Abstract
Alcohol (ethanol), presumably consumed as wine as far back as 7000 BC, is most likely the first addictive substance known to man. In modern days, its abuse leading to neurotoxicity and a myriad of organ damages, is of considerable social and medical concern. In the United States alone, approximately 180,000 people die yearly because of alcohol-related accidents and diseases. Given its ubiquitous nature, alcohol may interact with many cellular components. In this chapter, we specifically concentrate on its neurotoxic mechanisms involving glial cells and their role in neuroinflammation. Moreover, exploitation of this knowledge for potential novel interventions in alcohol-induced neurotoxicity are touched upon.
1. Introduction
Globally, more than 2.6 million die annually because of ethanol (alcohol) consumption, rendering it a worldwide medical and social concern. Abuse of alcohol leads to alcohol use disorder (AUD), defined as a “chronic relapsing brain disease characterized by an impaired ability to stop or control alcohol use despite adverse social, occupational, or health consequences.” AUD afflicts more than 400 million people worldwide (WHO, June 2024), and it is estimated that more than 209 million people live with alcohol dependence, defined as not being able to stop drinking without experiencing withdrawal symptoms.
Alcohol consumption as well as death-related cases vary between different age groups, and between men and women. For example, adolescents are at a greater susceptibility to alcohol’s damaging effects, likely due to neuronal maturation and refinements that peak during adolescence (Getachew et al., 2024). Moreover, adolescent’s early consumption of alcohol enhances their risk of AUD in adulthood (Nixon and McClain, 2010; Crews et al., 2016). Thus, people of younger age (20–39 years) have the highest proportion (13 %) of alcohol-attributable deaths, and men consistently have higher rates of alcohol-related hospitalizations than women. On the other hand, women have a faster progression of AUD, probably due to lower average body weights and less total body water. Women are also at greater risk of alcohol-induced hangovers, liver inflammation and fibrosis, cardiovascular diseases and other organ damages compared to men of comparable weight, as they attain higher blood alcohol concentrations (BACs) and its toxic metabolite acetaldehyde (Gochfeld, 2017).
Women are particularly susceptible to alcohol-induced cancers, especially breast cancer. Indeed, in a large epidemiological study in the United Kingdom, it was found that for every 10 g of alcohol consumed per day, there was a 12 % increase in the risk of breast cancer in women (Allen et al., 2009). Finally, men commit suicide about four times more often than women and are much more likely to have been drinking alcohol when committing suicide (WHO, June 2024). However, the gap between men and women drinking appears to be slowly disappearing, at least in the United States. Indeed, a recent report indicates that adolescents and emerging adult females (18–25-year-old) are more likely to report drinking and getting drunk than their male peers (White, 2020).
Although extensive effort is being expended on elucidating the neurobiological bases of addiction in general, and alcohol in particular, only limited pharmacological tools in combating AUD are available. These include opioid receptor antagonists such as nalmefene or naltrexone that blunt the rewarding effects of alcohol, disulfiram (Antabuse), an aldehyde dehydrogenase inhibitor that induces aversion, and acamprosate, a synthetic gamma amino butyric acid (GABA) analog with functional antagonism of the glutamate N-methyl-d-aspartate (NMDA) receptor. For life-threatening withdrawal symptoms, benzodiazepine followed by behavioral cognitive therapy are applied (Airagnes et al., 2019).
In addition to the fact that the efficacy of these medications is not optimal, the treatment rate is rather low in that about one in six people globally receives treatment, with even lower rates in lower-middle-income countries (Mekonen et al., 2020). A further consideration in this regard is not only the damage during intoxication, e.g., from falls and other accidents but also damage due to poor diet and vitamin deficiencies that could lead to Wernicke-Korsakoff syndrome (WKS), characterized by changes in the vision, ataxia and impaired memory (Nutt et al., 2021). Thus, there is an urgent need for providing more efficacious treatments. In this regard, understanding the neurotoxic mechanisms of alcohol may provide better preventive and/or interventions in alcohol-induced neuronal damage. Here, we provide an up-to-date information on the role of glial cells in acute and chronic alcohol-induced neurotoxicity and potential novel interventions.
2. Alcohol
We use the term alcohol interchangeably with ethanol, an alcohol with chemical composition (C2H5OH), which has also been used for disinfection and sterilization, as well as managing the toxicities of methanol (CH3OH), and ethylene glycol (C2H6O2), both of which are metabolized by the same enzyme, alcohol dehydrogenase (ADH), which also metabolizes ethanol. Metabolism of methanol leads to formation of the neurotoxic substances such as formaldehyde and formic acid, and metabolism of ethylene glycol leads to formation of toxic substances such as glycolic and oxalic acids. Because the affinity of ethanol for ADH is much higher than the other two alcohols, its administration spares their metabolism and hence their toxicities (Le Daré et al., 2019; Mathew and Goyal, 2021).
3. Binge vs heavy drinking
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) defines binge drinking as a pattern of drinking alcohol that brings blood alcohol concentration (BAC) to 0.08 %—or 0.08 g of alcohol per deciliter—or more. This can be brought about in men if they have 5 dinks or more, and in women if they have 4 drinks or more in 2 h. In adolescent boys and girls this BAC may be achieved with less number of drinks. A drink is defined as a beverage containing approximately 14 g of alcohol, which can be equivalent to 12 ounces of beer, 5 ounces of wine or 1.5 ounces of spirits such as vodka, whiskey, etc.
Heavy drinking is defined as having five or more episodes of binge drinking in the past month. Heavy drinking increases one’s risk for longterm alcohol-related health problems and developing AUD. High-intensity drinking, which peaks around age 21, is defined as alcohol intake at levels twice the threshold for binge drinking, meaning 8 or more drinks for women and 10 or more drinks for men on one occasion. This pattern of drinking, most common among young adults attending college, is associated with an even greater risk of detrimental consequences. Moreover, repeated episodes of binge drinking during adolescence, a unique phase in brain development, can not only affect brain maturity but also result in lingering behavioral deficits (e.g., social interaction), as well as in cognitive functions including learning, memory, and attention (Crews et al., 2016; Hauser et al., 2023; Getachew et al., 2024).
Acute intoxication is influenced by the volume and percentage of alcohol, the interval over which it is ingested, weight, size, body fat percentage, sex of the individual as well as the level of tolerance of the individual. The latter, also referred to as tachyphylaxis may develop rapidly and can play a critical role in alcohol addiction (Haass-Koffler and Perciballi, 2020; Elvig et al., 2021). Thus, alcohol effects are dose dependent. At lower BAC (e.g., 0.08 %), impairments in judgment and attention; at moderate concentration (e.g., 0.1–0.3 %), slurred speech, ataxia, and nystagmus (uncontrollable eye movements); and at higher concentration (e.g., above 0.4 %), stupor, coma, and respiratory depression leading to death, may occur.
Complications associated with chronic alcohol abuse may include sleep disturbance, hypoglycemia, electrolyte disturbance, hypotension, acute or chronic myopathy, peripheral polyneuropathy, and WKS, characterized by ophthalmoplegia, ataxia, confusion, amnesia and confabulation. It is suspected that some alcohol-related neurological effects may be due to nutritional deficiencies such as thiamine (Fernandes et al., 2017).
Heavy alcohol use may not only increase the risk of ischemic and hemorrhagic stroke, but also that of Marchiafava-Bignami disease, a rare but fatal degenerative disorder (Fernandes et al., 2017; Xu et al., 2019; Nehring and Freeman, 2020).
4. Neurotoxicity
Neurotoxic effects of alcohol, whether induced by binge or chronic intake, involves several brain regions and numerous neurotransmitters. Elucidation of the molecular bases of these effects can lead to novel interventions.
5. Tolerance
Tolerance, defined as a reduction in the effects of alcohol due to previous exposure, may be considered a neuroadaptive response initially. However, because it leads to the escalation of drinking and dependence, it can precipitate neurotoxicity and/or AUD (Koob, 2021). Several types of tolerance to alcohol have been described. For example, dispositional or metabolic tolerance characterized by faster metabolism of the alcohol; acute tolerance or acute functional tolerance which may occur within a single session of alcohol exposure and is influenced by family and drinking history; and chronic tolerance which is associated with an increase in alcohol metabolism (i.e., pharmacokinetic tolerance); as well as pharmacodynamic tolerance which may involve receptor desensitization. It is believed that a number of neurotransmitter systems including cholinergic, glutamate/nitric oxide, GABA, opioids, dopamine (DA), norepinephrine, serotonin, adenosine, cannabinoids, and peptides such as vasopressin, neuropeptide Y, neurosteroids, and protein kinase C, all of which modulate tolerance, and the “intoxicating” effects of alcohol, play a role (Nunes et al., 2019; Elvig et al., 2021). Recent studies in invertebrate models show that tolerance to alcohol involves neuroadaptive changes in the neurotransmitter systems, ion channels, and synaptic proteins. Moreover, it has been hypothesized that changes in neuronal excitability are an adaptive response to ethanol (Bhandari et al., 2024).
It is also noteworthy that in general men tolerate alcohol better than women, likely due to less water content in women and hence less chance of diluting alcohol and therefore greater concentration reaching the tissues. In addition, alcohol history, liver function, use of other substances or drugs, or other comorbid conditions may affect the individual’s tolerance to alcohol. On the other hand, people with enzyme capacities to faster convert alcohol to acetaldehyde (e.g., higher ADH activity) and slower capacity to metabolize acetaldehyde (e.g., lower acetaldehyde dehydrogenase) would exhibit a much lower tolerance to alcohol and hence exhibit a reduced chance of excessive drinking and developing AUD (Wilson and Matschinsky, 2020; Elvig et al., 2021).
6. Brain regions
It is believed that the effects of alcohol on cerebellum is responsible for the myriads of motor impairments including general weakness, ataxia, dysarthria (motor speech disorder), and nystagmus (involuntary, rhythmic eye movements) (Dolbec et al., 2020). On the other hand, alcohol’s effects on prefrontal cortex and limbic system, is believed to be responsible for impaired executive function, addictive properties, and negative emotions including anxiety (Vetreno and Crews, 2014; Reglodi et al., 2019). Alcohol also affects the lateral orbitofrontal cortex, essential for flexible decision-making (Gioia and Woodward, 2021).
7. Mechanisms
Although alcohol does not appear to have any specific receptor, a number of neurotransmitter systems and cellular mechanisms mediate its varied effects including its neurotoxicity. Here, following a brief discussion of the neurotransmitter and receptor systems, and involvement of neuroplasticity and neurogenesis, we concentrate on the role of glial cells in relation to neuroinflammatory responses resulting in alcohol neurotoxicity.
8. Neurotransmitters and receptors
Alcohol’s interactions with both ionotropic and metabotropic glutamate receptors, the nicotinic cholinergic receptors, as well as with DAergic system are believed to play a critical role in its rewarding and addictive properties (Meyerhof et al., 2006; Kalejaiye et al., 2013; Wu et al., 2014; Rahman, 2015; Alasmari et al., 2018; Johnson and Lovinger, 2020; Miller and Kamens, 2020). On the other hand, its interaction with serotonergic receptors and corticotropin-releasing factor (CRF) are suspected to be related to alcohol-induced anxiety and depression (Forster et al., 2018; Pleil and Skelly, 2018). Alcohol’s interaction with GABA may have a pivotal role in the modulation of sleep and developmental plasticity (Granato and Dering, 2018).
Although several studies ascribe the neurotoxic properties of drugs of abuse including alcohol to the opioid sigma receptors and cannabinoid receptors (Rousseaux and Greene, 2016; Sabino et al., 2017; Kunos, 2020; García-Baos et al., 2021), glutamatergic hyperactivation and overstimulation of the NMDA receptors are believed to be primarily responsible for the excitotoxicity. Thus, neurodegeneration may be brought about by accumulation of cytosolic calcium, and oxidative/nitrosative stress due to excess glutamate release. It is suggested that chronic alcohol consumption causes astrocyte damage and hence impairs the ability of these glial cells to regulate extracellular glutamate concentration, leading to hyperglutamatergic excitotoxicity (Lovinger, 1993; Collins and Neafsey, 2016; Kamal et al., 2020). It is of relevance to note that glutamate excitotoxicity has also been implicated in neurodegenerative diseases such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) (Kamal et al., 2020; Egunlusi and Joubert, 2024).
Given the extensive involvement of many neurotransmitters in AUD, it is not surprising that several receptor systems may provide viable interventional targets. Indeed, potential exploitation of both muscarinic and nicotinic cholinergic receptors, both metabotropic and ionotropic glutamate receptors, GABA, opioid, cannabinoid, as well as tyrosine kinase receptors in AUD was recently reviewed (Tizabi et al., 2021). Specifically, it was shown that ketamine, an NMDA receptor antagonist, differentially attenuated alcohol intake in male versus female Alcohol Preferring rats (Rezvani et al., 2017), and that both ketamine and NBQX, an AMPA/kainate receptor antagonist reduced alcohol drinking in male Wistar rats (Ruda-Kucerova et al., 2018). Moreover, administration of GluN2B-containing NMDAR antagonist reduced the neuronal loss and the behavioral effects following alcohol withdrawal in adult mice (Gakare et al. 2022). These findings together with ample evidence implicating NMDA receptors in excitotoxicity, suggest that NMDA receptors could be a promising target in countering alcohol-induced neurotoxicity (Carles et al., 2024; Egunlusi and Joubert, 2024).
9. Neurotrophic factors
Neurotrophic factors (NFs) are varied protein molecules that play a key role in mediating all aspects of neuronal functions including their development, maintenance, plasticity, neurogenesis, and survival at various stages of life (Bothwell, 2016; Ledda and Paratcha, 2016; Vilar and Mira, 2016; Ibanez and Andressoo, 2017; Fursa et al., 2024; Hernández-Del Caño et al., 2024). The two most studied factors are brain-derived NF (BDNF), and glial-derived NF (GDNF), where the association of the former with neurodegenerative and neuropsychiatric conditions such as PD and major depressive disorder (MDD) is well documented (Tizabi et al., 2024). For example, BDNF and its receptor TrkB have been implicated in mood disorders due to their regulation of synaptic plasticity (Manji et al., 2003; Caviedes et al., 2017). It is postulated that alcohol-induced impairments in cognitive behavior, sleep, and mood regulation are due to reduction of BDNF and elevation of inflammatory markers such as interleukin (IL)–1β and tumor necrosis factor (TNF)-α in discrete brain regions. Curiously, neuroinflammation precipitated by alcohol can further impair neuroplasticity and neurogenesis and hence contribute to more drinking (Hurley and Tizabi, 2013; Crews et al., 2017; de Timary et al., 2017). Thus, a vicious cycle where alcohol-induced reduction in BDNF results in neuroinflammation which reduces BDNF ensues. This inverse relationship between inflammation and neuroplasticity can therefore result in symptom manifestation following alcohol toxicity.
10. Neurogenesis
Neurogenesis is a rather complex neurobiological process whereby new neurons are generated from neural stem cells (NSCs) or neural progenitor cells (NPCs). This occurs primarily in the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus, where the destination of the latter is mainly the striatum (Borsini et al., 2015). Although approximately 700 new neurons are generated daily in adult human hippocampus, they fall short of repairing the damaged ones brought about by toxicants, infection, injury, etc. The reason for the limited capacity to replace the damaged neurons is that pathological perturbations via activation of microglia for example, cause the release of pro-inflammatory cytokines that not only can lead to neuronal death but can also hinder the neurogenesis process (Das and Basu, 2008; Shigemoto-Mogami et al., 2014; Borsini et al., 2015).
Regarding alcohol’s effect on neurogenesis, it is estimated that alcohol exposure can reduce neurogenesis in the hippocampus by almost 40 % (Anderson et al., 2012; Geil et al., 2014). It is noteworthy that adult neurogenesis is comprised of four distinct components (e.g., cell proliferation, differentiation, migration, and survival), any one of which or their combination can be affected by alcohol (Geil et al., 2014). Moreover, using rodent models, it was shown that alcohol reduces proliferation and neurogenesis in the hippocampus through apoptosis and that chronic binge alcohol consumption increases neural degeneration months after abstinence via necrosis. In sum, intoxication may lead to neurodegeneration via several mechanisms including increased oxidative stress and release of proinflammatory proteins that are neurotoxic (Crews and Nixon, 2009; Geil et al., 2014). On the other hand, abstinence after binge ethanol intoxication may lead to normalization of brain function via enhanced neurogenesis (Crews and Nixon, 2009; Geil et al., 2014).
11. Other mechanisms
It is noteworthy that many other mechanisms may contribute to alcohol-induced neurotoxicity. These include perturbations of the hypothalamic–pituitary-adrenal (HPA) axis, mitochondrial metabolism leading to reactive oxygen species (ROS) and oxidative stress, endothelial disruption leading to nitric oxide and neuroinflammation, disruption of cellular bioenergetics, lipid membrane impairment, induction of edema and heat shock proteins, enhanced apoptosis, autophagy dysregulation, gene and epigenetic dysregulation, disturbance in the gut microbiota (dysbiosis) and the gut-brain axis, among others (Geil et al., 2014; Collins, 2015; Kondela et al., 2017; Tajuddin et al., 2018; Kouzoukas et al., 2019; Nakayama and Hasegawa, 2022; Gervasi and Mandalari, 2023; McMahan et al., 2023; Crews et al., 2024). However, here we mainly concentrate on neuroinflammation and the role of glial cells as this may be a common denominator among at least several mechanisms mentioned above (Guerri and Pascual, 2019).
12. Neuroinflammation
As mentioned above, alcohol-induced neuroinflammation, due to increase in release of cytokines such as IL-1β and TNF-α, and decreased neurogenesis, may enhance alcohol-drinking behavior and alcohol-induced neurotoxicity (Hurley and Tizabi, 2013; Crews et al., 2017; de Timary et al., 2017). Mechanisms by which alcohol induces cytokine release and neuroinflammation have been extensively investigated. It is apparent now that alcohol’s interaction with glial cells in general, and microglia in particular, is responsible for neuroinflammation. Thus, here following a brief description of various glial cells, we delve into alcohol’s effect on each type. Various stages of alcohol-induced neurotoxicity with emphasis on neuroinflammation is depicted in Fig. 1.
Fig. 1.
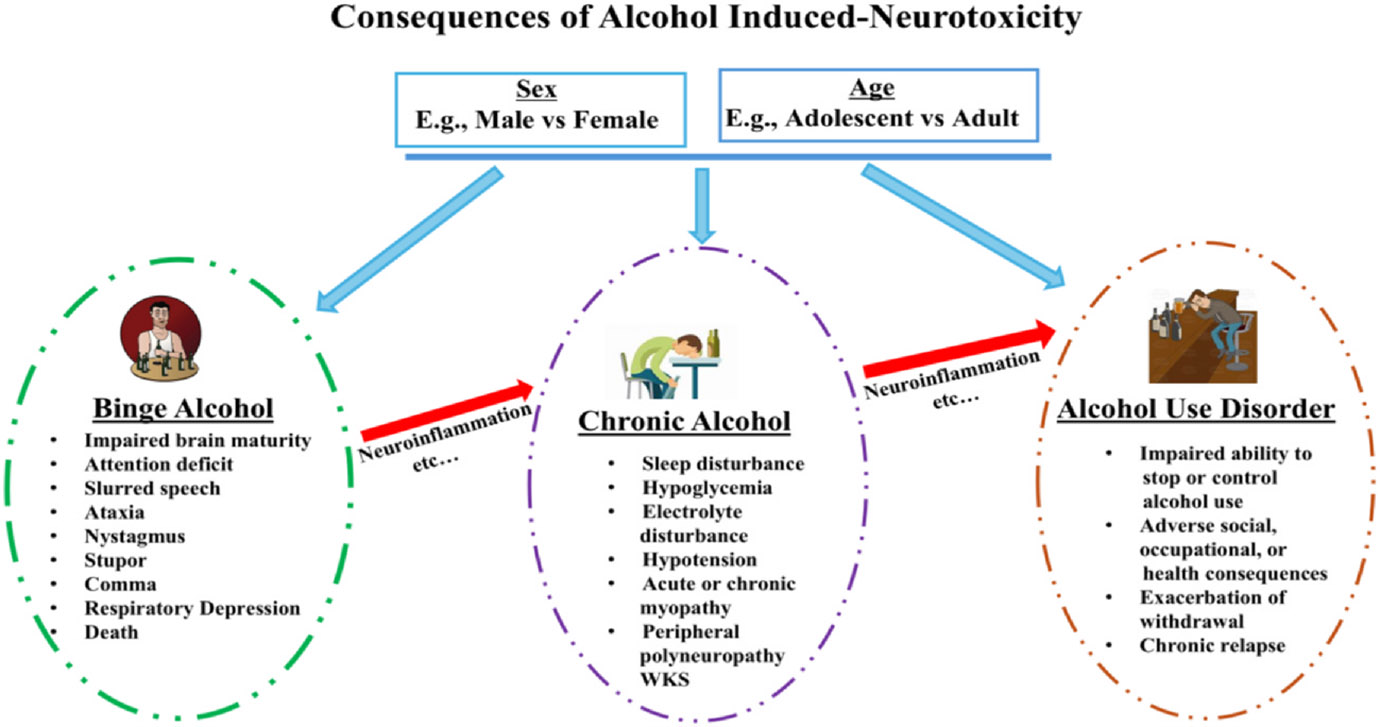
Schematic diagram depicting the consequences of heavy alcohol use either as binge or chronic on motor, nonmotor and cognitive functions. The neurotoxic effects are underscored by inflammation and are influenced by sex and age at every stage. WKS = Wernicke-Korsakoff Syndrome.
13. Glial cells
The name “glia” derived from the Greek word “glue” was ascribed to these cells because it was thought that they provide the scaffolding or the support system to keep the neurons together. It is now well established that glial cells are much more than “glue” with diverse and vital functions (Souza et al., 2019; Bonvento and Bolaños, 2021; Kim and Picciotto, 2023) These include regulation of neurotransmitters (Allen and Eroglu, 2017; Andersen and Schousboe, 2023; Novikov et al., 2023), control of metabolism and energetics (Kim and Picciotto, 2023; Ebling and Lewis, 2018; Chamberlain et al., 2021), fluid and electrolyte homeostasis (Reed and Blazer-Yost, 2022), myelination (Sanchez-Petidier et al., 2022; Wies Mancini et al., 2024), formation of the blood-brain barrier (BBB) (Manu et al., 2023; Fernandes et al., 2024), development and remodeling of synapses (Lalo et al., 2021; Rasia-Filho et al., 2023), neuroendocrine function (Clayton et al., 2022), innate immunity response (Kofler and Wiley, 2011; Chen and Holtzman, 2022), as well as detoxification (Dringen et al., 2015; Ioannou et al., 2019).
14. Relevance to alcohol-induced neurotoxicity
Although glia-mediated brain damage by alcohol is likely multifactorial, consisting of direct actions of alcohol or its metabolites on the glial cells or indirectly via hormonal, hepatic and hemodynamic factors (Miguel-Hidalgo, 2018), it is now well documented that alcohol can significantly influence the function as well as the survival of the glial cells, and induce neurotoxicity (Sushma et al., 2023). It is noteworthy that glial cells play a crucial role in protecting the neurons from the toxic effects of alcohol. However, with exposure to chronic alcohol, uncontrollable levels of ROS may be produced leading to neurotoxicity (Sushma et al., 2023). For example, by producing ROS, alcohol can damage the mitochondria and activate the endoplasmic reticulum (ER) stress pathway, which can lead to cellular dysfunction and/or cell death (Lin et al., 2008; Chen et al., 2023; Sushma et al., 2023). Brain’s unique susceptibility to ROS is believed to be due to several factors including its high oxygen demand, relatively low concentration of the antioxidant enzymes, and poly unsaturated fatty acids (PUFAs) (Sushma et al., 2023). Moreover, alcohol-induced alteration of glial cells in utero has been implicated in the pathophysiology of fetal alcohol spectrum disorder (FASD) (Wilhelm and Guizzetti, 2016; Popova et al., 2023).
15. Microglia
Microglia, representing 10–15 % of all the central nervous system (CNS) cells, are called the resident immune cells of the CNS, as through rapid movements of their fine tenacles called filopodia, they constantly survey the environment to provide rapid protection to any insult (Jäkel and Dimou, 2017; Nebeling et al., 2023). They accomplish this by rapid change in their morphology. Hence, by eliminating pathogens and cell residues, microglia play a key role in maintaining brain homeostasis. Microglia are also involved in neurogenesis, formation and elimination of synapses, and mediating T-cell infiltration into the brain (Pathak and Sriram, 2023). Interestingly, their concentration is particularly high in the hippocampus and the substantia nigra. On the other hand, overactivation of microglia can cause neuroinflammation and subsequent damage or death of neurons leading to neurodegenerative and/or neuropsychiatric conditions (Saitgareeva et al., 2020; Costa et al., 2021; de Marchi et al., 2023; Gao et al., 2023). Microglia can become overactivated due to persistent stress which causes the release of pro-inflammatory cytokines such as IL-1β and IL-6, and induction of a vicious cycle where microglia themselves further produce pro-inflammatory cytokines (Stein et al., 2017; Schramm and Waisman, 2022; Chen et al., 2024). Previously, based on the markers found on their cell surface, microglia were categorized into two main phenotypes: M1 or neurotoxic; and M2 or neuroprotective. However, use of these terminologies is no longer recommended because the same markers may appear in both M1 and M2 phenotype (Wang et al., 2023). Therefore, depending on the environment, microglia can be either protective or harmful.
16. Relevance to alcohol-induced neurotoxicity
Chronic consumption of alcohol can result in neuroinflammation and neuronal damage. Thus, alcohol increases the expression of proinflammatory cytokines such as TNFα, IL-1β, and chemokine CCL2, and microglial activation. Recently, it was suggested that consumption of chronic alcohol by recruitment of peripheral macrophages into the CNS, alters microglia through the CCR2/5 axis. Hence, manipulation of the CCR2/5 axis my offer a therapeutic potential for the treatment of alcohol-associated neuroinflammation (Lowe et al., 2020).
Regarding the differential reaction of adolescent vs adult to either binge or chronic alcohol, the available evidence indicates that adolescents tend to be more vulnerable to conditioned aversion, dopaminergic transmission in reward-related regions, neurogenesis, neurodegeneration, and are more likely to be impacted during adulthood (Crews et al., 2016; Hauser et al., 2023; Getachew et al., 2024). On the other hand, adolescents may be more resilient to motor-impairing, sedative effects, or some cognitive outcomes of alcohol (Weiland et al., 2012; Cousijn et al., 2018; Kuhns et al., 2022). However, in some respects, alcohol may similarly affect adolescent and adults as evidenced by reduced number of microglia in several brain areas including the hippocampus of both adolescent and adult rats (Marshall et al., 2020).
In another study it was revealed that microglia might not be the primary effector cell responsible for regulation of acute and voluntary alcohol behaviors. However, in a model of repeated immune activation using polyinosinic:polycytidylic acid (poly(I:C)) to activate microglia, it was shown that microglia depletion blocked poly(I:C)-induced escalations in alcohol intake, indicating that microglia regulate drinking behaviors with sufficient immune activation (Warden et al., 2021).
Chronic alcohol consumption also induces changes in microglial and neuroimmune-related gene expressions as seen in both murine and human brains after chronic alcohol consumption (Kapoor et al., 2019). Importantly, in a male rat model of four days binge alcohol exposure, it was found that both pro-inflammatory and antiinflammatory microglia are present in the brain, suggesting that anti-inflammatory microglia are likely activated to mitigate the pro-inflammatory response to alcohol (Peng et al., 2017). Furthermore, depletion of microglia by the colony stimulating factor 1 receptor inhibitor, PLX5622, inhibited expression of pro-inflammatory genes and enhanced expression of anti-inflammatory genes in a mouse model of acute binge alcohol withdrawal (Walter and Crews, 2017). Interestingly, similar findings were reported in a human study where microglia activity in the basal ganglia of humans was shown to be altered in AUD and reversed with remission from alcohol (Rasool et al., 2024).
Although it is still unsettled whether activated microglia are key contributor to disease pathogenesis or merely a consequence of exposure to chronic alcohol consumption (Portis and Haass-Koffler, 2020), a recent study does suggest that manipulation of microglia, specifically in the putamen, globus pallidus, and ventral pallidum may offer novel targets for AUD treatment (Rasool et al., 2024).
17. Astroglia (astrocytes)
Astrocytes or astroglia, named because of their star-like shape, are the most abundant cells in the brain with key functional roles in maintaining neuronal integrity. Recent findings indicate a heterogeneity in astrocyte physiology and gene expression (Kim et al., 2022). Astrocytes play a vital function in the CNS by a variety of mechanisms including providing nutrients and removing waste from the neurons, modulating blood flow, maintaining pH homeostasis, regulating the glymphatic network (the fluid dynamics within the CNS), buffering extracellular ions, particularly calcium, releasing gliotransmitters, recycling neurotransmitters, detoxifying ammonia, regulating synaptic plasticity, participating in synaptogenesis and neurogenesis, and being a key component of the blood-brain barrier (BBB) (Stoklund Dittlau and Freude, 2024; Verkhratsky and Nedergaard, 2018; Kim et al., 2022; Purushotham and Buskila, 2023; Das et al., 2024; Ngoc et al., 2024). As such, astrocytes are implicated in a variety of behavioral measurements including cognitive functions (Albini et al., 2023; Soares et al., 2024), and AUD as discussed below.
18. Relevance to alcohol-induced neurotoxicity
Like microglia, astrocytes also play a key role in regulating the brain’s response to alcohol. Indeed, astrocytes are considered critical regulators of alcohol-intake, and that alcohol effect on astrocytes has crucial downstream consequences on neurotransmission and neuronal health (Adermark and Bowers, 2016). Also similar to microglia, astrocytes may impart a beneficial or detrimental effect depending on the nature of the stimulus, (Jiwaji and Hardingham, 2022; Edison, 2024). For example, disruption of their trophic support to neurons may lead to neurological conditions (Rodrigues-Amorim et al., 2018). On the other hand, their role in maintaining glutamate homeostasis by uptake of glutamate from the synaptic cleft, is critical in protecting the neurons from excitotoxicity (Mahmoud et al., 2019). This process is regulated by an astrocyte-rich manganese (Mn)-dependent enzyme, namely glutamine synthetase (GS), that catalyzes the conversion of the glutamate into glutamine in the astrocytes. Glutamine is then released from the astrocytes and taken up by neurons where it is converted back to glutamate. Thus, if astrocytes fail to adequately regulate the glutamate uptake, excitotoxicity will ensue (Sidoryk-Wegrzynowicz and Aschner, 2013; Melkumyan et al., 2022; Qian et al., 2023). Additionally, it is known that GABAergic neurons synthesize GABA from glutamate and that astrocytes can also synthesize and release GABA. Thus, astrocytes play a crucial role in regulating GABA homeostasis and synaptic neurotransmission as well. Moreover, astrocytic GABA dysregulation has been implicated in a variety of psychiatric disorders, including AUD (Ali et al., 2024).
Astrocytes in different brain areas appear to be mediating the varied effects of alcohol. For example, cortical astrocytes have been shown to regulate ethanol consumption and intoxication in mice (Erickson et al. 2021), whereas astrocytes in the lateral central amygdala may be related to alcohol-induced anxiety and/or increases in proinflammatory cytokine in this area (Melkumyan et al., 2022; Brewton et al., 2023).
Recently, combining calcium signaling measurements and gene expression, alcohol-specific astrocyte subtypes were identified. Thus, various alcohol doses increased intracellular calcium levels in a subset of astrocytes only (Kim et al., 2022). Moreover, effects of alcohol on astrocytes appear to be sex dependent. Thus, GFAP expression is reduced in the extended amygdala of male, but not female mice following chronic or binge drinking (Brewton et al., 2023).
Finally, the well documented bidirectional communication between astrocytes and microglia in inflammatory responses are likely to contribute to glial response to various doses of alcohol (Kim et al., 2022; Bhusal et al., 2023; Brewton et al., 2023; Chen et al., 2023; Xiong et al., 2023).
19. Role of astrocytes in hepatic encephalopathy
Hepatic encephalopathy (HE), a neurological complication resulting from loss of hepatic function and accumulation of ammonia in the brain is characterized by brain edema (swelling), and behavioral changes including confusion, forgetfulness, personality or mood changes, poor concentration and judgment, and sleep disturbance (Hamdani et al., 2021). Glial cells, particularly astrocytes, play a significant role in this condition as they are primarily responsible for detoxifying ammonia (Jaeger et al., 2019; Claeys et al., 2021).
Chronic alcohol via damage to the liver, may further impair the liver’s ability to metabolize ammonia, resulting in hyperammonemia, consequently higher ammonia levels in the brain. Since astrocytes are the most important ammonia-metabolizing cells in the brain, and alcohol can directly damage astrocytes, this can lead to further buildup of ammonia. The higher the ammonia levels, the more inhibition of the transport of glutamine from the astrocytes to neurons and further disruption of normal neurotransmission. Moreover, because of a build-up of glutamine and ammonia in the astrocytes, they can accumulate water and swell, leading to increased pressure in the brain (Hamdani et al., 2021; Claeys et al., 2021).
In addition, there is an interplay between hyperammonemia and microglia that can lead to neuroinflammation and hence further exacerbate HE development. To gain an insight into the cellular perturbations driving HE pathology, cell-type specific transcriptomic changes were investigated in an animal model of HE. It was revealed that astrocytes exhibited increased corticoid receptor and oxidative stress signaling, whereas microglial transcriptome changes were linked to immune cell attraction, thus the suggestion that glial astrocytes and microglia may offer novel targets for development of novel therapeutic strategies in HE (Claeys et al., 2023).
20. Oligodendrocytes
Oligodendrocytes (OLs), constituting approximately 75 % of all glial cells in certain brain areas, are the major source of myelination in the CNS (Michalski and Kothary, 2015). OLs, not only provide the myelin sheath which is critical in insulating axons and facilitate conduction, but they also provide metabolic and trophic supply, regulate extracellular potassium concentration, and modulate axonal growth via their GDNF and BDNF (Michalski and Kothary, 2015; Han et al., 2022; Zhou and Zhang, 2023). It is noteworthy that white matter, a network of nerve fibers in the brain and spinal cord, gets its color from myelin and that “White Matter Disease” referring to damaged white matter, is characterized by memory, balance and mobility impairments which can be brought about by alcohol and is usually due to reduced blood supply as might also occur during aging (McEvoy et al., 2018; Sorond and Gorelick, 2019; Hoffmann and Miron, 2024). Moreover, it was recently suggested that CNS macrophages, consisting of CNS-resident microglia and cells associated with CNS border regions (e.g., the meninges, vasculature, and choroid plexus), as well as macrophages derived from infiltrating monocytes from the blood, influence the development, maintenance, and regeneration of myelin, and hence further understanding of these interactions could provide novel therapies in White Matter Diseases (Hoffmann and Miron, 2024).
21. Relevance to alcohol-induced neurotoxicity
Although it is well known that alcohol consumption affects oligodendrocytes and the white matter, few studies have attempted to elucidate the mechanism and more importantly determine a dose-response relationship. Whereas most studies have focused on FASD and severe AUD (Marguet et al., 2022; Bazzi et al., 2023), a recent study in mice evaluated transcriptomic changes in mature oligodendrocytes following exposure to moderate and high concentrations of alcohol (Bazzi et al., 2023). It was concluded that alcohol concentration-dependently induced transcriptomic changes in oligodendrocytes with critical downstream impacts on myelin production. Hence, it was suggested that elucidation of changes including changes in cell cycle regulation and inflammation following alcohol exposure, may provide novel targets for modulating myelin production or inhibition (Bazzi et al., 2023).
Alterations in oligodendrocytes as well as astrocytes have been observed in the prefrontal cortex (PFC) of postmortem human subjects with chronic alcohol abuse or dependence, which may explain changes detected by neuroimaging techniques in subjects with AUD (Miguel-Hidalgo, 2018). Importantly, significant interaction or crosstalk between astrocytes and oligodendrocytes exists (Nutma et al., 2020). This communication which is important for glial development, regeneration and repair, may occur directly via direct cell-cell contact or via secreted cytokines, chemokines, exosomes, and signaling molecules. Given the critical role of this communication in maintaining homeostasis, it is not surprising that a failure or miscommunication in this crosstalk can result in CNS diseases (Nutma et al., 2020). Additionally, AUD can affect the expression of astrocyte and myelin proteins and of oligodendrocyte transcription factors. Furthermore, epigenetic modifications and alterations in regulatory microRNAs (miRNAs) may also be occurring that could have profound effects on gene expression and protein translation in these glial cells (Nutma et al., 2020). It was also observed that chronic exposure to alcohol can inhibit myelinogenesis in the adult mouse brain, contributing to alcohol-related cognitive impairments (Guo et al., 2021).
More recently it was reported that adolescent alcohol exposure significantly affected myelin formation, and mature OLs in the medial-PFC of a mouse model with age- and subregional-specificity (Huang et al., 2024). Thus, AUD may be associated with connectivity disturbances between the PFC and other brain regions accompanied by alterations in gap-junction proteins, glutamate transporters or enzymes related to glutamate and GABA metabolism (Nutma et al., 2020; Guo et al., 2021; Huang et al., 2024).
22. Synantocytes (NG2 cells)
Synantocytes also called NG2 cells due to their expression of the protein “NG2” (nerve/glia antigen 2, chondroitin sulfate proteoglycan), which is a marker used to identify this cell population, may be considered the fourth type of glial cells in the CNS (Butt et al., 2005). The term “synantocyte” derived from Greek word refers to their unique characteristic of making numerous contacts with neurons (Butt et al., 2005). NG2 cells also considered as OL progenitor cells (OPCs) are widely distributed throughout the brain, continue to proliferate in adult brain (Hill and Nishiyama, 2014; Somkuwar et al., 2014; Michalski and Kothary, 2015; Kirdajova and Anderova, 2020), and can also be transformed into astrocytes and neurons (Hill and Nishiyama, 2014; Michalski and Kothary, 2015; Kirdajova and Anderova, 2020). NG2 cells have been implicated in many neurodegenerative diseases including AD, multiple sclerosis (MS), glioma, experimental autoimmune encephalomyelitis (EAE), a disease associated with increased BBB permeability, traumatic brain injury, epilepsy, acute ischemic stroke, neurovascular unit formation during development, neuroinflammation, as well as in neuropsychiatric diseases (Vélez-Fort et al., 2009; Xu et al., 2011; Dimou and Gallo, 2015; Ferrara et al., 2016; Nakano et al., 2017; Yu et al., 2022; Zhang et al., 2019; Hu et al., 2023; Poggi et al., 2023; Timmermann et al., 2023). These cells maintain a crosstalk with microglia and astrocytes (Zhang et al., 2022), and by inhibiting microglia-to-neuron prostaglandin E2 signaling may also provide protection against prion neurotoxicity (Liu et al., 2024).
23. Relevance to alcohol-induced neurotoxicity
Alcohol exposure can negatively affect the development, morphology, and gene expression of NG2 cells and be toxic to their survival. In contrast, abstinence from alcohol can increase NG2 differentiation, remyelination and lead to brain growth. Alcohol inhibition of myelinogenesis can contribute to cognitive impairments. Thus, regular heavy drinking can cause brain cells to die and brain tissue to shrink. Moreover, NG2 cells may also contribute to addictive component of alcohol (Somkuwar et al., 2014; Guo et al., 2021).
It was recently reported that NG2 cells form synaptic complexes with hippocampal interneurons and that selective photostimulation of NG2 cells can stimulate GABA release and enhance inhibitory synaptic transmission, triggering anxiety-like behavior (Zhang et al., 2021). Moreover, this action involved glutamic acid decarboxylase 67 (GAD67) and vesicle-associated membrane protein 2 (VAMP-2), involved in GABA synthesis, and in the fusion of synaptic vesicles and the release of neurotransmitter, respectively (Zhang et al., 2021; Agarwal et al., 2024). Whether the same mechanism might involve alcohol’s effect is yet to be determined.
24. Ependymal cells
Ependymal cells, derived from radial glial cells in the embryo, are neuroepithelial, nonmotile, multiciliated cells at birth before they mature to motile multiciliated cells during the first two postnatal weeks (Deng et al., 2023). Initially, these cells project short and randomly oriented cilia into the ventricles, but during maturation, as the cilia length increases, they start beating and orienting in the same direction (Deng et al., 2023). Ependymal cells form the epithelial lining of the central canal of the spinal cord and the ventricles, and as glial cells in the CNS, play key roles in brain metabolism, waste clearance, and production and flow of cerebrospinal fluid (CSF). Specifically, these cells form critical components of the blood-CSF barrier and the CSF-brain barrier, which provide protection against the entry of foreign substances into the CSF (Aschner and Philbert, 2010; Nelles and Hazrati, 2022; Deng et al., 2023; Groh et al., 2024).
Three distinct types of ependymal cilia, type-I, type-II and type-III based upon their beating frequency, their beating angle, and their distinct localization within the mouse brain (lateral ventricle) have been identified (Omran et al., 2017; Deng et al., 2023). Additionally, they have a prominent role in energy metabolism due to containing a large number of mitochondria and have been implicated in several neurological conditions such as spinal cord injury and hydrocephalus (Nelles and Hazrati, 2022; Deng et al., 2023). Indeed, their potential manipulation as therapeutic intervention in these conditions has been suggested (Deng et al., 2023).
25. Relevance to alcohol-induced neurotoxicity
Alcohol has been shown to act as a risk factor in the impairment of the motility of ependymal cilia in the brain (Omran et al., 2017). Specifically, it was reported that oral gavage of alcohol decreased the beating frequency of all three types of ependymal cilia in both the third and the lateral ventricles of rats (Omran et al., 2017). Moreover, chronic alcohol use can impair ependymal cilia mobility in the ventricles and disrupt the flow of CSF in the brain. This can lead to alcohol-associated encephalopathy or as described above, WKS, which occurs in around 1–2 % of the population in the United States. and is most often seen in males, and patients of lower socioeconomic status (Patel et al., 2023).
Since alcohol exposure may cause hydrocephalus, a recent study was conducted to determine the effect of vaporized nasal alcohol exposure on the choroid plexus, and ependymal cells. It was observed that rats exposed to vaporized nasal alcohol had degeneration of choroid plexus and ependymal cells with ciliopathy and enlarged lateral ventricles or hydrocephalus (Kanat et al. 2024). The authors contended that because alcohol is often used for hygiene and specifically for prevention of transmission of the Sars-Cov-2-virus, the results could have human implication (Kanat et al., 2024).
26. Therapeutic implications and future directions
Neurotoxicity of alcohol may involve a myriad of cellular and molecular mechanisms, many of which have been amply reviewed (Tizabi et al., 2021; LaHood and Kok, 2023). Moreover, novel interventions based on our current understanding of the subject have been proposed (Tizabi et al., 2021; LaHood and Kok, 2023). Here, we concentrated on the role of various glial cells in alcohol-induced neurotoxicity (Fig. 2). Since alcohol’s detrimental effects could involve any of these glial cells, which have also been implicated in a variety of neuropsychiatric/neurodegenerative disorders (Soares et al., 2024; Tizabi et al., 2024), further elucidation of their structure and function could provide novel targets in mitigating alcohol-induced neurotoxicity as well as potential co-morbid conditions.
Fig. 2.
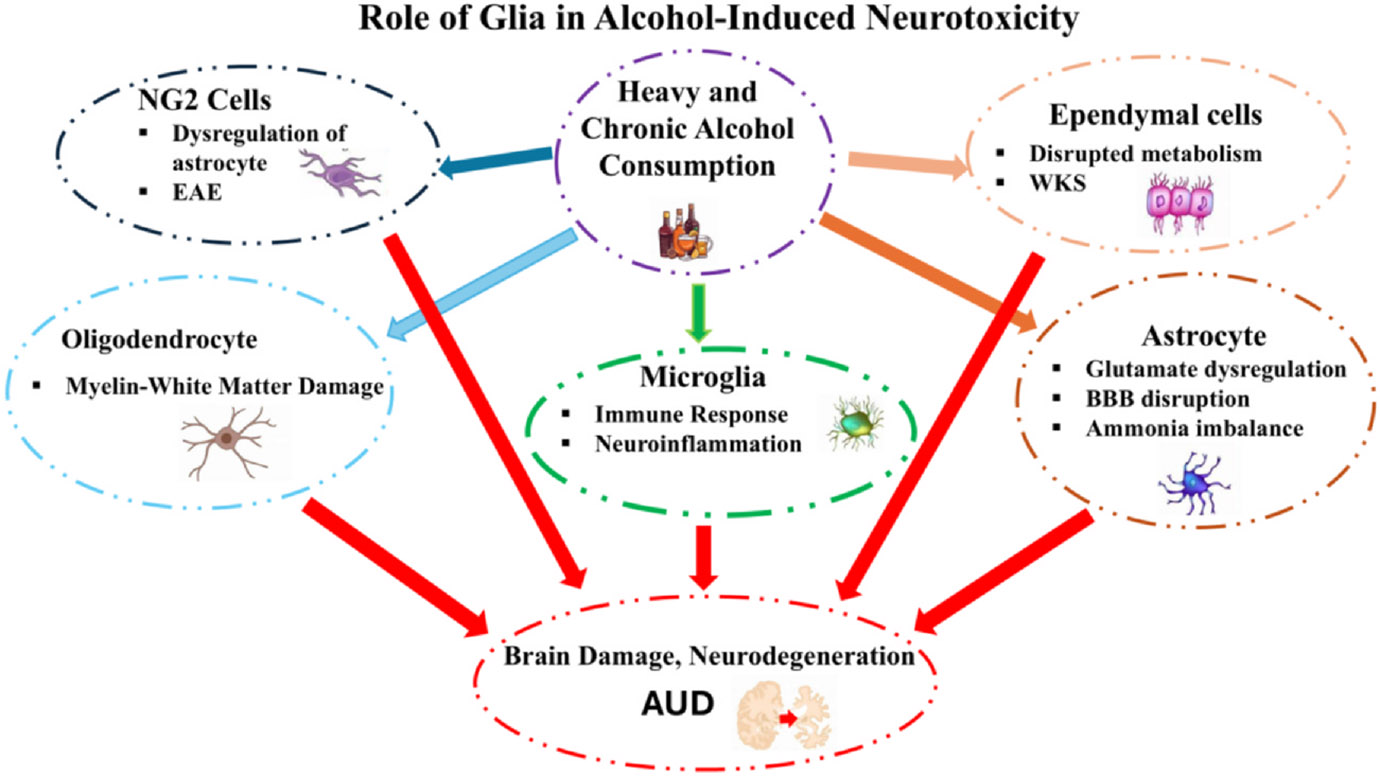
Schematic diagram depicting the role of various glial cells on alcohol-induced neurotoxicity. Specific effects of alcohol on each glial cell type are highlighted. Not shown are the extensive interactions between the glial cells. AUD = alcohol use disorder, BBB = blood-brain barrier, EAE = experimental autoimmune encephalomyelitis, WKS = Wernicke-Korsakoff Syndrome.
Acknowledgment
YT was supported in part by NIH/NIAAA R03AA022479; BG was supported by Toffler foundation; MAC was supported in part by NIAAA U01AA018279; MA was supported in part by NIEHS R01ES10563 and R01ES07331. Dedicated to George Koob.
Abbreviations
- ADH
alcohol dehydrogenase.
- AD
Alzheimer’s disease.
- AUD
Alcohol-Use Disorder.
- BAC
blood alcohol concentration.
- BBB
blood-brain barrier.
- BDNF
brain-derived neurotrophic factors.
- CNS
central nervous system.
- CREB
cAMP response element-binding protein.
- CRF
corticotropin-releasing factor.
- CSF
cerebrospinal fluid.
- DG
dentate gyrus.
- EAE
experimental autoimmune encephalomyelitis.
- ER
endoplasmic reticulum.
- FASD
fetal alcohol spectrum disorder.
- GABA
gamma aminobutyric acid.
- GAD67
glutamic acid decarboxylase 67 glial-derived neurotrophic factor.
- GPCRs
G protein-coupled receptors.
- HE
hepatic encephalopathy.
- HPA
hypothalamic–pituitary-adrenal.
- IL
interleukin.
- MDD
major depressive disorder.
- miRNA
microRNA.
- MS
multiple sclerosis.
- NBQX
2,3-dioxo-6-nitro-7-sulfamoyl-benzo[f]quinoxaline.
- NF
neurotrophic factor.
- NIAAA
National Institute on Alcohol Abuse and Alcoholism.
- NMDA
N-methyl-d-aspartate.
- NPC
neural progenitor cell.
- NSC
neural stem cell.
- OL
oligodendrocyte.
- OPC
oligodendrocyte progenitor cell.
- PARP-1
poly (ADP-ribose) polymerase-1.
- PD
Parkinson’s disease.
- PFC
prefrontal cortex.
- PUFA
poly unsaturated fatty acid.
- ROS
reactive oxygen species.
- SGZ
subgranular zone.
- SVZ
subventricular zone.
- TNF-α
tumor necrosis factor.
- VAMP-2
vesicle-associated membrane protein 2.
- WHO
world health organization.
- WKS
Wernicke-Korsakoff syndrome.
References
- Adermark L, Bowers MS, 2016. Disentangling the role of astrocytes in alcohol use disorder. Alcohol. Clin. Exp. Res 40 (9), 1802–1816. 10.1111/acer.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Chandran A, Raza F, Ungureanu IM, Hilcenko C, Stott K, et al. , 2024. VAMP2 regulates phase separation of α-synuclein. Nat. Cell Biol 26 (8), 1296–1308. 10.1038/s41556-024-01451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airagnes G, Ducoutumany G, Laffy-Beaufils B, et al. , 2019. Alcohol withdrawal syndrome management: is there anything new? Rev. Med. Interne 40 (6), 373–379. 10.1016/j.revmed.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Alasmari F, Goodwani S, McCullumsmith RE, Sari Y, 2018. Role of glutamatergic system and mesocorticolimbic circuits in alcohol dependence. Prog. Neurobiol 171, 32–49. 10.1016/j.pneurobio.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini M, Krawczun-Rygmaczewska A, Cesca F, 2023. Astrocytes and brain-derived neurotrophic factor (BDNF). Neurosci. Res 197, 42–51. 10.1016/j.neures.2023.02.001. [DOI] [PubMed] [Google Scholar]
- Ali DN, Ali HM, Lopez MR, Kang S, Choi DS, 2024. Astrocytic GABAergic regulation in alcohol use and major depressive disorders. Cells 13 (4), 318. 10.3390/cells13040318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NE, Beral V, Casabonne D, et al. , 2009. on behalf of the Million Women Study Collaborators. Moderate alcohol intake and cancer incidence in women. J. Natl Cancer Inst 101 (5), 296–305. 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- Allen NJ, Eroglu C, 2017. Cell biology of astrocyte-synapse interactions. Neuron 96, 697–708. 10.1016/j.neuron.2017.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JV, Schousboe A, 2023. Glial glutamine homeostasis in health and disease. Neurochem. Res 48 (4), 1100–1128. 10.1007/s11064-022-03771-1. [DOI] [PubMed] [Google Scholar]
- Anderson ML, Nokia MS, Govindaraju KP, Shors TJ, 2012. Moderate drinking? Alcohol consumption significantly decreases neurogenesis in the adult hippocampus. Neuroscience 224, 202–209. 10.1016/j.neuroscience.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Philbert A, 2010. 13.12 – Glial cells. In: McQueen CA (Ed.), Comprehensive Toxicology, second ed. Elsevier, pp. 199–219. 10.1016/B978-0-08-046884-6.01313-0. [DOI] [Google Scholar]
- Bazzi SA, Maguire C, Mayfield RD, Melamed E, 2023. Alcohol induces concentration-dependent transcriptomic changes in oligodendrocytes. 2023.09.22.559075. bioRxiv [Prepr.] 10.1101/2023.09.22.559075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari A, Seguin A, Rothenfluh A, 2024. Synaptic mechanisms of ethanol tolerance and neuroplasticity: insights from invertebrate models. Int. J. Mol. Sci 25 (13), 6838. 10.3390/ijms25136838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhusal A, Afridi R, Lee WH, Suk K, 2023. Bidirectional communication between microglia and astrocytes in neuroinflammation. Curr. Neuropharmacol 21 (10), 2020–2029. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvento G, Bolaños JP, 2021. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab. 33 (8), 1546–1564. 10.1016/j.cmet.2021.07.006. [DOI] [PubMed] [Google Scholar]
- Borsini A, Zunszain PA, Thuret S, Pariante CM, 2015. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 38 (3), 145–157. 10.1016/j.tins.2014.12.006.(E). [DOI] [PubMed] [Google Scholar]
- Bothwell M., 2016. Recent advances in understanding neurotrophin signaling. F1000 Faculty Rev. F1000Research 5, 1885. 10.12688/f1000research.8434.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewton HW, Robinson SL, Thiele TE, 2023. Astrocyte expression in the extended amygdala of C57BL/6J mice is sex-dependently affected by chronic intermittent and binge-like ethanol exposure. Alcohol 108, 55–64. 10.1016/j.alcohol.2022.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M, 2005. Synantocytes: the fifth element. J. Anat 207 (6), 695–706. 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carles A, Freyssin A, Perin-Dureau F, Rubinstenn G, Maurice T, 2024. Targeting N-methyl-d-aspartate receptors in neurodegenerative diseases. Int. J. Mol. Sci 25 (7), 3733. 10.3390/ijms25073733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviedes A, Lafourcade C, Soto C, Wyneken U, 2017. BDNF/NF-κB signaling in the neurobiology of depression. Curr. Pharm. Des 23 (21), 3154–3163. 10.2174/1381612823666170111141915. [DOI] [PubMed] [Google Scholar]
- Chamberlain KA, Huang N, Xie Y, et al. , 2021. Oligodendrocytes enhance axonal energy metabolism by deacetylation of mitochondrial proteins through transcellular delivery of SIRT2. Neuron 109 (21), 3456–3472.e8. 10.1016/j.neuron.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Holtzman DM, 2022. Emerging roles of innate and adaptive immunity in Alzheimer’s disease. Immunity 55 (12), 2236–2254. 10.1016/j.immuni.2022.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Lou Q, Song XJ, et al. , 2024. Microglia govern the extinction of acute stress-induced anxiety-like behaviors in male mice. Nat. Commun 15 (1), 449. 10.1038/s41467-024-44704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shi C, He M, et al. , 2023. Endoplasmic reticulum stress: molecular mechanism and therapeutic targets. Sig. Transduct. Target. Ther 8, 352. 10.1038/s41392-023-01570-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeys W, Van Hoecke L, Lernout H, et al. , 2023. Experimental hepatic encephalopathy causes early but sustained glial transcriptional changes. J. Neuroinflammation 20, 130. 10.1186/s12974-023-02814-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeys W, Van Hoecke L, Lefere S, Geerts A, Verhelst X, Van Vlierberghe H, et al. , 2021. The neurogliovascular unit in hepatic encephalopathy. JHEP Rep. 3 (5), 100352. 10.1016/j.jhepr.2021.100352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton RW, Lovell-Badge R, Galichet C, 2022. The properties and functions of glial cell types of the hypothalamic median eminence. Front. Endocrinol 13. 10.3389/fendo.2022.953995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, 2015. Alcohol abuse and docosahexaenoic acid: effects on cerebral circulation and neurosurvival. Brain Circ (1), 63–68. 10.1007/s12640-012-9360-5. [DOI] [Google Scholar]
- Collins MA, Neafsey EJ, 2016. Alcohol, excitotoxicity and adult brain damage: an experimentally unproven chain-of-events. Front. Mol. Neurosci 9, 8. 10.3389/fnmol.2016.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa T, Fernandez-Villalba E, Izura V, et al. , 2021. Combined 1-deoxynojirimycin and ibuprofen treatment decreases microglial activation, phagocytosis and dopaminergic degeneration in MPTP-treated mice. J. Neuroimmune Pharmacol 16 (2), 390–402. 10.1007/s11481-020-09925-8. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Luijten M, Feldstein Ewing SW, 2018. Adolescent resilience to addiction: a social plasticity hypothesis. Lancet Child. Adolesc. Health 2 (1), 69–78. 10.1016/S2352-4642(17)30148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ, Coleman LG Jr., 2017. The role of neuroimmune signaling in alcoholism. Neuropharmacology 122, 56–73. 10.1016/j.neuropharm.2017.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Macht V, Vetreno RP, 2024. Epigenetic regulation of microglia and neurons by proinflammatory signaling following adolescent intermittent ethanol (AIE) exposure and in human AUD. Adv. Drug. Alcohol. Res 4, 12094. 10.3389/adar.2024.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K, 2009. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol. Alcohol 44 (2), 115–127. 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA, Robinson DL, 2016. Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharmacol. Rev 68 (4), 1074–1109. 10.1124/pr.115.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Basu A, 2008. Inflammation: a new candidate in modulating adult neurogenesis. J. Neurosci. Res 86 (6), 1199–1208. 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- Das N, Dhamija R, Sarkar S, 2024. The role of astrocytes in the glymphatic network: a narrative review. Metab. Brain Dis 39 (3), 453–465. 10.1007/s11011-023-01327-y. [DOI] [PubMed] [Google Scholar]
- de Marchi F, Munitic I, Vidatic L, et al. , 2023. Overlapping neuroimmune mechanisms and therapeutic targets in neurodegenerative disorders. Biomedicines 11 (10), 2793. 10.3390/biomedicines11102793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Timary P, Stärkel P, Delzenne NM, et al. , 2017. A role for the peripheral immune system in the development of alcohol use disorders? Neuropharmacology 122, 148–160. 10.1016/j.neuropharm.2017.04.013. [DOI] [PubMed] [Google Scholar]
- Deng S, Gan L, Liu C, Xu T, Zhou S, Guo Y, et al. , 2023. Roles of ependymal cells in the physiology and pathology of the central nervous system. Aging Dis. 14 (2), 468–483. 10.14336/AD.2022.0826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Gallo V, 2015. NG2-glia and their functions in the central nervous system. Glia 63 (8), 1429–1451. 10.1002/glia.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolbec K, Dobbs MR, Ibraheem M, 2020. Toxin-induced cerebellar disorders. Neurol. Clin 38 (4), 843–852. 10.1016/j.ncl.2020.07.003. [DOI] [PubMed] [Google Scholar]
- Dringen R, Brandmann M, Hohnholt MC, Blumrich EM, 2015. Glutathione-dependent detoxification processes in astrocytes. Neurochem. Res 40 (12), 2570–2582. 10.1007/s11064-014-1481-1. [DOI] [PubMed] [Google Scholar]
- Ebling FJP, Lewis JE, 2018. Tanycytes and hypothalamic control of energy metabolism. Glia 66 (6), 1176–1184. 10.1002/glia.23303. [DOI] [PubMed] [Google Scholar]
- Edison P, 2024. Astroglial activation: current concepts and future directions. Alzheimers Dement. 20 (4), 3034–3053. 10.1002/alz.13678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egunlusi AO, Joubert J, 2024. NMDA receptor antagonists: emerging insights into molecular mechanisms and clinical applications in neurological disorders. Pharm. (Basel) 17 (5), 639. 10.3390/ph17050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvig SK, McGinn MA, Smith C, et al. , 2021. Tolerance to alcohol: a critical yet understudied factor in alcohol addiction. Pharmacol. Biochem. Behav 204, 173155. 10.1016/j.pbb.2021.173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson EK, DaCosta AJ, Mason SC, et al. , 2021. Cortical astrocytes regulate ethanol consumption and intoxication in mice. Neuropsychopharmacol 46, 500–508. 10.1038/s41386-020-0721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes VM, Auld V, Klämbt C, 2024. Glia as functional barriers and signaling intermediaries. Cold Spring Harb. Perspect. Biol 16 (1), a041423. 10.1101/cshperspect.a041423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes LMP, Bezerra FR, Monteiro MC, et al. , 2017. Thiamine deficiency, oxidative metabolic pathways and ethanol-induced neurotoxicity: how poor nutrition contributes to the alcoholic syndrome, as marchiafava-bignami disease. Eur. J. Clin. Nutr 71 (5), 580–586. 10.1038/ejcn.2016.267. [DOI] [PubMed] [Google Scholar]
- Ferrara G, Errede M, Girolamo F, Morando S, Ivaldi F, Panini N, et al. , 2016. NG2, a common denominator for neuroinflammation, blood–brain barrier alteration, and oligodendrocyte precursor response in EAE, plays a role in dendritic cell activation. Acta Neuropathologica 132 (1), 23–42. 10.1007/s00401-016-1563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster GL, Anderson EM, Scholl J, et al. , 2018. Negative consequences of early-life adversity on substance use as mediated by corticotropin-releasing factor modulation of serotonin activity. Neurobiol. Stress 9, 29–39. 10.1016/j.ynstr.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fursa GA, Andretsova SS, Shishkina VS, Voronova AD, Karsuntseva EK, Chadin AV, et al. , 2024. The use of neurotrophic factors as a promising strategy for the treatment of neurodegenerative diseases (review). Bull. Exp. Biol. Med 177 (4), 517–527. 10.1007/s10517-024-06218-5. [DOI] [PubMed] [Google Scholar]
- Gakare SG, Varghese SS, Patni PP, Wagh SA, Ugale RR, 2022. Prevention of glutamate excitotoxicity in lateral habenula alleviates ethanol withdrawal-induced somatic and behavioral effects in ethanol dependent mice. Behav. Brain Res 416, 113557. 10.1016/j.bbr.2021.113557. [DOI] [PubMed] [Google Scholar]
- Gao C, Jiang J, Tan Y, Chen S, 2023. Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets. Signal. Transduct. Target. Ther 8 (1), 359. 10.1038/s41392-023-01588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Baos A, Alegre-Zurano L, Cantacorps L, et al. , 2021. Role of cannabinoids in alcohol-induced neuroinflammation. Prog. Neuropsychopharmacol. Biol. Psychiatry 104, 110054. 10.1016/j.pnpbp.2020.110054. [DOI] [PubMed] [Google Scholar]
- Geil CR, Hayes DM, McClain JA, Liput DJ, Marshall SA, Chen KY, et al. , 2014. Alcohol and adult hippocampal neurogenesis: promiscuous drug, wanton effects. Prog. Neuropsychopharmacol. Biol. Psychiatry 54, 103–113. 10.1016/j.pnpbp.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi T, Mandalari G, 2023. The interplay between gut microbiota and central nervous system. Curr. Pharm. Des 29 (41), 3274–3281. 10.2174/0113816128264312231101110307. [DOI] [PubMed] [Google Scholar]
- Getachew B, Hauser SR, Bennani S, El Kouhen N, Sari Y, Tizabi Y, 2024. Adolescent alcohol drinking interaction with the gut microbiome: implications for adult alcohol use disorder. Adv. Drug. Alcohol. Res 4, 11881. 10.3389/adar.2024.11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia DA, Woodward JJ, 2021. Altered activity of lateral orbitofrontal cortex neurons in mice following chronic intermittent ethanol exposure. eNeuro 8 (2). 10.1523/ENEURO.0503-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochfeld M., 2017. Sex differences in human and animal toxicology. Toxicol. Pathol 45 (1), 172–189. 10.1177/0192623316677327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato A, Dering B, 2018. Alcohol and the developing brain: why neurons die and how survivors change. Int. J. Mol. Sci 19 (10), 2992. 10.3390/ijms19102992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh AMR, Song YL, Tea F, et al. , 2024. Multiciliated ependymal cells: an update on biology and pathology in the adult brain. Acta Neuropathol. 148, 39. 10.1007/s00401-024-02784-0. [DOI] [PubMed] [Google Scholar]
- Guerri C, Pascual M, 2019. Role of neuroinflammation in ethanol neurotoxicity. In: Aschner M, Costa LG (Eds.), Adv. Neurotoxicology, vol. 3. Academic Press, pp. 259–294. ISSN 2468-7480, ISBN 9780128157176. [Google Scholar]
- Guo F, Zhang YF, Liu K, Huang X, Li RX, Wang SY, et al. , 2021. Chronic exposure to alcohol inhibits new myelin generation in adult mouse brain. Front. Cell Neurosci 15, 732602. 10.3389/fncel.2021.732602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Perciballi R, 2020. Alcohol tolerance in human laboratory studies for development of medications to treat alcohol use disorder. Alcohol. Alcohol 55 (2), 129–135. 10.1093/alcalc/agz103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdani EH, Popek M, Frontczak-Baniewicz M, Utheim TP, Albrecht J, Zielińska M, et al. , 2021. Perturbation of astroglial Slc38 glutamine transporters by NH4+ contributes to neurophysiologic manifestations in acute liver failure. FASEB J. 35 (7), e21588. 10.1096/fj.202001712RR. [DOI] [PubMed] [Google Scholar]
- Han S, Gim Y, Jang EH, Hur EM, 2022. Functions and dysfunctions of oligodendrocytes in neurodegenerative diseases. Front. Cell. Neurosci 16, 1083159. 10.3389/fncel.2022.1083159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Waeiss RA, Deehan GA Jr, Engleman EA, Bell RL, Rodd ZA, 2023. Adolescent alcohol and nicotine exposure alters the adult response to alcohol use. Adv. Drug. Alcohol. Res 3, 11880. 10.3389/adar.2023.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Del Caño C, Varela-Andrés N, Cebrián-León A, Deogracias R, 2024. Neurotrophins and their receptors: BDNF’s role in GABAergic neurodevelopment and disease. Int. J. Mol. Sci 25 (15), 8312. 10.3390/ijms25158312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Nishiyama A, 2014. NG2 cells (polydendrocytes): listeners to the neural network with diverse properties. Glia 62 (8), 1195–1210. 10.1002/glia.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Miron VE, 2024. CNS macrophage contributions to myelin health. Immunol. Rev 10.1111/imr.13416. [DOI] [PubMed] [Google Scholar]
- Hu X, Geng P, Zhao X, et al. , 2023. The NG2-glia is a potential target to maintain the integrity of neurovascular unit after acute ischemic stroke. Neurobiol. Dis 180, 106076. 10.1016/j.nbd.2023.106076. [DOI] [PubMed] [Google Scholar]
- Huang D, Li M, Qiao Z, Zhou H, Cai Y, Li X, et al. , 2024. Effects of adolescent alcohol exposure on oligodendrocyte lineage cells and myelination in mice: age and subregion differences. IBRO Neurosci. Rep 17, 220–234. 10.1016/j.ibneur.2024.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LL, Tizabi Y, 2013. Neuroinflammation, neurodegeneration, and depression. Neurotox. Res 23 (2), 131–144. 10.1007/s12640-012-9348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou MS, Jackson J, Sheu SH, et al. , 2019. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell 177 (6), 1522–1535.e14. 10.1016/j.cell.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Jaeger V, DeMorrow S, McMillin M, 2019. The direct contribution of astrocytes and microglia to the pathogenesis of hepatic encephalopathy. J. Clin. Transl. Hepatol 7 (4), 352–361. 10.14218/JCTH.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäkel S, Dimou L, 2017. Glial cells and their function in the adult brain: a journey through the history of their ablation. Front. Cell. Neurosci 11. 10.3389/fncel.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiwaji Z, Hardingham GE, 2022. Good, bad, and neglectful: astrocyte changes in neurodegenerative disease. Free. Radic. Biol. Med 182, 93–99. 10.1016/j.freeradbiomed.2022.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Lovinger DM, 2020. Allosteric modulation of metabotropic glutamate receptors in alcohol use disorder: insights from preclinical investigations. Adv. Pharmacol 88, 193–232. 10.1016/bs.apha.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalejaiye O, Bhatti BH, Taylor RE, et al. , 2013. Nicotine blocks the depressogenic effects of alcohol: implications for drinking-smoking co-morbidity. J. Drug. Alcohol. Res 2, 235709. 10.4303/jdar/235709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal H, Tan GC, Ibrahim SF, Shaikh MF, Mohamed IN, Mohamed RMP, Hamid AA, Ugusman A, Kumar J, 2020. Alcohol use disorder, neurodegeneration, Alzheimer’s and Parkinson’s disease: interplay between oxidative stress, neuroimmune response and excitotoxicity. Front. Cell Neurosci 14, 282. 10.3389/fncel.2020.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanat A, Aydin MD, Sahin B, Daltaban IS, Gel MS, Guvercin AR, et al. , 2024. Important finding for COVID-19 pandemic: hydrocephalus-producing effect of vaporized alcohol disinfectant. J. Neurol. Surg. A Cent. Eur. Neurosurg 85 (4), 355–360. 10.1055/a-1962-1491. [DOI] [PubMed] [Google Scholar]
- Kapoor M, Wang J-C, Farris SP, Liu Y, McClintick J, Gupta I, et al. , 2019. Analysis of whole genome-transcriptomic organization in brain to identify genes associated with alcoholism. Transl. Psychiatry 9, 89 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HB, Lu Y, Oh SC, Morris J, Miyashiro K, Kim J, et al. , 2022. Astrocyte ethanol exposure reveals persistent and defined calcium response subtypes and associated gene signatures. J. Biol. Chem 298 (8), 102147. 10.1016/j.jbc.2022.102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Picciotto MR, 2023. Nicotine addiction: more than just dopamine. 2019. Curr. Opin. Neurobiol 83, 102797. 10.1016/j.conb.2023.102797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirdajova D, Anderova M, 2020. NG2 cells and their neurogenic potential. Curr. Opin. Pharmacol 50, 53–60. 10.1016/j.coph.2019.11.005. [DOI] [PubMed] [Google Scholar]
- Kofler J, Wiley CA, 2011. Microglia. Toxicol. Pathol 39 (1), 103–114. 10.1177/0192623310387619. [DOI] [PubMed] [Google Scholar]
- Kondela T, Gallová J, Hauß T, Barnoud J, Marrink SJ, Kučerka N, 2017. Alcohol interactions with lipid bilayers. Molecules 22 (12), 2078. 10.3390/molecules22122078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2021. Drug addiction: hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol. Rev 73 (1), 163–201. 10.1124/pharmrev.120.000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzoukas DE, Schreiber JA, Tajuddin NF, Kaja S, Neafsey EJ, Kim HY, et al. , 2019. PARP inhibition in vivo blocks alcohol-induced brain neurodegeneration and neuroinflammatory cytosolic phospholipase A2 elevations. Neurochem. Int 129, 104497. 10.1016/j.neuint.2019.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhns L, Kroon E, Lesscher H, Mies G, Cousijn J, 2022. Age-related differences in the effect of chronic alcohol on cognition and the brain: a systematic review. Transl. Psychiatry 12 (1), 345. 10.1038/s41398-022-02100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunos G., 2020. Interactions between alcohol and the endocannabinoid system. Alcohol. Clin. Exp. Res 44 (4), 790–805. 10.1111/acer.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaHood AJ, Kok SJ, 2023. Ethanol toxicity. StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL. [PubMed] [Google Scholar]
- Lalo U, Koh W, Lee CJ, Pankratov Y, 2021. The tripartite glutamatergic synapse. Neuropharmacology 199, 108758. 10.1016/j.neuropharm.2021.108758. [DOI] [PubMed] [Google Scholar]
- Le Daré B, Lagente V, Gicquel T, 2019. Ethanol and its metabolites: update on toxicity, benefits, and focus on immunomodulatory effects. Drug. Metab. Rev 51 (4), 545–561. 10.1080/03602532.2019.1679169. [DOI] [PubMed] [Google Scholar]
- Ledda F, Paratcha G, 2016. Assembly of neuronal connectivity by neurotrophic factors and leucine-rich repeat proteins. Front. Cell Neurosci 10, 199. 10.3389/fncel.2016.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Walter P, Yen TS, 2008. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol 3, 399–425. 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Guo J, Matoga M, Korotkova M, Jakobsson PJ, Aguzzi A, 2024. NG2 glia protect against prion neurotoxicity by inhibiting microglia-to-neuron prostaglandin E2 signaling. Nat. Neurosci 27 (8), 1534–1544. 10.1038/s41593-024-01663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, 1993. Excitotoxicity and alcohol-related brain damage. Alcohol. Clin. Exp. Res 17 (1), 19–27. 10.1111/j.1530-0277.1993.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Lowe PP, Morel C, Ambade A, et al. , 2020. Chronic alcohol-induced neuroinflammation involves CCR2/5-dependent peripheral macrophage infiltration and microglia alterations. J. Neuroinflammation 17 (1), 296. 10.1186/s12974-020-01972-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud S, Gharagozloo M, Simard C, Gris D, 2019. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells 8 (2), 184. 10.3390/cells8020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, Gray A, et al. , 2003. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol. Psychiatry 53 (8), 707–742. 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Manu DR, Slevin M, Barcutean L, Forro T, Boghitoiu T, Balasa R, 2023. Astrocyte involvement in blood–brain barrier function: a critical update highlighting novel, complex, neurovascular interactions. Int. J. Mol. Sci 24 (24), 17146. 10.3390/ijms242417146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguet F, Brosolo M, Friocourt G, et al. , 2022. Oligodendrocyte lineage is severely affected in human alcohol-exposed foetuses. Acta Neuropathol. Commun 10, 74. 10.1186/s40478-022-01378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Wooden JI, Nixon K, 2020. Microglia dystrophy following binge-like alcohol exposure in adolescent and adult male rats. Front. Neuroanat 14, 52. 10.3389/fnana.2020.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew F, Goyal A, 2021. Ethanol. StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL. [Google Scholar]
- McEvoy LK, Fennema-Notestine C, Elman JA, Eyler LT, Franz CE, Hagler DJ, et al. , 2018. Alcohol intake and brain white matter in middle aged men: microscopic and macroscopic differences. Neuroimage Clin. 18, 390–398. 10.1016/j.nicl.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan RH, Anton P, Coleman LG, et al. , 2023. Alcohol and immunology: mechanisms of multi-organ damage. Summary of the 2022 alcohol and Immunology research interest group (AIRIG) meeting. Alcohol 110, 57–63. 10.1016/j.alcohol.2023.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekonen T, Chan GCK, Connor J, et al. , 2020. Treatment rates for alcohol use disorders: a systematic review and meta-analysis. Addiction. 10.1111/add.15357. [DOI] [PubMed] [Google Scholar]
- Melkumyan M, Snyder AE, Bingaman SS, Arnold AC, Silberman Y, 2022. Astrocytes play a critical role in mediating the effect of acute ethanol on central amygdala glutamatergic transmission. Neuropharmacology 205, 108918. 10.1016/j.neuropharm.2021.108918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof DJ, Tizabi Y, Staley JK, et al. , 2006. Smoking comorbidity in alcoholism: neurobiological and neurocognitive consequences. Alcohol. Clin. Exp. Res 30 (2), 253–264. 10.1111/j.1530-0277.2006.00034.x. [DOI] [PubMed] [Google Scholar]
- Michalski JP, Kothary R, 2015. Oligodendrocytes in a nutshell. Front. Cell. Neurosci 9, 340. 10.3389/fncel.2015.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, 2018. Molecular neuropathology of astrocytes and oligodendrocytes in alcohol use disorders. Front. Mol. Neurosci 11, 78. 10.3389/fnmol.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CN, Kamens HM, 2020. The role of nicotinic acetylcholine receptors in alcohol-related behaviors. Brain Res. Bull 163, 135–142. 10.1016/j.brainresbull.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Tamura Y, Yamato M, et al. , 2017. NG2 glial cells regulate neuroimmunological responses to maintain neuronal function and survival. Sci. Rep 7 (1), 42041. 10.1038/srep42041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Hasegawa H, 2022. Blood vessels as a key mediator for ethanol toxicity: implication for neuronal damage. Life (Basel) 12 (11), 1882. 10.3390/life12111882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebeling FC, Poll S, Justus LC, et al. , 2023. Microglial motility is modulated by neuronal activity and correlates with dendritic spine plasticity in the hippocampus of awake mice. eLife 12, e83176. 10.7554/eLife.83176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehring SM, Freeman AM, 2020. Alcohol use disorder. StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL. [Google Scholar]
- Nelles DG, Hazrati LN, 2022. Ependymal cells and neurodegenerative disease: outcomes of compromised ependymal barrier function. Brain Commun. 4 (6), fcac288. 10.1093/braincomms/fcac288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoc KH, Jeon Y, Ko J, Um JW, 2024. Multifarious astrocyte-neuron dialog in shaping neural circuit architecture. Trends Cell Biol. S0962-8924 (24), 00098–00099. 10.1016/j.tcb.2024.05.002. [DOI] [PubMed] [Google Scholar]
- Nixon K, McClain JA, 2010. Adolescence as a critical window for developing an alcohol use disorder: current findings in neuroscience. Curr. Opin. Psychiatry 23 (3), 227–232. 10.1097/YCO.0b013e32833864fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikov NI, Brazhnik ES, Kitchigina VF, 2023. Pathological correlates of cognitive decline in Parkinson’s disease: from molecules to neural networks. Biochem. (Mosc.) 88 (11), 1890–1904. 10.1134/S0006297923110172. [DOI] [PubMed] [Google Scholar]
- Nunes PT, Kipp BT, Reitz NL, Savage LM, 2019. Aging with alcohol-related brain damage: Critical brain circuits associated with cognitive dysfunction. Int. Rev. Neurobiol 148, 101–168. 10.1016/bs.irn.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutma E, van Gent D, Amor S, Peferoen LAN, 2020. Astrocyte and oligodendrocyte cross-talk in the central nervous system. Cells 9 (3), 600. 10.3390/cells9030600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, Hayes A, Fonville L, Zafar R, Palmer EOC, Paterson L, et al. , 2021. Alcohol and the brain. Nutrients 13 (11), 3938. 10.3390/nu13113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omran AJ, Saternos HC, Althobaiti YS, et al. , 2017. Alcohol consumption impairs the ependymal cilia motility in the brain ventricles. Sci. Rep 7, 13652. 10.1038/s41598-017-13947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Malhotra V, Scwartz S, Smith T, Malhotra V, 2023. Chronic alcohol use associated encephalopathy with a nearly identical presentation to normal pressure hydrocephalus. Cureus 15 (5), e38977. 10.7759/cureus.38977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak D, Sriram K, 2023. Neuron-astrocyte omnidirectional signaling in neurological health and disease. Front. Mol. Neurosci 16, 1169320. 10.3389/fnmol.2023.1169320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Geil Nickell CR, Chen KY, McClain JA, Nixon K, 2017. Increased expression of M1 and M2 phenotypic markers in isolated microglia after four-day binge alcohol exposure in male rats. Alcohol 62, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil KE, Skelly MJ, 2018. CRF modulation of central monoaminergic function: implications for sex differences in alcohol drinking and anxiety. 72, 33–47. 10.1016/j.alcohol.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi G, Wennström M, Müller MB, Treccani G, 2023. NG2-glia: rising stars in stress-related mental disorders? Mol. Psychiatry 28 (2), 518–520. 10.1038/s41380-022-01838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova S, Charness ME, Burd L, et al. , 2023. Fetal alcohol spectrum disorders. Nat. Rev. Dis. Primers 9, 11. 10.1038/s41572-023-00420-x. [DOI] [PubMed] [Google Scholar]
- Portis SM, Haass-Koffler CL, 2020. New microglial mechanisms revealed in alcohol use disorder: how does that translate? Biol. Psychiatry 88 (12), 893–895. 10.1016/j.biopsych.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushotham SS, Buskila Y, 2023. Astrocytic modulation of neuronal signalling. Front. Netw. Physiol 3, 1205544. 10.3389/fnetp.2023.1205544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian K, Jiang X, Liu ZQ, et al. , 2023. Revisiting the critical roles of reactive astrocytes in neurodegeneration. Mol. Psychiatry 28, 2697–2706. 10.1038/s41380-023-02061-8. [DOI] [PubMed] [Google Scholar]
- Rahman S., 2015. Targeting brain nicotinic acetylcholine receptors to treat major depression and co-morbid alcohol or nicotine addiction. CNS Neurol. Disord. Drug. Targets 14 (5), 647–653. 10.2174/1871527314666150429112954. [DOI] [PubMed] [Google Scholar]
- Rasia-Filho AA, Calcagnotto ME, von Bohlen und Halbach O, 2023. Glial cell modulation of dendritic spine structure and synaptic function. Rev. Adv. Neurobiol 34, 255–310. 10.1007/978-3-031-36159-3_6. [DOI] [PubMed] [Google Scholar]
- Rasool AE, Furlong T, Prasad AA, 2024. Microglia activity in the human basal ganglia is altered in alcohol use disorder and reversed with remission from alcohol. Addict. Biol 29 (2), e13374. 10.1111/adb.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MM, Blazer-Yost B, 2022. Channels and transporters in astrocyte volume regulation in health and disease. Cell. Physiol. Biochem 56 (S2), 12–30. 10.33594/000000495. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Toth D, Vicena V, Manavalan S, Brown D, Getachew B, et al. , 2019. Therapeutic potential of PACAP in alcohol toxicity. Neurochem. Int 124, 238–244. 10.1016/j.neuint.2019.01.017. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED, Cauley M, et al. , 2017. Ketamine differentially attenuates alcohol intake in male versus female alcohol preferring (P) rats. J. Drug. Alcohol. Res 6, 236030. 10.4303/jdar/236030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Amorim D, Rivera-Baltanás T, Bessa J, et al. , 2018. The neurobiological hypothesis of neurotrophins in the pathophysiology of schizophrenia: a meta-analysis. J. Psychiatr. Res 106, 43–53. 10.1016/j.jpsychires.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Rousseaux CG, Greene SF, 2016. Sigma receptors [σRs]: biology in normal and diseased states. J. Recept. Signal. Transduct. Res 36 (4), 327–388. 10.3109/10799893.2015.1015737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruda-Kucerova J, Babinska Z, Luptak M, et al. , 2018. Both ketamine and NBQX attenuate alcohol drinking in male wistar rats. Neurosci. Lett 666, 175–180. 10.1016/j.neulet.2017.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Hicks C, Cottone P, 2017. Sigma receptors and substance use disorders. Adv. Exp. Med. Biol 964, 177–199. 10.1007/978-3-319-50174-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitgareeva AR, Bulygin KV, Gareev IF, Beylerli OA, Akhmadeeva LR, 2020. The role of microglia in the development of neurodegeneration. Neurological Sci. 41 (12), 3609–3615. 10.1007/s10072-020-04468-5. [DOI] [PubMed] [Google Scholar]
- Sanchez-Petidier M, Guerri C, Moreno-Manzano V, 2022. Toll-like receptors 2 and 4 differentially regulate the self-renewal and differentiation of spinal cord neural precursor cells. Stem Cell Res. Ther 13 (1), 117. 10.1186/s13287-022-02798-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm E, Waisman A, 2022. Microglia as central protagonists in the chronic stress response. Neuro. Neuroimmunol. Neuroinflamm 9, e200023. 10.1212/NXI.0000000000200023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K, 2014. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci 34 (6), 2231–2243. 10.1523/JNEUROSCI.1619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Aschner M, 2013. Manganese toxicity in the central nervous system: the glutamine/glutamate-γ-aminobutyric acid cycle. J. Intern. Med 273 (5), 466–477. 10.1111/joim.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares ÉN, Costa AC, Dos S, Ferrolho G, De J, et al. , 2024. Nicotinic acetylcholine receptors in glial cells as molecular target for Parkinson’s disease. Cells 13 (6), 474. 10.3390/cells13060474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Staples MC, Galinato MH, Fannon MJ, Mandyam CD, 2014. Role of NG2 expressing cells in addiction: a new approach for an old problem. Front. Pharmacol 5, 279. 10.3389/fphar.2014.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Gorelick PB, 2019. Brain white matter: a substrate for resilience and a substance for subcortical small vessel disease. Brain Sci. 9 (8), 193. 10.3390/brainsci9080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza DG, Almeida RF, Souza DO, Zimmer ER, 2019. The astrocyte biochemistry. Semin. Cell Developmental Biol 95, 142–150. 10.1016/j.semcdb.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Vasconcelos MF, Albrechet-Souza L, Ceresér KMM, de Almeida RMM, 2017. Microglial over-activation by social defeat stress contributes to anxiety- and depressive-like behaviors. Front. Behav. Neurosci 11. 10.3389/fnbeh.2017.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoklund Dittlau K, Freude K, 2024. Astrocytes: the stars in neurodegeneration? Biomolecules 14 (3), 289. 10.3390/biom14030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sushma, Mishra S, Kanchan S, Divakar A, Jha G, Sharma D, et al. , 2023. Alcohol induces ER stress and apoptosis by inducing oxidative stress and disruption of calcium homeostasis in glial cells. Food Chem. Toxicol 182, 114192. 10.1016/j.fct.2023.114192. [DOI] [PubMed] [Google Scholar]
- Tajuddin N, Kim HY, Collins MA, 2018. PARP inhibition prevents ethanol-induced neuroinflammatory signaling and neurodegeneration in rat adult-age brain slice cultures. J. Pharmacol. Exp. Ther 365 (1), 117–126. 10.1124/jpet.117.245290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann A, Tascio D, Jabs R, et al. , 2023. Dysfunction of NG2 glial cells affects neuronal plasticity and behavior. Glia 71 (6), 1481–1501. 10.1002/glia.24352. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Getachew B, Collins MA, 2021. Ethanol neurotoxicity. In: Kostrzewa RM (Ed.), Handbook of Neurotoxicity. Springer, Cham, pp. 1–23. 10.1007/978-3-030-71519-9_205-1. [DOI] [Google Scholar]
- Tizabi Y, Getachew B, Hauser SR, Tsytsarev V, Manhães AC, da Silva VDA, 2024. Role of glial cells in neuronal function, mood disorders, and drug addiction. Brain Sci. 14 (6), 558. 10.3390/brainsci14060558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez-Fort M, Audinat E, Angulo MC, 2009. Functional α7-containing nicotinic receptors of NG2-expressing cells in the hippocampus. Glia 57 (10), 1104–1114. 10.1002/glia.20834. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M, 2018. Physiology of astroglia. Physiological Rev. 98 (1), 239–389. 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT, 2014. Current hypotheses on the mechanisms of alcoholism. Handb. Clin. Neurol 125, 477–497. 10.1016/B978-0-444-62619-6.00027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar M, Mira H, 2016. Regulation of neurogenesis by neurotrophins during adulthood: expected and unexpected roles. Front. Neurosci 10, 26. 10.3389/fnins.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter TJ, Crews FT, 2017. Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. J. Neuroinflamm 14, 86. 10.1186/s12974-017-0856-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zong S, Cui X, Wang X, Wu S, Wang L, et al. , 2023. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Front. Immunol 14, 1117172. 10.3389/fimmu.2023.1117172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden AS, Triplett TA, Lyu A, Grantham EK, Azzam MM, DaCosta A, et al. , 2021. Microglia depletion and alcohol: Transcriptome and behavioral profiles. Addict. Biol 26 (2), e12889. 10.1111/adb.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Nigg JT, Welsh RC, et al. , 2012. Resiliency in adolescents at high risk for substance abuse: flexible adaptation via subthalamic nucleus and linkage to drinking and drug use in early adulthood. Alcoholism: Clin. Exp. Res 10.1111/j.1530-0277.2012.01741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, 2020. Gender differences in the epidemiology of alcohol use and related harms in the United States. Alcohol. Res 40 (2), 01. 10.35946/arcr.v40.2.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wies Mancini VSB, Mattera VS, Pasquini JM, Pasquini LA, Correale JD, 2024. Microglia-derived extracellular vesicles in homeostasis and demyelination/remyelination processes. J. Neurochem 168 (1), 3–25. 10.1111/jnc.16011. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Guizzetti M, 2016. Fetal alcohol spectrum disorders: an overview from the glia perspective. Front. Integr. Neurosci 9, 65. 10.3389/fnint.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DF, Matschinsky FM, 2020. Ethanol metabolism: the good, the bad, and the ugly. Med. Hypotheses 140, 109638. 10.1016/j.mehy.2020.109638. [DOI] [PubMed] [Google Scholar]
- Wu J, Gao M, Taylor DH, 2014. Neuronal nicotinic acetylcholine receptors are important targets for alcohol reward and dependence. Acta Pharmacol. Sin 35, 311–315. 10.1038/aps.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Chen J, Li Y, 2023. Microglia and astrocytes underlie neuroinflammation and synaptic susceptibility in autism spectrum disorder. Front. Neurosci 17, 1125428. 10.3389/fnins.2023.1125428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Li C, Parsiola AL, et al. , 2019. Dose-dependent influences of ethanol on ischemic stroke: role of inflammation. Front. Cell Neurosci 13, 6. 10.3389/fncel.2019.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JP, Zhao J, Li S, 2011. Roles of NG2 glial cells in diseases of the central nervous system. Neurosci. Bull 27 (6), 413–421. 10.1007/s12264-011-1838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Su Y, Guo C, Yi C, Yu B, Chen H, et al. , 2022. Pathological oligodendrocyte precursor cells revealed in human schizophrenic brains and trigger schizophrenia-like behaviors and synaptic defects in genetic animal model. Mol. Psychiatry 27 (12), 5154–5166. 10.1038/s41380-022-01777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Lin H, Zou M, et al. , 2022. Nicotine in inflammatory diseases: antiinflammatory and pro-inflammatory effects. Front. Immunol 13. 10.3389/fimmu.2022.826889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liu Y, Hong X, et al. , 2021. NG2 glia-derived GABA release tunes inhibitory synapses and contributes to stress-induced anxiety. Nat. Commun 12, 5740. 10.1038/s41467-021-25956-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang Q, Yang Q, Gu HY, Yin YQ, Li YD, et al. , 2019. NG2 glia regulate brain innate immunity via TGF-β2/TGFBR2 axis. BMC Med. 17 (1), 204. 10.1186/s12916-019-1439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhang J, 2023. Neuronal activity and remyelination: new insights into the molecular mechanisms and therapeutic advancements. Front. Cell Developmental Biol 11. 10.3389/fcell.2023.1221890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- Becker HC, Veatch LM, Diaz-Granados JL, 1998. Repeated ethanol withdrawal experience selectively alters sensitivity to different chemoconvulsant drugs in mice. Psychopharmacology. 10.1007/s002130050699. (in press.). [DOI] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, 2012. Neuroinflammatory pathways in binge alcohol-induced neuronal degeneration: oxidative stress cascade involving aquaporin, brain edema, and phospholipase A2 activation. Neurotox Res. 21 (1), 70–8. 10.1007/s12640-011-9276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew B, Hudson T, Heinbockel T, Csoka AB, Tizabi Y, 2018. Protective effects of donepezil against alcohol-induced toxicity in cell culture: role of caspase-3. Neurotox Res. 34 (3), 757–762. 10.1007/s12640-018-9913-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew B, Csoka AB, Garden AR, Copeland RL, Tizabi Y, 2021. Sodium butyrate protects against ethanol-induced toxicity in SH-SY5Y cell line. Neurotox Res. 39 (6), 2186–2193. 10.1007/s12640-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew B, Csoka AB, Tizabi Y, 2022. Dihydromyricetin protects against ethanol-induced toxicity in SH-SY5Y cell line: role of GABAA receptor. Neurotox Res. 40 (3), 892–899. 10.1007/s12640-02. [DOI] [PubMed] [Google Scholar]
- Hu XJ, Ticku MK, 1997. Functional characterization of a kindling-like model of ethanol withdrawal in cortical cultured neurons after chronic intermittent ethanol exposure. Brain Res. 767, 228–234. 10.1016/s0006-8993(97)00581-7. [DOI] [PubMed] [Google Scholar]
- Hurley TD, Edenberg HJ, Li T-K, 2002. Pharmacogenomics: the search for individualized therapies. The Pharmacogenomics of Alcoholism. Wiley-VCH, Weinheim, Germany, pp. 417–441. 10.1002/3527600752.ch18. [DOI] [Google Scholar]
- Ibáñez CF, Andressoo JO, 2017. Biology of GDNF and its receptors – relevance for disorders of the central nervous system. Neurobiol. Dis 97 (Pt B), 80–89. 10.1016/j.nbd.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Luo J., 2014. Autophagy and ethanol neurotoxicity. Autophagy 10 (12), 2099–108. 10.4161/15548627.2014.981916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Jeliazkova V, et al. , 2008. Alcohol exposure regulates heat shock transcription factor binding and heat shock proteins 70 and 90 in monocytes and macrophages: implication for TNF-α regulation. J. Leukoc. Biol 84, 1335–1345. 10.1189/jlb.0407256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KH, Tajuddin N, Brown J, et al. , 2014. Phospholipase A2, oxidative stress, and neurodegeneration in binge ethanol-treated organotypic slice cultures of developing rat brain. Alcohol Clin. Exp. Res 38, 161–169. 10.1111/acer.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD, 2010. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc. Natl. Acad. Sci. U. S. A 107 (24), 11104–9. 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]


