Abstract
Background
Stroke is a leading cause of death and disability worldwide, especially in China, where its incidence is rising. Post-stroke rehabilitation is crucial for restoring neurological function and improving quality of life. Light therapy, a non-pharmacological intervention, is gaining attention for its potential to promote neuroplasticity and enhance brain circulation. Warm and cold light, characterized by different color temperatures, have demonstrated beneficial effects on sleep quality, neurological recovery, and emotional well-being in stroke patients. However, the underlying mechanisms remain incompletely understood. This study explores the effects of different light color temperatures on stroke recovery to optimize rehabilitation approaches.
Methods
The study was designed as a prospective, single-center, randomized controlled trial. 48 patients with ischemic stroke were randomly divided into three groups (1:1:1): warm light group (WLG, 500 lx, 3000 K, n = 16), cold light group (CLG, 500 lx, 6500 K, n = 16), and control group (CG, 500 lx, 5000 K, n = 16). Patients in all three groups received 60 min of rehabilitation therapy daily, from Monday to Friday between 8:30 and 9:30 AM, under light environments with different color temperatures. National Institutes of Health Stroke Scale (NIHSS), Short Form-36 (SF-36), Self-Rating Sleep Scale (SRSS), and levels of interleukin 6 (IL-6), norepinephrine (NE) and melatonin (MT) were measured before and after 4 weeks of intervention.
Results
After 4 weeks intervention, CLG showed a significant reduction of NIHSS when compared to CG(p < 0.001, partial η2 = 0.316) and WLG(p = 0.003, partial η2 = 0.237). Although all three groups showed significant reductions in SRSS scores in the within-group comparisons, between-group comparisons revealed that CLG demonstrated a significantly greater reduction in SRSS compared to CG (p = 0.004, partial η2 = 0.253) and WLG (p = 0.007, partial η2 = 0.241). Regarding the SF-36, the physical component summary (PCS) score significantly decreased in both CG (Δ = –5.34 ± 7.35, p = 0.011) and WLG (Δ = –3.63 ± 4.68, p = 0.015), while CLG showed a significant improvement (Δ = 4.28 (8.78), p = 0.024). Between-group analysis demonstrated that post-intervention PCS scores were significantly higher in CLG compared to CG (p = 0.001, partial η2 = 0.323) and WLG (p = 0.002, partial η2 = 0.326), with no significant difference between CG and WLG (p = 0.104). Mental component summary (MCS) scores showed no statistically significant differences either within or between groups. No significant changes in NE and IL-6 levels were observed across all groups. CLG showed a significant reduction of MT when compared to WLG(p = 0.018, partial η2 = 0.174). No apparent adverse events were reported.
Conclusions
This study demonstrated that cold light therapy significantly improves neurological function, sleep quality, physical health status, including better performance on the physical component of quality of life in post-stroke patients, while warm light shows moderate benefits in sleep. These results support the integration of light-based interventions as adjunctive strategies in post-stroke care.
Trial registration The study was registered in Chinese Clinical Trial Registry as a clinical trial ID (ChiCTR2200057541), March 14, 2022.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12984-025-01679-9.
Keywords: Light therapy, Ischemic stroke, Cold light, Warm light, Rehabilitation
Introduction
Since 2015, stroke has been a leading cause of death and disability globally, especially in China [1, 2], where its incidence is predicted to rise significantly by 2030 [3, 4]. As a major neurological disorder that leads to multiple complications, it has a profound impact on patients' quality of life [5]. The 2018 Chinese guidelines for acute ischemic stroke management recommend various treatments such as enhancing cerebral circulation, statins, and traditional Chinese medicine [6]. Alongside these pharmacological treatments, post-stroke rehabilitation therapy also holds paramount importance. Effective rehabilitation is crucial for reinstating a healthy lifestyle and mitigating the burden of disease [7]. Advancing post-stroke rehabilitation therapy not only provides significant clinical benefits but also improves the efficiency of medical resource utilization and enhances the quality of life of patients [8].
To further enhance the effectiveness of rehabilitation therapy, reduce side effects, ensure high safety, and improve patient compliance and convenience, there is a growing recognition of the need for innovative non-pharmacological or non-invasive treatment methods in post-stroke rehabilitation. Light therapy, which integrates medical science with physics, engineering, and technology, is an excellent example of such innovation. As an alternative to traditional drug therapy, light therapy is gaining significant interest and is rapidly emerging as an active area of research [9, 10]. Light interacts with the human body through both visual and non-visual neural pathways upon exposure to the eyes and, to a lesser extent, the skin, thereby exerting profound influences on a wide range of physiological functions. The visual pathway is primarily mediated by rod and cone cells, which are responsible for spatial vision and color perception. While the non-visual pathway is primarily mediated by intrinsically photosensitive retinal ganglion cells (ipRGCs), which are responsible for regulating circadian rhythms, pupillary constriction, and melatonin secretion. ipRGCs contain melanopsin, a photopigment that is particularly sensitive to short-wavelength light (447–484 nm) [11].
Light therapy serves distinct roles across medical fields. In neonatology, it effectively treats neonatal jaundice by converting bilirubin into a more excretable form [12]. Dermatology employs ultraviolet light therapy to treat conditions such as psoriasis and vitiligo, capitalizing on its immunomodulatory and antiproliferative effects on skin cells [13]. Additionally, low-level laser therapy, another form of light therapy, is increasingly recognized for its ability to promote wound healing, alleviate pain, and reduce inflammation, illustrating light's complex influence on cellular functions [14–16]. The adaptability of light therapy extends to treating circadian rhythm disorders, mood disorders such as seasonal affective disorder (SAD), and it is even being explored as an adjunct in cancer treatment to enhance patients’ quality of life [17–19]. In neurological disease treatment, 40 Hz light stimulation has been shown to induce gamma brain wave frequencies in Alzheimer's disease mouse models and reduce beta-amyloid protein accumulation in the brain [20].
Light therapy is gaining recognition as an effective non-pharmacological intervention in stroke rehabilitation. It enhances neurological function, improves quality of life, and alleviates sleep disorders [21, 22]. It is hypothesized to aid in the reconstruction of blood vessels and enhance brain blood circulation. In addition, it may promote neuroplasticity, the brain's ability to reorganize and adapt after injury [23]. Interestingly, the therapy involves the use of two distinct types of light—warm and cold—each with unique spectral characteristics and physiological effects. Warm light, characterized by a lower color temperature and a spectrum leaning towards red light, contrasts with cold light, which has a higher color temperature and is biased towards blue light [24, 25]. The mechanisms by which warm and cold light aid stroke rehabilitation remain under study. Research suggested that warm light enhanced sleep quality, stimulated neuronal activity, promoted synaptic plasticity, supported neurological recovery, and reduced emotional disorders [26–32]. In contrast, cold light was thought to boost alertness and improve attention [33].Both warm and cold light, despite their differing mechanisms and effects, are recognized as non-pharmacological interventions capable of improving neurological function and quality of life in stroke rehabilitation [34].
Based on these findings, we hypothesized that exposure to different light color temperatures—specifically warm light (3000 K) and cold light (6500 K)—would differentially influence neurological function, sleep conditions, quality of life, and biological markers in patients recovering from ischemic stroke. To test this hypothesis, we conducted a prospective, randomized controlled clinical trial to investigate the effects of varying light color temperatures during rehabilitation sessions. This study aimed to assess whether tailored light interventions could optimize post-stroke recovery through modulation of both neural and circadian processes. The findings from this trial may contribute to the development of more refined and personalized therapeutic strategies, better aligned with the specific rehabilitation needs of ischemic stroke patients.
Methods
Study design
An investigator-initiated, randomized clinical trial was conducted. The trial protocol was approved by the Ethics Committee of the Shanghai Yangzhi Rehabilitation Hospital (Shanghai Sunshine Rehabilitation Center) (Approval No. 2022-001) [see Additional file 1]. In addition, this study was conducted in compliance with the Helsinki Declaration and CONSORT (Consolidated Standards of Reporting Trials) guidelines. This trial was registered with the Chinese Clinical Trial Registry (No. ChiCTR2200057541). All patients provided voluntary informed consent after receiving information about the study's purpose and methods [see Additional file 2].
Participants
Patients were recruited from the Neurological Rehabilitation Department of Shanghai Yangzhi Rehabilitation Hospital (Shanghai Sunshine Rehabilitation Center) between April 2022 and August 2024. All selected patients met the inclusion criteria, which included males and females aged 40–70 years with a first-ever ischemic stroke diagnosed according to the Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke (2018) [35] who were in the recovery phase (1–3 months post-stroke), possessed effective communication abilities, and voluntarily signed informed consent [6, 35–37].
Patients with unstable or decompensated chronic diseases, including severe or uncontrolled central nervous, cardiovascular, pulmonary, hepatic, renal, metabolic, gastrointestinal, genitourinary, endocrine (e.g., poorly controlled diabetes or thyroid disorders), or hematopoietic disorders were excluded. Patients with well-controlled chronic conditions such as hypertension, diabetes, or hyperlipidemia were not excluded. Additional exclusion criteria included clinical symptoms that could compromise patient safety or study validity; inability to participate in rehabilitation training or to provide informed consent, as determined by the research team; inability to walk and a National Institutes of Health Stroke Scale (NIHSS) score greater than 20 [38].
Randomization and blinding
This study incorporated three distinct groups. Thus, the block randomization method was employed to mitigate the variability inherent in simple randomization and to ensure a roughly equal distribution of subjects across all groups throughout this trial. Given the three-group design, a block size of six was chosen. The 51 subjects were divided into nine blocks. Initially, each subject within a block was assigned a unique number from 1 to 6. Subsequently, six random numbers were sequentially drawn from a designated section of a random number table for each block. These numbers were then ordered by magnitude. The assignment of subjects to groups was based on these numbers: subjects with random numbers 1 and 2 were allocated to test group 1 (warm light group), those with numbers 3 and 4 to test group 2 (cold light group), and subjects with numbers 5 and 6 to test group 3 (control group). Apart from the physicians and therapists, other researchers were blinded to the allocation scheme. Moreover, the physicians and therapists were not involved in the final outcome assessment or data analysis.
Treatment
All patients underwent rehabilitation in controlled light environments corresponding to their assigned groups. The intervention time was specifically set between 8:30 and 9:30 a.m., based on recommendations from the International Commission on Illumination (CIE) [39, 40]and the WELL Building Standard[41], which suggest that adequate circadian stimulation in the morning—defined as CS ≥ 0.3, melanopic Equivalent Daylight Illuminance (EDI) ≥ 250 lx, or Equivalent Melanopic Lux (EML) ≥ 275 lx—can support circadian entrainment and improve sleep quality.
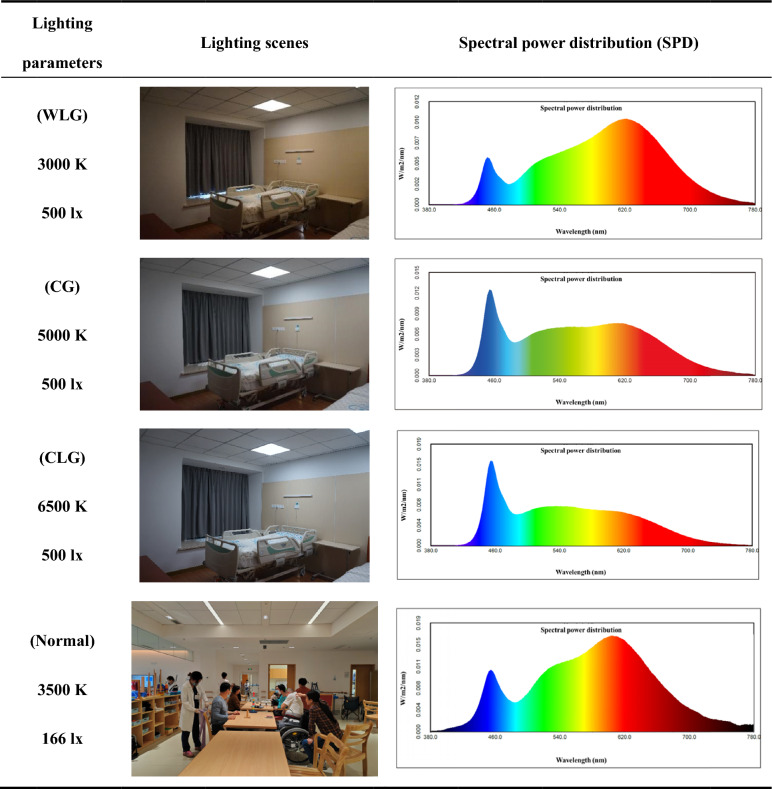
Patients were randomly allocated into three groups for this study: warm light group (WLG), cold light group (CLG), and control group (CG), as detailed in Table 1 Each hospital bed was equipped with an LED panel light directly above the bed (600*600 mm, customized by Shanghai keey group co.,ltd). The general color rendering index (Ra) of the lighting was 95. All rehabilitation sessions were conducted in the morning in light-controlled rooms. The WLG was exposed to a light environment with an illuminance of 500 lx and a color temperature of 3000 K, the CLG to 500 lx and 6500 K, and the CG to standard rehabilitation room conditions with 500 lx and 5000 K. The illuminance at the eyes was the average horizontal illuminance at the eye position about 1.2 m above the floor. And the spectral power distribution (SPD) of different lighting scenes was measured by a spectroradiometer (Konica Minolta CL-500A). After 9:30 a.m. each day, and during the rest of the daytime, the patients performed their routine training in the rehabilitation hall with an ocular illuminance of approximately 166.14 lx and a color temperature of 3500 K [see Table 1, Normal].
Table 1.
Lighting scenes and spectral power distribution of 3 lighting groups
All patients in the three groups were instructed to participate in daily rehabilitation sessions for 4 weeks, conducted on weekdays (Monday to Friday) from 8:30 a.m. to 9:30 a.m., with each session lasting 60 min. Considering the logistical requirements related to the transport of rehabilitation equipment, each session during this time frame was limited to physical therapy aimed at improving motor function and occupational therapy focused on enhancing independence in activities of daily living.
Outcome measurements
National Institutes of Health Stroke Scale (NIHSS)
A standardized and objective tool used to assess neurological function in stroke patients [42]. The NIHSS evaluates six domains, including level of consciousness, eye movements, facial symmetry, limb motor function, language, and sensory response, providing a quantitative measure of stroke severity. The total score ranges from 0 to 42 with higher scores indicating more severe neurological impairment. Stroke severity is categorized as follows: 0–1 points, normal or near normal; 1–4 points, minor stroke; 5–15 points, moderate stroke; 15–20 points, moderate to severe stroke; and 21–42 points, severe stroke[43]. NIHSS assessments were performed at baseline and again after 4 weeks of intervention. To ensure consistency and accuracy, both evaluating physicians received uniform professional training prior to the assessments.
Self-Rating Sleep Scale (SRSS)
A measurement test used to evaluate sleep quality and disturbances over a recent period, typically the past month. The SRSS consists of 10 items assessing various aspects of sleep, including sleep latency, duration, depth, frequency of awakenings, and the impact of sleep on daytime functioning. Each item is scored on a 5-point Likert scale (1 to 5), with higher scores indicating more severe sleep disturbances. The total score ranges from 10 to 50. where 10 indicates essentially no sleep problems and 50 represents the most severe level of disturbance [44]. SRSS assessments were administered at baseline and again after the 4-week intervention period.
Short Form-36 (SF-36)
A measurement test used to assess health-related quality of life across multiple physical and mental health domains. The SF-36 is a widely validated self-reported questionnaire consisting of 36 items that evaluate eight dimensions: physical functioning, role limitations due to physical health, role limitations due to emotional problems, energy/fatigue, emotional well-being, social functioning, pain, general health. Each domain score ranges from 0 to 100, where 0 indicates the poorest health status and 100 indicates the best possible health status [45]. In this study, the SF-36 was administered at baseline and after the 4-week intervention period.
Interleukin 6 (IL-6)
A pro-inflammatory cytokine involved in the body’s immune response and inflammatory processes [46]. Studies have demonstrated that in patients with ischemic stroke, IL-6 is independently associated with an increased risk of stroke recurrence and poor functional outcomes [47]. Therefore, in this study, serum IL-6 levels were measured to assess the post-stroke inflammatory status. Blood samples were collected at 6:30 a.m. under fasting conditions and in complete darkness to avoid light-induced hormonal fluctuations. Serological analysis was performed using a dual-antibody, single-step sandwich enzyme-linked immunosorbent assay (ELISA). The Human Interleukin-6 (IL-6) ELISA Kit (96 T, JN18468, Shanghai Jining Biotech) was used according to the manufacturer’s protocol. IL-6 levels were assessed at baseline and again after the 4-week intervention period.
Norepinephrine (NE)
A key neurotransmitter involved in the regulation of mood, stress response, and autonomic nervous system function [48–50]. Studies have shown initially increased serum norepinephrine concentrations are independent predictors of poor long-term outcome in patients with stroke [51]. Therefore, in this study, serum NE levels were measured to evaluate the neurochemical status of patients during the recovery phase following stroke. Blood samples were collected at 6:30 a.m. under fasting conditions and in the absence of any light exposure. Serological analysis was conducted using a dual-antibody, single-step sandwich enzyme-linked immunosorbent assay (ELISA). The Human Norepinephrine (NE) ELISA Kit (96 T, JN6218, Shanghai Jining Biotech) was used in accordance with the manufacturer’s instructions. NE levels were assessed at baseline and again at the end of the 4-week intervention period.
Melatonin (MT)
A neurohormone primarily secreted by the pineal gland, playing a crucial role in the regulation of circadian rhythms, sleep–wake cycles, and neuroprotection [52]. It exhibits strong antioxidant and anti-inflammatory properties and has been implicated in modulating neuroplasticity and immune responses following central nervous system injuries, including stroke [53]. In this study, serum melatonin levels were measured as a biomarker to assess circadian rhythm stability and endogenous neuroprotective status during stroke recovery. Blood samples were collected at 6:30 a.m. under fasting conditions and in complete darkness to prevent acute suppression of melatonin release. Quantification of melatonin concentration was performed using a competitive enzyme-linked immunosorbent assay (ELISA). The Human Melatonin ELISA Kit (96 T, EK-DSM003, Enzyme-linked Biotech, Shanghai, China) was utilized following the manufacturer’s standard protocol. Serum MT levels were evaluated at baseline and after the 4-week intervention to examine potential correlations between treatment and circadian neuroendocrine regulation.
Power analysis
Sample size calculation was performed using PASS version 15.0.5 software (NCSS, LLC, Kaysville, Utah, USA), based on previous related studies and preliminary research findings [54]. Fourteen patients in each group were the minimum sample size. After adjusting for an anticipated 5% dropout rate, the final planned sample size was increased to 15 participants per group, totaling 45 participants [see Additional file 3]. Depending on previous research results with an effect size of 0.51, two-sided (two tails) type I error of 0.05, and power of 80%.
Statistical analysis
All randomized patients diagnosed with ischemic stroke were included for analysis using a modified intent-to-treat approach. Data were processed with SPSS 26.0 (SPSS Inc., USA), ensuring a complete dataset with no missing values and maintaining the integrity of treatment-related variables by coding them undisclosed to investigators during primary analysis. Descriptive statistics were reported as mean ± standard deviation (SD) for normally distributed continuous variables and median with interquartile range for non-normally distributed ones.
At baseline, continuous variables with normal distribution and variance homogeneity underwent One-Way ANOVA, while the Kruskal–Wallis Test was applied for non-normally distributed variables for between-group comparisons. Categorical variables were analyzed using the Chi-Square test.
After a four-week intervention, paired comparisons were conducted using either paired T-tests or paired Wilcoxon tests, depending on the normality of data distribution, to assess changes within each group. Additionally, changes between groups were compared using T-tests or Mann–Whitney U tests based on the distribution of data. Statistical significance thresholds were set at P < 0.05, with higher levels denoted by P < 0.01 and P < 0.001, indicating progressively greater differences.
Results
Participant characteristics
Out of eighty screened individuals, fifty-one were recruited, yielding a recruitment rate of 63.8%. These patients were evenly distributed into three groups: CG, WLG, and CLG, each comprising 17 individuals, achieving a randomization rate of 100%. Prior to the intervention, one participant from both the CG and WLG withdrew their informed consent, while one individual from the CLG was lost to follow-up. Consequently, 48 patients, representing a retention rate of 94.1%, successfully completed the follow-up assessment (Fig. 1). Table 2 illustrates that there was no statistically significant difference (p > 0.05) between three groups in age, gender, height, weight, BMI, medical history (hypertension, hyperlipidemia, diabetes) and location of cerebral ischemic. No apparent adverse events were reported.
Fig. 1.
Flow chart diagram
Table 2.
Baseline characteristics of patients
| CG | WLG | CLG | p value | |
|---|---|---|---|---|
| Age (years) | 63.00 ± 7.16 | 62.44 ± 6.54 | 62.31 ± 5.25 | 0.948 |
| Male/Female (total) | 12/4 (16) | 12/4 (16) | 9/7(16) | 0.574 |
| Height (m) | 1.76 (0.16) | 1.73 (0.16) | 1.67 (0.19) | 0.264 |
| Weight (kg) | 69.50 (16.50) | 71.00 (22.75) | 72.00 (15.00) | 0.713 |
| BMI (kg/m2) | 23.84 ± 2.69 | 23.43 ± 2.84 | 25.06 ± 3.08 | 0.259 |
| Medical history | ||||
| Hypertension (total) | 16 (16) | 12(16) | 12 (16) | 0.100 |
| Hyperlipidemia (total) | 4 (16) | 2 (16) | 4 (16) | 0.738 |
| Diabetes (total) | 9 (16) | 8 (16) | 9(16) | 1.000 |
| Location of cerebral ischemic | ||||
| Basal ganglia region(total) | 2 (16) | 4(16) | 3 (16) | 0.574 |
| Specific cerebral lobe region (total) | 5 (16) | 5 (16) | 2 (16) | |
| Multiple brain regions (total) | 9 (16) | 7 (16) | 11 (16) | |
CG Control Group, WLG Warm Light Group, CLG Cold Light Group, BMI Body Mass Index, Specific cerebral lobe region, including the frontal lobe, temporal lobe, occipital lobe, or parietal lobe, Multiple brain regions, infarctions involving multiple brain regions, NIHSS National Institute of Health stroke scale, SRSS Self-Rating Scale of Sleep, SF-36 medical outcomes study-short from, IL-6 Interleukin-6, NE Norepinephrine, MT Melatonin
All data are presented as the mean ± standard error of the mean (SEM)for normal distribution or median (interquartile range) for abnormal distribution. Differences between groups (CG, WLG, and CLG) were tested using either one-way ANOVA or the Kruskal–Wallis H test, in addition to sexual analyzed by the chi-square test
Clinical efficacy
National institutes of health stroke scale (NIHSS)
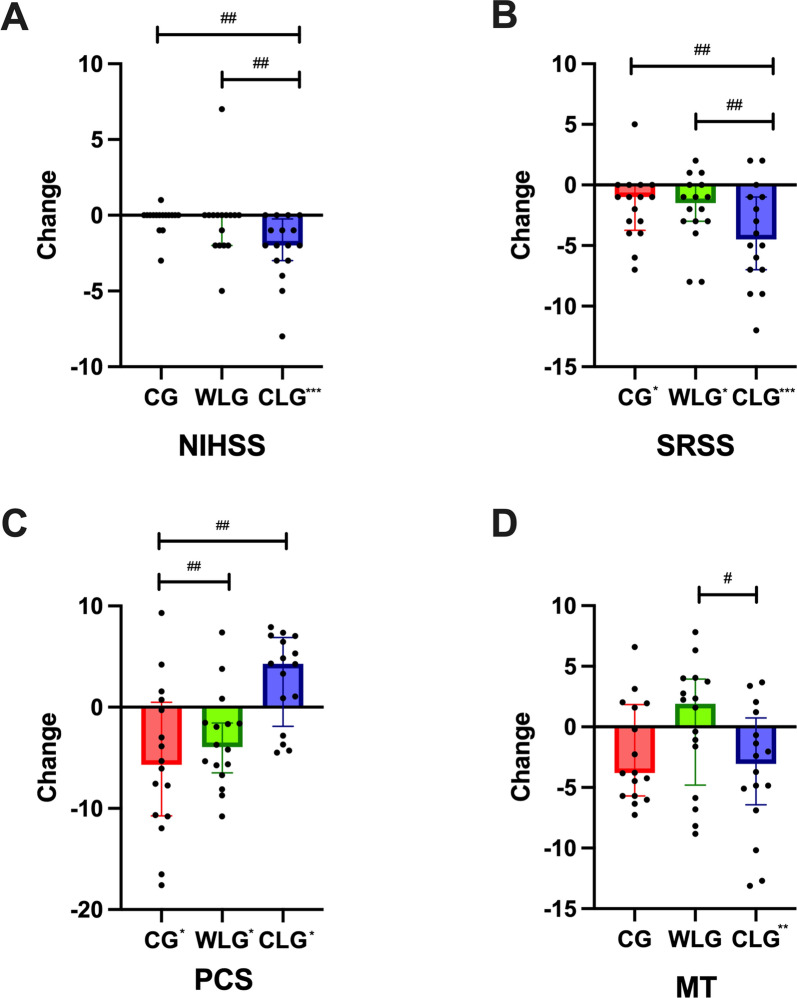
Within-Group Comparisons: In the CLG, the follow-up median (IQR) NIHSS score was 4.50 (8.00), showing a significant reduction compared to the baseline score of 7.13 ± 6.70 (Δ = − 2.00, p < 0.001). Pairwise comparisons revealed significant differences between CLG and CG (p < 0.001, partial η2 = 0.316), as well as between CLG and WLG (p = 0.003, partial η2 = 0.237). (Fig. 2A and Table 3).
Fig. 2.
Change in clinical efficacy among three groups. A–D Change in (A) National Institute of Health stroke scale (NIHSS), B Self-Rating Scale of Sleep (SRSS), C Physical Component Summary, D Melatonin (MT) among control group (CG), warm light group (WLG), and cold light group (CLG) after 4 weeks of the intervention. Analyses were conducted using all participants (intention-to-treat), using a multiple imputation approach for other missing data. Each black data point represents an individual participant (CG, n = 16; WLG, n = 16; CLG, n = 16). Change from baseline is presented as mean ± standard error of the mean for normally distributed variables or the median (interquartile range) for abnormal distribution. #p < 0.05, ##p < 0.01, ###p < 0.001: pairwise comparisons of change scores between the groups were evaluated by t test or Mann–Whitney U test. *p < 0.05, **p < 0.01, ***p < 0.001: significant differences shown at x axis compared with baseline (paired t test or paired Wilcoxon test)
Self-rating sleep scale (SRSS)
Within-Group Comparisons: In the CG, the SRSS score at 4 weeks post-treatment was 25.50 ± 5.85, with a significant reduction compared to the baseline value of 26.88 ± 6.24 (Δ = − 1.00, p = 0.040). In the WLG, the follow-up SRSS score was 22.38 ± 5.38, also showing a statistically significant reduction from the baseline value of 23.88 ± 5.94 (Δ = − 1.00, p = 0.026). In the CLG, the follow-up SRSS score was 21.19 ± 4.92 compared to the baseline value of 26.25 ± 6.14, with a significant reduction observed (Δ = − 5.50, p < 0.001). Pairwise comparisons revealed significant differences between CLG and CG (p = 0.004, partial η2 = 0.253), as well as between CLG and WLG (p = 0.007, partial η2 = 0.241). (Fig. 2B and Table 3).
Table 3.
Change in Primary Outcomes after 4-week intervention among participants
| CG | p value | WLG | p value | CLG | p value | WLG vs. CG | CLG vs. CG | CLG vs. WLG | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 16 | n = 16 | n = 16 | p value | Effect size(partial η2) | p value | Effect size (partial η2) |
p value | Effect size (partial η2) |
|||||
| NIHSS | Baseline | 11.69 ± 7.64 | – | 9.56 ± 7.72 | – | 7.13 ± 6.70 | – | – | – | – | – | – | – |
| Follow-up | 13.00(9.00) | – | 8.00(9.25) | – | 4.50(8.00) | – | – | – | – | – | – | – | |
| △ | 0.00(0.00) | 0.257 | 0.00(1.75) | 0.332 | − 2.00(2.75) | < 0.001* | 0.462 | 0.002 | < 0.001* | 0.316 | 0.003* | 0.237 | |
| SRSS | Baseline | 26.88 ± 6.24 | – | 23.88 ± 5.94 | – | 26.25 ± 6.14 | – | – | – | – | – | – | – |
| Follow-up | 25.50 ± 5.85 | – | 22.38 ± 5.38 | – | 21.19 ± 4.92 | – | – | – | – | – | – | – | |
| △ | − 1.00(3.00) | 0.040* | − 1.00(3.00) | 0.026* | − 5.50(5.50) | < 0.001* | 0.803 | 0.001 | 0.004* | 0.253 | 0.007* | 0.241 | |
| SF-36 | |||||||||||||
| PCS | Baseline | 41.07 ± 7.11 | 39.25 ± 4.71 | 36.55 ± 8.07 | |||||||||
| Follow-up | 35.73 ± 4.40 | 35.07(7.92) | 39.33 ± 4.21 | ||||||||||
| △ | − 5.34 ± 7.35 | 0.011* | − 3.36 ± 4.68 | 0.015* | 4.28(8.78) | 0.024* | 0.104 | 0.027 | 0.001* | 0.323 | 0.002* | 0.326 | |
| MCS | Baseline | 35.56 ± 7.09 | 35.07 ± 6.36 | 34.79 ± 8.24 | |||||||||
| Follow-up | 32.76(6.89) | 35.74 ± 9.07 | 39.45 ± 7.01 | ||||||||||
| △ | − 1.34 ± 10.01 | 0.535 | 0.67 ± 10.77 | 0.806 | 4.66 ± 12.27 | 0.149 | 0.576 | 0.010 | 0.140 | 0.071 | 0.336 | 0.031 | |
| Serological indicators | |||||||||||||
| IL-6 | Baseline | 66.65 ± 12.94 | – | 64.78 ± 15.91 | – | 58.13 ± 12.42 | – | – | – | – | – | – | – |
| Follow-up | 60.15 ± 16.52 | – | 60.07 ± 19.06 | – | 51.55 ± 11.20 | – | – | – | – | – | – | – | |
| △ | − 6.50 ± 19.04 | 0.192 | − 4.71 ± 25.70 | 0.475 | − 6.58 ± 17.47 | 0.153 | 0.824 | 0.002 | 0.990 | 0.000 | 0.811 | 0.002 | |
| NE | Baseline | 2446.50 (929.00) | – | 1937.00 (751.25) | – | 1949.50 (613.75) | – | – | – | – | – | – | – |
| Follow-up | 2263.50.00(732.75) | – | 2144.00(600.25) | – | 2133.50(605.50) | – | – | – | – | – | – | – | |
| △ | 195.00(1205.00) | 0.796 | 175.50(799.25) | 0.109 | 350.00(931.50) | 0.326 | 0.734 | 0.010 | 0.763 | 0.000 | 0.970 | 0.006 | |
| MT | Baseline | 12.28 ± 2.86 | – | 11.91 ± 3.71 | – | 11.75 ± 3.85 | – | – | – | – | – | – | – |
| Follow-up | 10.47 ± 2.85 | – | 12.24 ± 4.93 | – | 7.63 ± 3.78 | – | – | – | – | – | – | – | |
| △ | − 1.81 ± 4.17 | 0.102 | 0.33 ± 4.92 | 0.795 | − 4.13 ± 5.10 | 0.006* | 0.195 | 0.055 | 0.170 | 0.062 | 0.018* | 0.174 | |
NIHSS National Institute of Health stroke scale, SRSS Self-Rating Scale of Sleep, SF-36 Short Form-36, PCS Physical component summary, MCS Mental component summary, IL-6 Interleukin-6, NE Norepinephrine, MT Melatonin
All data were presented as mean ± standard error of the mean (SEM) for normally distributed variables or the median (interquartile range) for abnormal distribution. Change scores from baseline were represented by “△” in the table. After 4-week of intervention, pairwise comparisons of change scores between the groups (e.g., WLG vs. CG, CLG vs. CG, CLG vs. WLG) were evaluated by t test or Mann–Whitney U test. All parameters: significant differences compared with baseline (paired t test or paired Wilcoxon test). *p < 0.05
Short form-36 (SF-36)
(1) Physical component summary(PCS)
Within-Group Comparisons: In the CG, the mean PCS score significantly decreased from 41.07 ± 7.11 at baseline to 35.73 ± 4.40 at follow-up (Δ = – 5.34 ± 7.35, p = 0.011). Similarly, the WLG also showed a significant decrease in PCS scores, dropping from 39.25 ± 4.71 to 35.07 (7.92) (Δ = – 3.63 ± 4.68, p = 0.015). In contrast, the CLG demonstrated a significant improvement, with PCS increasing from 36.55 ± 8.07 to 39.33 ± 4.21 (Δ = 4.28(8.78), p = 0.024).
Between-Group Comparisons: Post-intervention comparisons revealed that PCS scores in the CLG were significantly higher than those in the CG (p = 0.001, partial η2 = 0.323) and the WLG (p = 0.002, partial η2 = 0.326), while no significant difference was found between WLG and CG (p = 0.104). (Fig. 2C and Table 3).
(2) Mental component summary(MCS)
Within-Group Comparisons In the CG, the mean MCS score declined from 35.56 ± 7.09 at baseline to 32.76 ( 6.89) at follow-up, resulting in a non-significant change (Δ = – 1.34 ± 10.01, p = 0.535).In the WLG, the score slightly increased from 35.07 ± 6.36 to 35.74 ± 9.07, also without statistical significance (Δ = 0.67 ± 10.77, p = 0.806). In contrast, the CLG showed an increase in MCS from 34.79 ± 8.24 to 39.45 ± 7.01, with a mean change of 4.66 ± 12.27, although the change did not reach statistical significance (p = 0.149).
Between-Group Comparisons Post-intervention comparisons revealed no significant difference in MCS scores between the WLG and CG groups (p = 0.576, partial η2 = 0.010). Similarly, the difference between CLG and CG was not statistically significant (p = 0.140, partial η2 = 0.071). Likewise, no significant difference was observed between the CLG and WLG groups (p = 0.336, partial η2 = 0.031).
Interleukin 6 (IL-6)
There were no significant differences between baseline and 4 weeks post-treatment in either within-group or pairwise comparisons across all three groups.
Norepinephrine (NE)
There were no significant differences between baseline and 4 weeks post-treatment in either within-group or pairwise comparisons across all three groups.
Melatonin (MT)
As compared with baseline, after 4 weeks of intervention a significant reduction of MT was observed only in CLG (-4.13 ± 5.10, p = 0.006), and a significant difference in MT reduction was also observed compared with WLG (p = 0.018, partial η2 = 0.174) (Fig. 2D and Table 3).
Discussion
The objective of this study was to evaluate the efficacy of different light color temperatures on neurological function, sleep conditions, quality of life, and biological markers in patients recovering from ischemic stroke. After 4 weeks of intervention, assessment with the NIHSS demonstrated significant improvements in neurological function in the cold light group (CLG) compared to both the control group (CG) and the warm light group (WLG). Previous research has indicated that each one-point increase in NIHSS score reduces the likelihood of achieving excellent outcomes by 24% at 7 days and 17% at 3 months post-stroke [55]. In our study, patients in the CLG exhibited an average 2-point reduction in NIHSS scores from baseline, suggesting that cold light therapy may offer a promising approach to enhancing neurological rehabilitation outcomes in patients within three months of ischemic stroke onset. The observed improvements may be attributed to several underlying mechanisms associated with cold light therapy. Studies have demonstrated that specific wavelengths of light, particularly in the blue spectrum (e.g., 465 nm), can significantly enhance neurite outgrowth [56]. This suggests that cold light may promote neural regeneration by stimulating the extension and growth of nerve cells. Additionally, cold light has been shown to exert neuroprotective effects by reducing oxidative stress—primarily through its action on mitochondrial function—which helps preserve neuronal integrity and function [57]. These neuroprotective effects may be mediated via the suppression of reactive oxygen species (ROS) and the activation of pro-survival cellular pathways. For instance, although blue laser irradiation (405 nm) has been associated with increased ROS levels in various cell types [58], certain wavelengths of cold light (e.g., 472 nm) have been found to activate the Raf/MEK/ERK signaling cascade, thereby inducing significant neurite outgrowth in PC12 rat pheochromocytoma cells, even in the absence of exogenous growth factors [59]. This highlights the potential of cold light to promote intrinsic neuronal repair processes. Our study provides pioneering evidence of the multifaceted benefits of light therapy in stroke rehabilitation.
Our study demonstrated that both cold and warm light interventions significantly improved SRSS scores, with the cold light group exhibiting more notable improvements. These findings indicate that light therapy, particularly cold light, may be beneficial in regulating sleep patterns in patients recovering from ischemic stroke within three months. The potential mechanisms underlying these improvements in sleep quality can be attributed to the non-visual pathways of light perception. Light-induced non-visual responses are primarily mediated by intrinsically photosensitive retinal ganglion cells (ipRGCs), which are directly connected to the suprachiasmatic nucleus (SCN)—the brain’s central circadian pacemaker. Among various wavelengths, short-wavelength blue light (around 480 nm) and light intensity play crucial roles in modulating ipRGC activity [24]. These non-visual responses are triggered when ipRGCs transmit photic signals to various brain regions, including the suprachiasmatic nucleus (SCN) and pineal gland, modulating circadian rhythms, melatonin secretion, and thereby influencing sleep quality and cognitive performance [60, 61]. Notably, the impact of light on sleep varies depending on the correlated color temperature (CCT) and spectral composition of the light source [62]. Warm light (3000 K), which contains less short-wavelength light, has a relatively weaker activation effect on ipRGCs. This weaker stimulation is associated with increased melatonin secretion, promoting relaxation and enhancing sleep onset and continuity. Therefore, warm light is often considered supportive of sleep regulation, particularly when applied during evening hours or in low-stimulation environments [63]. In contrast, cold light (6500 K) is enriched in short-wavelength light and activates ipRGCs more strongly, typically leading to suppression of melatonin secretion, enhanced alertness, and improved attention and cognitive performance [64]. However, our study observed that morning exposure to cold light also led to notable improvements in nighttime sleep quality, particularly in the cold light group. This seemingly contradictory outcome may be explained by the phase-shifting effect of blue-enriched light when applied in the early hours of the day. Evidence suggests that light exposure in the early morning can produce a forward shift in the circadian phase, advancing the timing of endogenous rhythms such as melatonin secretion, sleep onset, and wakefulness [40]. The cold light group (CLG), which received high-CCT illumination with enhanced short-wavelength components during morning rehabilitation, likely experienced a more pronounced circadian phase advance, thereby facilitating earlier and more consolidated nighttime sleep [65]. This hypothesis is further supported by our sampling protocol, where both melatonin levels and SRSS scores were collected at 6:30 a.m. under fasting and dark conditions, minimizing external light interference. Clinical evidence further supports the benefits of controlled morning light exposure. One study found that exposure to 500 lx for one hour in the morning using either warm (3500 K) or cold light (6500 K) resulted in a more pronounced reduction in melatonin levels in the cold light group [66]. Similarly, Andrea Yoo et al. demonstrated that 3000 K warm light at > 400 lx delivered via table or floor lamps for two hours before 9:00 a.m. significantly advanced sleep onset and increased total sleep time by approximately 20 min in patients with Parkinson’s disease [67]. A clinical trial involving 56 patients with post-stroke insomnia found that 30 min of bright light therapy (10,000 lx) in the morning significantly improved daytime sleepiness, fatigue, mood, and quality of life [34]. Collectively, these findings suggest that both warm and cold light interventions, when appropriately timed and applied, can beneficially modulate sleep regulation via non-visual pathways.
Our results from the SF-36 questionnaire showed that, after 4 weeks of intervention, the Physical Component Summary (PCS) score significantly improved in CLG. Between-group comparisons indicated that the PCS score in the CLG was significantly higher than in both the CG and WLG. Although the present analysis focused on total PCS scores rather than its individual subdomains, the overall improvement observed in the CLG may reflect favorable changes in components such as physical functioning, pain perception, and general health. These domains are commonly affected by stroke and responsive to rehabilitation efforts. Given that PCS encompasses physical functioning, role limitations due to physical health, bodily pain, and general health, the significant group differences observed may indicate that cold light intervention supported recovery in one or more of these aspects. Improved sleep quality, facilitated by light-induced circadian alignment, may indirectly contribute to enhanced motor recovery by supporting restorative sleep and daytime alertness—both essential for active participation in rehabilitation sessions. In particular, cold light, via enhanced stimulation of intrinsically photosensitive retinal ganglion cells (ipRGCs), may promote increased morning arousal and motivation, enabling patients to engage more effectively in physical therapy. In the pain dimension, although the analgesic effects of light therapy are less frequently discussed, some evidence suggests that light can influence pain perception via modulation of the central nervous system and inflammation-related signaling pathways [14]. Cold light, by improving mood and reducing fatigue, may also increase patients’ pain tolerance and reduce perceived discomfort during recovery. Regarding social functioning, morning exposure to blue-rich light may promote better mood, sociability, and cognitive flexibility, all of which are important for social engagement. Previous studies have shown that light therapy improves affective states and interpersonal functioning, particularly in neurological and mood disorder populations [68].
In contrast, the Mental Component Summary (MCS) scores did not show statistically significant changes either within or between groups. Although a numerical increase was observed in the CLG, this improvement did not reach statistical significance. As MCS includes domains such as role limitations due to emotional problems, energy/fatigue, emotional well-being, and social functioning, the lack of overall significant change may reflect limited sensitivity of the intervention to psychological or psychosocial dimensions within the relatively short study period. Notably, previous studies have suggested that cold light exposure may positively influence components such as fatigue and social engagement by modulating circadian rhythms and enhancing morning alertness. For instance, blue-enriched light has been shown to suppress melatonin and increase cortical activation, thereby reducing fatigue and improving subjective energy levels [40]. It is therefore possible that some components of MCS were more responsive than others, contributing to the observed but non-significant trend toward improvement in the cold light group. Further investigation is warranted to determine whether specific MCS subdomains may benefit from longer or more targeted light-based interventions, and whether these effects translate into meaningful improvements in overall mental health–related quality of life.
While our study has not yet established a direct link between light therapy and improvement in IL-6 and NE. Many studies have shown that IL-6 concentration is a predictor of stroke severity and clinical outcome [69, 70]. Research indicates that IL-6, shortly after ischemic stroke injury, stimulates inflammatory reaction through the trans-signaling pathway to eliminate dying and dead neurons in the damaged areas of the brain, leading its high expression in plasma of patients. This can improve the symptoms of brain inflammation and edema in the early stages, but prolonged activation may exacerbate brain damage [71]. Clinical studies have revealed that inhibiting IL-6 action can preserve cerebral blood flow and limit ischemic damage, with research indicating an approximately 19% increase in ischemic stroke risk per standard deviation increase in log-IL-6 levels [72, 73]. Interestingly, many studies have shown that IL-6 plays a protective role in the nervous system [74, 75]. IL-6 secreted by astrocytes facilitates the polarization of Th1 to Th2 cells, thereby creating an immunosuppressive microenvironment conducive to neurogenesis, angiogenesis, and neuronal differentiation. However, these studies do not specify the precise temporal window for this effect. Norepinephrine (NE) seems to play a significant role in the brain's response to stroke and the recovery processes that follow. Research indicates that norepinephrine is involved in the regulation of neural circuits within the amygdala, an area crucial for emotional memory [76]. The levels of NE can influence how memories, especially emotionally significant ones, are encoded and retained [77].
After 4 weeks of intervention, a significant reduction in melatonin (MT) levels was observed only in the cold light group (CLG), and this reduction was also significantly greater than that in the warm light group (WLG), suggesting that light with higher color temperature (6500 K) exerts a stronger suppressive effect on melatonin secretion. This finding is consistent with prior evidence that blue-enriched light, particularly in the short-wavelength range (around 460–480 nm), can inhibit melatonin production more effectively than warm light [78].
Melatonin is a neurohormone synthesized primarily by the pineal gland, and plays a pivotal role in circadian rhythm regulation, sleep–wake cycles, and central nervous system modulation [52]. The synthesis and release of melatonin follow a distinct circadian pattern regulated by the suprachiasmatic nucleus (SCN), with levels peaking during the night and suppressed by exposure to light—especially short-wavelength light—during the day [61]. Our sampling of melatonin at 6:30 a.m. under fasting and dark conditions ensured minimal external influence, thereby providing a reliable snapshot of endogenous circadian rhythm phase. The observed reduction in melatonin in the CLG may reflect a phase-advancing effect of morning blue-enriched light exposure. Exposure to high-CCT lighting in the early morning hours has been shown to advance the melatonin rhythm, resulting in earlier melatonin offset and promoting better sleep consolidation at night [79]. This circadian phase shift could partially explain the simultaneous improvement in sleep quality (as measured by SRSS) and decrease in MT levels in the cold light group, as these outcomes may reflect a realignment of circadian timing rather than a pathological suppression of melatonin secretion. In contrast, the warm light group showed no significant change in melatonin levels. Warm light (3000 K), which emits minimal blue light, induces only weak ipRGC activation and thus exerts a much milder influence on circadian phase and melatonin dynamics [61]. However, it still supports relaxation and improves sleep onset via indirect mechanisms such as creating a restful environment and minimizing circadian disruption.
The rehabilitation process of stroke patients is complicated and affected by many factors. Current studies may not cover all relevant populations, such as patients with different levels of injury and different stages of recovery. Therefore, this study enrolled patients who had experienced an ischemic stroke within the past 1 to 3 months experiencing their first stroke as patients. A limitation of this study is the small sample size and the treatment duration of four weeks, which may affect the outcomes. This study focused solely on three color temperatures in terms of illumination, acknowledging that other factors like light intensity, exposure timing, and duration can impact results. Additionally, it is recognized that previous light exposure history may alter melatonin sensitivity to subsequent light, suggesting this aspect be addressed in future research.
Conclusion
This study demonstrates that both cold and warm light therapies exert beneficial effects on the recovery of patients within three months of ischemic stroke. Cold light therapy (6500 K), in particular, significantly improved neurological function, physical health status, including better performance on the physical component of quality of life, likely through enhanced non-visual stimulation of ipRGCs, circadian phase advancement, and modulation of neurophysiological processes. These effects may be mediated by improved neural plasticity, reduced oxidative stress, and the entrainment of sleep–wake rhythms via early-morning exposure to blue-enriched light.
Supplementary Information
Acknowledgements
We extend our gratitude to Shanghai keey group co., ltd for providing the residential health lighting system used in this trial, as well as for their contribution to the environmental modification of the rehabilitation training facilities.
Abbreviations
- WLG
Warm light group
- CLG
Cold light group
- CG
Control group
- NIHSS
National institutes of health stroke scale
- SF-36
Short form-36
- PCS
Physical component summary
- MCS
Mental component summary
- SRSS
Self-rating sleep scale
- NE
Norepinephrine
- IL-6
Interleukin 6
- MT
Melatonin
Author contributions
The authors' contributions were as follows: WN, XY and WTY prepared the first draft of the manuscript. WHJ conceived and designed the study. SYQ and HQL managed the ethical approval and clinical trial registration. QFX and LC led the patient recruitment efforts. WZY was in charge of collecting clinical blood samples. JJK, LY, and BZF carried out the questionnaire distribution and patient follow-up. Data analysis was conducted by WN and XY, who had unrestricted access to all data. LDM and WHJ were responsible for reviewing and editing the manuscript. All authors reviewed, approved the final manuscript, and assume accountability for the content, ensuring the accuracy of the data and adherence to the registered protocol and its statistical analysis plan.
Funding
This study was supported by Health Management Outstanding Talents of Shanghai Association of Rehabilitation Medical (2023JGYQ08); Traditional Chinese Medicine Research Project of Shanghai Municipal Health Commission (2022QN029); China Postdoctoral Science Foundation (2023M742642); National Key Clinical Specialty Discipline Construction Project of China (Z155080000004); Shanghai Research Center of Rehabilitation Medicine (Top Priority Research Center of Shanghai) (2023ZZ02027); Shanghai Clinical Research Ward (SHDC2023CRW018B); Shanghai Hospital Development Center Foundation-Shanghai Municipal Hospital Rehabilitation Medicine Specialty Alliance (SHDC22023304); Shanghai Shenkang Hospital Development Center Research Physician Innovation and Translational Capability Training Program (SHDC2023CRS045); Research Project (Medical and Health Category) of the Shanghai Songjiang District Science and Technology (2023SJKJGG078).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was registered on Chinese Clinical Trial Registry (ChiCTR2200057541). The trial protocol was approved by the Ethics Committee of the Shanghai Yangzhi Rehabilitation Hospital (Shanghai Sunshine Rehabilitation Center) (Approval No. 2022–001) and study procedures were conducted according to the principles outlined in the Declaration of Helsinki. All parents or guardians provided written informed consent after thorough explanations of the study goals and procedures were given.
Consent for publication
All the authors provided their consent for publication of the present version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nan Wang, Yan Xue and Tongyue Wang have contributed equally to this work.
Contributor Information
Danmei Lan, Email: landanmei2013@163.com.
Qilong Hu, Email: hqlhz@163.com.
Hengjing Wu, Email: whjdata@126.com.
References
- 1.Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–210. [DOI] [PMC free article] [PubMed]
- 2.Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70. [DOI] [PubMed] [Google Scholar]
- 3.Tu WJ, Wang LD. China stroke surveillance report 2021. Mil Med Res. 2023;10(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383(9913):245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Sui M, Yan T, You L, Li K, Gao Y. A study in persons later after stroke of the relationships between social participation, environmental factors and depression. Clin Rehabil. 2017;31(3):394–402. [DOI] [PubMed] [Google Scholar]
- 6.Chinese Society of Neurology CSS. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin J Neurol. 2018;51(09):666–82. [Google Scholar]
- 7.Cieza A, Causey K, Kamenov K, Hanson SW, Chatterji S, Vos T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;396(10267):2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodgers H, Howel D, Bhattarai N, Cant R, Drummond A, Ford GA, et al. Evaluation of an extended stroke rehabilitation service (EXTRAS): a randomized controlled trial and economic analysis. Stroke. 2019;50(12):3561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terman M. Evolving applications of light therapy. Sleep Med Rev. 2007;11(6):497–507. [DOI] [PubMed] [Google Scholar]
- 10.van Hoof J, Westerlaken AC, Aarts MP, Wouters EJ, Schoutens AM, Sinoo MM, et al. Light therapy: methodological issues from an engineering perspective. Technol Health Care. 2012;20(1):11–23. [DOI] [PubMed] [Google Scholar]
- 11.Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lancet T. Detection and treatment of neonatal jaundice. Lancet. 2010;375(9729):1845. [DOI] [PubMed] [Google Scholar]
- 13.Hart PH, Norval M, Byrne SN, Rhodes LE. Exposure to ultraviolet radiation in the modulation of human diseases. Annu Rev Pathol. 2019;14:55–81. [DOI] [PubMed] [Google Scholar]
- 14.Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897–908. [DOI] [PubMed] [Google Scholar]
- 15.Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M. Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg. 2005;31(3):334–40. [DOI] [PubMed] [Google Scholar]
- 16.Wickenheisser VA, Zywot EM, Rabjohns EM, Lee HH, Lawrence DS, Tarrant TK. Laser light therapy in inflammatory, musculoskeletal, and autoimmune disease. Curr Allergy Asthma Rep. 2019;19(8):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med Rev. 2016;29:52–62. [DOI] [PubMed] [Google Scholar]
- 18.Nussbaumer-Streit B, Forneris CA, Morgan LC, Van Noord MG, Gaynes BN, Greenblatt A, et al. Light therapy for preventing seasonal affective disorder. Cochrane Database Syst Rev. 2019;3(3):011269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An K, Zhao H, Miao Y, Xu Q, Li YF, Ma YQ, et al. A circadian rhythm-gated subcortical pathway for nighttime-light-induced depressive-like behaviors in mice. Nat Neurosci. 2020;23(7):869–80. [DOI] [PubMed] [Google Scholar]
- 20.Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540(7632):230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mårtensson B, Pettersson A, Berglund L, Ekselius L. Bright white light therapy in depression: a critical review of the evidence. J Affect Disord. 2015;182:1–7. [DOI] [PubMed] [Google Scholar]
- 22.Glickman G, Byrne B, Pineda C, Hauck WW, Brainard GC. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs). Biol Psychiatry. 2006;59(6):502–7. [DOI] [PubMed] [Google Scholar]
- 23.Naeser MA, Saltmarche A, Krengel MH, Hamblin MR, Knight JA. Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reports. Photomed Laser Surg. 2011;29(5):351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatori M, Gronfier C, Van Gelder RN, Bernstein PS, Carreras J, Panda S, et al. Global rise of potential health hazards caused by blue light-induced circadian disruption in modern aging societies. NPJ Aging Mech Dis. 2017;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman SA, St Hilaire MA, Lockley SW. The effects of spectral tuning of evening ambient light on melatonin suppression, alertness and sleep. Physiol Behav. 2017;177:221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei H, Wang W, Cao Y, Ma Y, Xue X. Efficacy and safety evaluation of bright light therapy in patients with post-stroke insomnia: a protocol for systematic review and meta-analysis. Medicine (Baltimore). 2021;100(50): e27937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamblin MR. Photobiomodulation for traumatic brain injury and stroke. J Neurosci Res. 2018;96(4):731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woelders T, Beersma DGM, Gordijn MCM, Hut RA, Wams EJ. Daily light exposure patterns reveal phase and period of the human circadian clock. J Biol Rhythms. 2017;32(3):274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S, Kim D. Effect of color light stimulation using LED on sleep induction time. J Healthc Eng. 2017;2017:6030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizza S, Luzi A, Mavilio M, Ballanti M, Massimi A, Porzio O, et al. Impact of light therapy on rotating night shift workers: the EuRhythDia study. Acta Diabetol. 2022;59(12):1589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahidi R, Golmohammadi R, Babamiri M, Faradmal J, Aliabadi M. Effect of warm/cool white lights on visual perception and mood in warm/cool color environments. Excli j. 2021;20:1379–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chellappa SL, Steiner R, Oelhafen P, Lang D, Götz T, Krebs J, et al. Acute exposure to evening blue-enriched light impacts on human sleep. J Sleep Res. 2013;22(5):573–80. [DOI] [PubMed] [Google Scholar]
- 34.Kim WH, Joa KL, Kim CB, Lee HS, Kang SG, Jung HY, et al. The effect of bright light therapy on sleep and quality of life in patients with poststroke insomnia. Psychosom Med. 2022;84(1):123–30. [DOI] [PubMed] [Google Scholar]
- 35.CSS CSoN. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2014. Chinese J Neurol. 2015;48(04):246–57. [Google Scholar]
- 36.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50(12):e344–418. [DOI] [PubMed] [Google Scholar]
- 37.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2016;47(6):e98–169. [DOI] [PubMed] [Google Scholar]
- 38.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2018;49(3):e46–110. [DOI] [PubMed] [Google Scholar]
- 39.l’Eclairage CId, CIE system for metrology of optical radiation for ipRGC-influenced responses to light2018: CIE Vienna, Austria.
- 40.Schlangen LJ, Price LL. The lighting environment, its metrology, and non-visual responses. Front Neurol. 2021;12: 624861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Standard WB. Circadian lighting design. New York: International Well Building Institute; 2022. [Google Scholar]
- 42.Muir KW, Weir CJ, Murray GD, Povey C, Lees KR. Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke. 1996;27(10):1817–20. [DOI] [PubMed] [Google Scholar]
- 43.Kwah LK, Diong J. National institutes of health stroke scale (NIHSS). J Physiother. 2014;60(1):61. [DOI] [PubMed] [Google Scholar]
- 44.Xie D, Qin H, Dong F, Wang X, Liu C, Xue T, et al. Functional connectivity abnormalities of brain regions with structural deficits in primary insomnia patients. Front Neurosci. 2020;14:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu V, Weatherall M, McNaughton H. Estimating the minimal clinically important difference for the physical component summary of the short form 36 for patients with stroke. J Int Med Res. 2021;49(12):3000605211067902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kerkis I, da Silva ÁP, Araldi RP. The impact of interleukin-6 (IL-6) and mesenchymal stem cell-derived IL-6 on neurological conditions. Front Immunol. 2024;15:1400533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, Lin J, Pan Y, Wang M, Meng X, Li H, et al. Interleukin-6 and YKL-40 predicted recurrent stroke after ischemic stroke or TIA: analysis of 6 inflammation biomarkers in a prospective cohort study. J Neuroinflammation. 2022;19(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42(1):33–84. [DOI] [PubMed] [Google Scholar]
- 49.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. [DOI] [PubMed] [Google Scholar]
- 50.Hermans EJ, Henckens MJ, Joëls M, Fernández G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37(6):304–14. [DOI] [PubMed] [Google Scholar]
- 51.Sander D, Winbeck K, Klingelhöfer J, Etgen T, Conrad B. Prognostic relevance of pathological sympathetic activation after acute thromboembolic stroke. Neurology. 2001;57(5):833–8. [DOI] [PubMed] [Google Scholar]
- 52.Alghamdi BS. The neuroprotective role of melatonin in neurological disorders. J Neurosci Res. 2018;96(7):1136–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J, Gao S, Lenahan C, Gu Y, Wang X, Fang Y, et al. Melatonin as an antioxidant agent in stroke: an updated review. Aging Dis. 2022;13(6):1823–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tseng CY, Hsu PS, Lee CT, Huang HF, Lan CC, Hsieh TH, et al. Acupuncture and traditional Chinese herbal medicine integrated with conventional rehabilitation for post-stroke functional recovery: a retrospective cohort study. Front Neurosci. 2022;16: 851333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adams HP Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53(1):126–31. [DOI] [PubMed] [Google Scholar]
- 56.Park S, Koppes RA, Froriep UP, Jia X, Achyuta AK, McLaughlin BL, et al. Optogenetic control of nerve growth. Sci Rep. 2015;5:9669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnstone DM, Moro C, Stone J, Benabid AL, Mitrofanis J. Turning on lights to stop neurodegeneration: the potential of near infrared light therapy in Alzheimer’s and Parkinson’s disease. Front Neurosci. 2015;9:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kushibiki T, Hirasawa T, Okawa S, Ishihara M. Blue laser irradiation generates intracellular reactive oxygen species in various types of cells. Photomed Laser Surg. 2013;31(3):95–104. [DOI] [PubMed] [Google Scholar]
- 59.Zhang K, Duan L, Ong Q, Lin Z, Varman PM, Sung K, et al. Light-mediated kinetic control reveals the temporal effect of the Raf/MEK/ERK pathway in PC12 cell neurite outgrowth. PLoS ONE. 2014;9(3): e92917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497(3):326–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fisk AS, Tam SKE, Brown LA, Vyazovskiy VV, Bannerman DM, Peirson SN. Light and cognition: roles for circadian rhythms, sleep, and arousal. Front Neurol. 2018;9:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Charles A, Czeisler OMB. The human circadian timing system and sleep-wake regulation. Amsterdam: Elsevier Inc.,; 2010. [Google Scholar]
- 63.Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74(2):246–60. [DOI] [PubMed] [Google Scholar]
- 64.Chellappa SL, Steiner R, Blattner P, Oelhafen P, Götz T, Cajochen C. Non-visual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert? PLoS ONE. 2011;6(1): e16429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hozer C, Pifferi F, Aujard F, Perret M. The biological clock in gray mouse lemur: adaptive, evolutionary and aging considerations in an emerging non-human primate model. Front Physiol. 2019;10:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi K, Shin C, Kim T, Chung HJ, Suk HJ. Awakening effects of blue-enriched morning light exposure on university students’ physiological and subjective responses. Sci Rep. 2019;9(1):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoo A, Ortega R, Raymond D, Gerber R, Markgraf M, Liang J, et al. Pilot Tailored Lighting Intervention on Sleep Disturbances in Parkinson disease (P7–11.014). AAN Enterprises; 2023.
- 68.Legenbauer T, Kirschbaum-Lesch I, Jörke C, Kölch M, Reis O, Berger C, et al. Bright light therapy as add-on to inpatient treatment in youth with moderate to severe depression: a randomized clinical trial. JAMA Psychiat. 2024;81(7):655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wytrykowska A, Prosba-Mackiewicz M, Nyka WM. IL-1β, TNF-α, and IL-6 levels in gingival fluid and serum of patients with ischemic stroke. J Oral Sci. 2016;58(4):509–13. [DOI] [PubMed] [Google Scholar]
- 70.Shaafi S, Sharifipour E, Rahmanifar R, Hejazi S, Andalib S, Nikanfar M, et al. Interleukin-6, a reliable prognostic factor for ischemic stroke. Iran J Neurol. 2014;13(2):70–6. [PMC free article] [PubMed] [Google Scholar]
- 71.Lockard GM, Alayli A, Monsour M, Gordon J, Schimmel S, Elsayed B, et al. Probing interleukin-6 in stroke pathology and neural stem cell transplantation. Int J Mol Sci. 2022;23(24):15453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Georgakis MK, Malik R, Gill D, Franceschini N, Sudlow CLM, Dichgans M. Interleukin-6 signaling effects on ischemic stroke and other cardiovascular outcomes: a mendelian randomization Study. Circ Genom Precis Med. 2020;13(3): e002872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Armstead WM, Hekierski H, Pastor P, Yarovoi S, Higazi AA, Cines DB. Release of IL-6 after stroke contributes to impaired cerebral autoregulation and hippocampal neuronal necrosis through NMDA receptor activation and upregulation of ET-1 and JNK. Transl Stroke Res. 2019;10(1):104–11. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki S, Tanaka K, Suzuki N. Ambivalent aspects of interleukin-6 in cerebral ischemia: inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab. 2009;29(3):464–79. [DOI] [PubMed] [Google Scholar]
- 75.Zhu H, Hu S, Li Y, Sun Y, Xiong X, Hu X, et al. Interleukins and Ischemic Stroke. Front Immunol. 2022;13: 828447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390(6660):604–7. [DOI] [PubMed] [Google Scholar]
- 77.Roozendaal B, Hermans EJ. Norepinephrine effects on the encoding and consolidation of emotional memory: improving synergy between animal and human studies. Curr Opin Behav Sci. 2017;14:115–22. [Google Scholar]
- 78.Morita T, Tokura H. The influence of different wavelengths of light on human biological rhythms. Appl Human Sci. 1998;17(3):91–6. [DOI] [PubMed] [Google Scholar]
- 79.Bani Issa W, Abdul Rahman H, Albluwi N, Samsudin ABR, Abraham S, Saqan R, et al. Morning and evening salivary melatonin, sleepiness and chronotype: a comparative study of nurses on fixed day and rotating night shifts. J Adv Nurs. 2020;76(12):3372–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.