeks, compared with nontransgenic littermates (Fig. 3A). MEKK1-null mice did not differ from wild-type controls; thus, MEKK1 is dispensable to achieving normal adult cardiac mass. In contrast, homozygous disruption of MEKK1 prevented all increase in the heart weight/body weight ratio in Gq transgenic mice. No change in body weight or liver weight/body weight occurred among the genotypes.
Figure 3.
MEKK1 is required for cardiac enlargement, myocyte enlargement, and ANF induction by Gq. (A) Heart and liver weight, corrected for body weight (n = 5–8 in each group). (B) Cardiac myocyte area. (Upper) Representative images. (Lower) Means ± SE (n = 3 in each group). (C) Dot blot analysis of ANF, β-MHC, α-MHC, and GAPDH gene expression in myocardium.
Left ventricular myocyte crosssectional area likewise was increased by αMHC-Gq (Fig. 3B) without necrosis, ventricular fibrosis, or myofibrillar disarray (not shown), as reported under these conditions (6). Homozygous disruption of MEKK1 had no effect on basal cardiac myocyte size, yet it blocked the increase evoked by Gq above the nontransgenic control level; thus, only the Gq-mediated increase in myocyte size requires MEKK1. Hemizygosity for MEKK1 did not impede the increase in heart mass or cell size by Gq.
ANF and β-MHC, molecular markers of hypertrophy, were highly induced by the Gq transgene as expected (ref. 6; Fig. 3C). However, ANF transcript levels were no greater in αMHC-Gq/MEKK1−/− mice than in normal ventricular myocardium. Thus, MEKK1 is required for induction of ANF by Gq. Nonetheless, induction of β-MHC by Gq was not affected by the absence of MEKK1. Thus, a subset of Gq effects are independent of this kinase, notwithstanding the requirement for MEKK1 in Gq-induced cardiac growth.
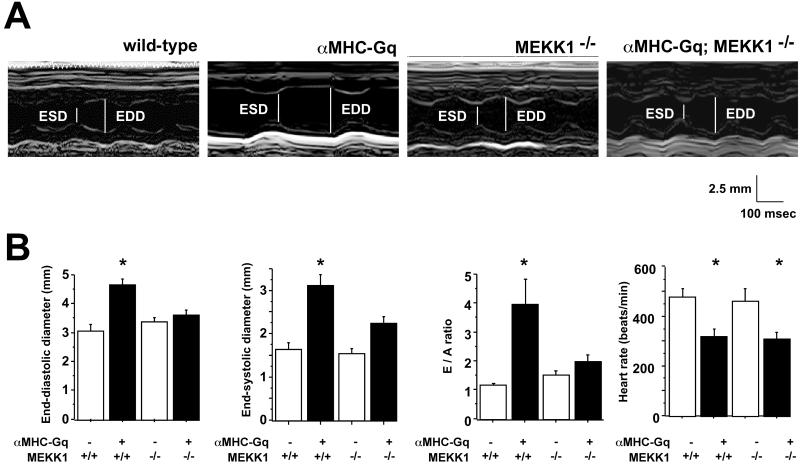
The functional characteristics of αMHC-Gq mice assessed by echocardiography were consistent with prior studies of this model (6). Left ventricular end-diastolic and end-systolic dimensions were increased, whereas fractional shortening, an index of systolic function, and heart rate were decreased (Fig. 4). The peak early-to-late transmitral flow velocity ratio (E/A ratio), an index of impaired diastolic function, also increased in αMHC-Gq mice. The absence of MEKK1 largely rescued the Gq-induced change in end-diastolic diameter, end-systolic diameter, and E/A ratio, despite no effect on baseline function. Because the reduction of heart rate by Gq was not corrected by deletion of MEKK1, the restoration of mechanical function to normal by all three parameters cannot be ascribed to mere rate-related effects.
Figure 4.
MEKK1 is required for Gq-induced mechanical dysfunction. (A) M-mode echocardiography. (B) Means ± SEM, compared with wild-type mice as the reference (n = 4–7 in each group). *, P < 0.05, compared with wild-type mice. ESD, end-systolic dimension; EDD, end-diastolic dimension.
MEKK1 Is Required for Gq-Induced JNK Activation.
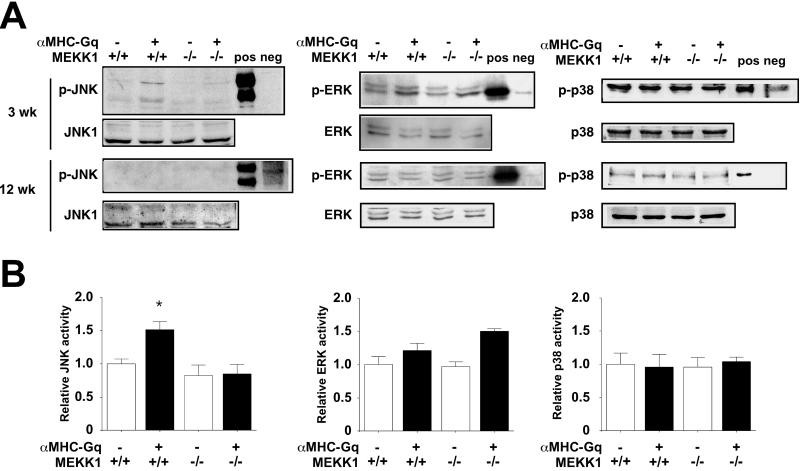
To compare the impact of Gq on MAPK function in myocardium in the presence or absence of MEKK1, Western blot and immune complex kinase assays were performed (Fig. 5). At the age of 3 weeks, JNK activation in αMHC-Gq mice was shown by using both phospho-specific antibodies and kinase activity against a recombinant substrate; levels of JNK expression were unaffected. In contrast, little or no increase was seen in the activation of ERK and p38 by either assay. The absence of MEKK1 specifically blunted the Gq-dependent activation of JNK, with no effect on other terminal MAPKs and no more than a 20% reduction in basal JNK activity. By 12 weeks, no difference was seen among the genotypes in phosphorylation of JNK, ERK, or p38, which is consistent with the lack of MAPK activation by Gq in published work on this model at the latter age only (6, 7).
Figure 5.
MEKK1 is required for Gq-induced JNK activation. (A) Western blot analysis. Cardiac samples from the genotypes shown were assayed at the ages of 3 and 12 weeks with antibodies to ERK, JNK, and p38 vs. the activated, phosphorylated forms of the kinases. Similar results were obtained in three independent experiments. Pos, positive control; neg, negative control. (B) Immune complex kinase assays. Cardiac samples from the genotypes shown were assayed at the age of 3 weeks. Numerical data are compared with wild-type littermate mice as the reference (n = 2–3 in each group). *, P < 0.05, compared with wild-type mice.
Discussion
Here, we have demonstrated that endogenous cardiac MEKK1 is activated by Gq in transgenic mice, and that homozygous disruption of MEKK1 prevents most hypertrophic responses to Gq, including growth, ANF induction, JNK activation, and mechanical dysfunction. Multiple signals suffice for cardiac hypertrophy in cultured myocytes and whole-animal gain-of-function models, and a smaller number have been subjected to functional verification with pharmacological inhibitors or dominant-negative proteins (1–4). Few examples exist, where single-gene deletion can rescue the cardinal features of pathological hypertrophy or heart failure in mice (29); to our knowledge, our findings are the first such proof of an obligatory role for any MAPK in cardiac disease. Notably, this requirement for MEKK1 was confined to the pathophysiological setting: MEKK1-deficient mice are phenotypically normal, apart from defects in eyelid closure (23), with normal cardiac structure and function by all parameters tested so far.
The present study thus reinforces the utility (and potential necessity) of assessing gene function under provocable, disease-related conditions and not merely basal, developmental ones (30). By contrast, MEKK3/MAP3K3, a related member of the MEKK/Ste11 family, is essential for angiogenesis in the embryo and yolk sac, and its absence directly or indirectly impairs cardiac trabeculation (31). Our study also reinforces the usefulness of myocytes formed in culture from null ES cells in linking genes to function in a cardiac context. Like oxidative stress (19), Gq requires endogenous MEKK1 for signaling to JNK specifically; other, more provisional methods imply its direct involvement in signaling to ERK and NF-κB (32). Conversely, ES cell-derived mast cells have been used to incriminate MEKK2/MAP3K2 in signaling to JNK by c-Kit or the IgE receptor, functions in which MEKK1 has no role (33).
Although strain-dependent effects could, in theory, modify responses to Gq or other trophic signals, it is now known that Gq-induced hypertrophy is unimpeded in a C57BL/6J background (9); moreover, we saw no inhibition in hemizygous MEKK1+/− mice. Because cogent technical advantages favor the use of FVB/N mice to make transgenic lines (34), an F2 intercross between parental strains, as used here, is one means to promote reproducibility when genetic complementation is sought involving a second background. Such an approach is most appropriate, where little or no overlap in phenotype exists between the genotypes, as is true in our study. Conversely, even a 10-generation backcross does not ensure absolute isogenicity: a flanking segment derived from the strain 129 ES cells can persist in the vicinity of targeted genes (35).
Gq-induced cardiac hypertrophy is among the best established pathways for pathological cardiac growth, especially given the multiplicity of G protein-coupled receptors that activate Gq and the unequivocal importance of their ligands (2, 5). Forced expression of Gq in cardiac myocytes evokes both the global and selective features of cardiac hypertrophy, including increased cardiac mass, myocyte size, and fetal gene expression (6, 7). The exact molecular mechanisms by which MEKK1 is activated downstream from Gq remain to be proven, despite progress in identifying proximal molecules that impinge upon this MAP3K (14–16). Candidates for which the strongest evidence exists include MAP4Ks (36), but also small G proteins like Ras or Rac and even other heterotrimeric G proteins (37). The potential involvement of protein kinase C (PKC) upstream of MEKK1 also should be noted, as PKC is activated in the αMHC-Gq mice (6) and can couple heterotrimeric G proteins to MEKK1 in other settings (38). At least as a formal possibility, a gain-of-function for Gαq might act via endogenous G protein β and γ subunits. More circuitously, activation of MEKK1 could involve Gq-induced autocrine growth factors or cytokines.
Although reported effectors for MEKK1 also include ERK (via MEK1/2), analysis of both the αMHC-Gq mice and ES cell-derived cardiac myocytes demonstrated here a preferential effect of MEKK1 on JNK. Evidence favoring a role for JNK in cardiac hypertrophy includes the use of dominant-negative mutations for viral gene transfer to cultured myocytes and the intact myocardium (39), as well as gain-of-function studies (40). In addition, JNK may be required for hypertrophic signaling by calcineurin (41), a pivotal mediator of calcium-dependent cardiac growth (4). Not all triggers for hypertrophy, however, necessarily involve the MEKK1-JNK signaling module, as shown by MEK1 signaling to ERK (40) and by transforming growth factor-β-activated kinase-1 (TAK1/MAP3K7) signaling to p38 (26). Compartmentalization within cells, including the selective assembly of MAPK cascades onto a variety of scaffolding proteins, may provide a partial explanation for such specificity (15, 16). The seeming paradox—that two or more independent signals may be needed for hypertrophic growth—could reflect the limitations of current knowledge, in areas distorted by the incomplete specificity of pharmacological inhibitors or dominant-negative proteins. Alternatively, a biological imperative might exist for costimulation of convergent or parallel pathways. At least in the case of Gq, although, loss of MEKK1 alone is sufficient to abrogate hypertrophic growth. This essential function concurs with defects in other stress responses, such as wound healing in adult mice lacking MEKK1 and defective responsiveness to phorbol esters and some growth factors in MEKK1-deficient cells (G.L.J., unpublished results). These properties are unique to MEKK1 and are not seen in mice or cells lacking MEKK2.
Genetic proof that MEKK1 is essential for hypertrophy and mechanical dysfunction induced by Gq suggests the logic of inhibiting this protein kinase in forms of heart disease involving this G protein. Given that MEKK1 activity was augmented by Gq only at the younger age examined, either MEKK1-dependent signals initiated by Gq subsequently function independently of this kinase, or basal MEKK1 activity is essential to maintain the hypertrophic phenotype even though it is dispensable for normal cardiac growth. Conditional disruption of MEKK1 in adult myocardium, using drug-dependent Cre recombinase, could discriminate between these alternatives (42). Most importantly, perhaps, the obligatory role of MEKK1 for this adult stress response could not have been predicted from the scant developmental features of the knockout and highlights the general need for explicit tests of gene function in the context of adult disease.
Acknowledgments
We are grateful to Gerald Dorn for the αMHC-Gq line and helpful discussions, and Bill Boerwinkle, Ivy Callahan, Qian Xiang, and Philip Papst for technical assistance. This work was supported in part by National Institutes of Health grants (to M.D.S. and G.L.J.) the RGK Foundation, and the M. D. Anderson Foundation Professorship (to M.D.S.).
Abbreviations
- ANF
atrial natriuretic factor
- Gq
Gαq
- MAPK
mitogen-activated protein kinases
- ERK
extracellular signal-regulated protein kinase
- JNK
c-Jun N-terminal kinase
- MEK
MAPK/ERK kinase
- MEKK1
MEK kinase-1
- ES
embryonic stem
- GST
glutathione S-transferase
- MHC
myosin heavy chain
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.MacLellan W R, Schneider M D. Annu Rev Physiol. 2000;62:289–320. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- 2.Molkentin J D, Dorn I G., 2nd Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 3.Chien K R. Cell. 1999;98:555–558. doi: 10.1016/s0092-8674(00)80043-4. [DOI] [PubMed] [Google Scholar]
- 4.Frey N, McKinsey T A, Olson E N. Nat Med. 2000;6:1221–1227. doi: 10.1038/81321. [DOI] [PubMed] [Google Scholar]
- 5.Dorn G W, Brown J H. Trends Cardiovasc Med. 1999;9:26–34. doi: 10.1016/s1050-1738(99)00004-3. [DOI] [PubMed] [Google Scholar]
- 6.D'Angelo D D, Sakata Y, Lorenz J N, Boivin G P, Walsh R A, Liggett S B, Dorn G W. Proc Natl Acad Sci USA. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams J W, Sakata Y, Davis M G, Sah V P, Wang Y B, Liggett S B, Chien K R, Brown J H, Dorn G W. Proc Natl Acad Sci USA. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, Lin F, Xu M, Hwa J, Graham R M. EMBO J. 2000;19:4265–4271. doi: 10.1093/emboj/19.16.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers J H, Tsirka A, Kovacs A, Blumer K J, Dorn G W, 2nd, Muslin A J. J Mol Cell Cardiol. 2001;33:209–218. doi: 10.1006/jmcc.2000.1307. [DOI] [PubMed] [Google Scholar]
- 10.Akhter S A, Luttrell L M, Rockman H A, Iaccarino G, Lefkowitz R J, Koch W J. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 11.Sakai S, Miyauchi T, Kobayashi M, Yamaguchi I, Goto K, Sugishita Y. Nature (London) 1996;384:353–355. doi: 10.1038/384353a0. [DOI] [PubMed] [Google Scholar]
- 12.Pitt B, Poole-Wilson P A, Segal R, Martinez F A, Dickstein K, Camm A J, Konstam M A, Riegger G, Klinger G H, Neaton J, et al. Lancet. 2000;355:1582–1587. doi: 10.1016/s0140-6736(00)02213-3. [DOI] [PubMed] [Google Scholar]
- 13.Clerk A, Sugden P H. Circ Res. 2000;86:1019–1023. doi: 10.1161/01.res.86.10.1019. [DOI] [PubMed] [Google Scholar]
- 14.Kyriakis J M, Avruch J. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 15.Chang L, Karin M. Nature (London) 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 16.Whitmarsh A J, Davis R J. Trends Biochem Sci. 1998;23:481–485. doi: 10.1016/s0968-0004(98)01309-7. [DOI] [PubMed] [Google Scholar]
- 17.Lange-Carter C A, Pleiman C M, Gardner A M, Blumer K J, Johnson G L. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 18.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 19.Minamino T, Yujiri T, Papst P J, Chan E D, Johnson G L, Terada N. Proc Natl Acad Sci USA. 1999;96:15127–15132. doi: 10.1073/pnas.96.26.15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogoyevitch M A, Gillespiebrown J, Ketterman A J, Fuller S J, Benlevy R, Ashworth A, Marshall C J, Sugden P H. Circ Res. 1996;79:162–173. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- 21.Thorburn J, Xu S C, Thorburn A. EMBO J. 1997;16:1888–1900. doi: 10.1093/emboj/16.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoki H, Richmond M, Izumo S, Sadoshima J. Biochem J. 2000;347:275–284. [PMC free article] [PubMed] [Google Scholar]
- 23.Yujiri T, Ware M, Widmann C, Oyer R, Russell D, Chan E, Zaitsu Y, Clarke P, Tyler K, Oka Y, et al. Proc Natl Acad Sci USA. 2000;97:7272–7277. doi: 10.1073/pnas.130176697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fanger G R, Johnson N L, Johnson G L. EMBO J. 1997;16:4961–4972. doi: 10.1093/emboj/16.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishizuka T, Kawasome H, Terada N, Takeda K, Gerwins P, Keller G M, Johnson G L, Gelfand E W. J Immunol. 1998;161:3624–3630. [PubMed] [Google Scholar]
- 26.Zhang D, Gaussin V, Taffet G E, Belaguli N S, Yamada M, Schwartz R J, Michael L H, Overbeek P A, Schneider M D. Nat Med. 2000;6:556–563. doi: 10.1038/75037. [DOI] [PubMed] [Google Scholar]
- 27.Oh H, Taffet G E, Youker K A, Entman M L, Overbeek P A, Michael L H, Schneider M D. Proc Natl Acad Sci USA. 2001;98:10308–10313. doi: 10.1073/pnas.191169098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yujiri T, Sather S, Fanger G R, Johnson G L. Science. 1998;282:1911–1914. doi: 10.1126/science.282.5395.1911. [DOI] [PubMed] [Google Scholar]
- 29.Minamisawa S, Hoshijima M, Chu G, Ward C A, Frank K, Gu Y, Martone M E, Wang Y, Ross J, Jr, Kranias E G, et al. Cell. 1999;99:313–322. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- 30.Hirota H, Chen J, Betz U A, Rajewsky K, Gu Y, Ross J, Jr, Muller W, Chien K R. Cell. 1999;97:189–198. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Boerm M, McCarty M, Bucana C, Fidler I J, Zhuang Y, Su B. Nat Genet. 2000;24:309–313. doi: 10.1038/73550. [DOI] [PubMed] [Google Scholar]
- 32.Yujiri T, Fanger G R, Garrington T P, Schlesinger T K, Gibson S, Johnson G L. J Biol Chem. 1999;274:12605–12610. doi: 10.1074/jbc.274.18.12605. [DOI] [PubMed] [Google Scholar]
- 33.Garrington T P, Ishizuka T, Papst P J, Chayama K, Webb S, Yujiri T, Sun W, Sather S, Russell D M, Gibson S B, et al. EMBO J. 2000;19:5387–5395. doi: 10.1093/emboj/19.20.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taketo M, Schroeder A C, Mobraaten L E, Gunning K B, Hanten G, Fox R R, Roderick T H, Stewart C L, Lilly F, Hansen C T, Overbeek P A. Proc Natl Acad Sci USA. 1991;88:2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanagawa O, Xu G, Tevaarwerk A, Vaupel B A. J Immunol. 2000;164:3919–3923. doi: 10.4049/jimmunol.164.7.3919. [DOI] [PubMed] [Google Scholar]
- 36.Su Y C, Han J, Xu S, Cobb M, Skolnik E Y. EMBO J. 1997;16:1279–1290. doi: 10.1093/emboj/16.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berestetskaya Y V, Faure M P, Ichijo H, Voyno-Yasenetskaya T A. J Biol Chem. 1998;273:27816–27823. doi: 10.1074/jbc.273.43.27816. [DOI] [PubMed] [Google Scholar]
- 38.Murasawa S, Matsubara H, Mori Y, Masaki H, Tsutsumi Y, Shibasaki Y, Kitabayashi I, Tanaka Y, Fujiyama S, Koyama Y, et al. J Biol Chem. 2000;275:26856–26563. doi: 10.1074/jbc.M909999199. [DOI] [PubMed] [Google Scholar]
- 39.Choukroun G, Hajjar R, Fry S, del Monte F, Haq S, Guerrero J L, Picard M, Rosenzweig A, Force T. J Clin Invest. 1999;104:391–398. doi: 10.1172/JCI6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bueno O F, De Windt L J, Tymitz K M, Witt S A, Kimball T R, Klevitsky R, Hewett T E, Jones S P, Lefer D J, Peng C F, et al. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Windt L J, Lim H W, Bueno O F, Liang Q, Delling U, Braz J C, Glascock B J, Kimball T F, del Monte F, Hajjar R J, Molkentin J D. Proc Natl Acad Sci USA. 2001;98:3322–3327. doi: 10.1073/pnas.031371998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minamino T, Gaussin V, DeMayo F J, Schneider M D. Circ Res. 2001;88:587–592. doi: 10.1161/01.res.88.6.587. [DOI] [PubMed] [Google Scholar]