Abstract
Trypanosoma cruzi, the causative agent of Chagas' disease, induces transient thymic aplasia early after infection—a phenomenon that stills lacks a molecular explanation. The parasite sheds an enzyme known as trans-sialidase (TS), which is able to direct transfer-sialyl residues among macromolecules. Because cell-surface sialylation is known to play a central role in the immune system, we tested whether the bloodstream-borne TS is responsible for the thymic alterations recorded during infection. We found that recombinant TS administered to naive mice was able to induce cell-count reduction mediated by apoptosis, mimicking cell subsets distribution and histologic findings observed during the acute phase of the infection. Thymocytes taken after TS treatment showed low response to Con A, although full ability to respond to IL-2 or IL-2 plus Con A was conserved, which resembles findings from infected animals. Alterations were found to revert several days after TS treatment. The administration of TS-neutralizing Abs to T. cruzi-infected mice prevented thymus alterations. Results indicate that the primary target for the TS-induced apoptosis is the so-called “nurse cell complex”. Therefore, we report here supporting evidence that TS is the virulence factor from T. cruzi responsible for the thymic alterations via apoptosis induction on the nurse cell complex, and that TS-neutralizing Abs elicitation during infection is associated with the reversion to thymic normal parameters.
Sialyl residues that decorate the cell surface glycoconjugates are involved, among other functions, in the cell–cell interactions that modulate the trafficking of cortical immature thymocytes to the medulla (1–3). Therefore, the possibility can be considered that exogenous alterations of the distribution or density of the cell surface sialylation pattern mediated by pathogen-derived products might strongly affect the normal development of thymocytes and, therefore, the production of T lymphocytes. Trypanosoma cruzi, the parasite protozoan agent of the Chagas' disease that affects about 20 million people in the Americas, seems to employ this immune system-disturbing strategy. The parasite expresses an enzyme known as trans-sialidase (TS), which is able to direct transfer α2,3-linked terminal sialyl residues among glycoconjugates (4–6) and to induce apoptosis in components of the immune system in vivo (7).
T. cruzi trypomastigote-derived TS is glycosylphosphatidyl inositol-anchored on the parasite cell surface (8); it is shed to the milieu and detected in blood during the acute stage of Chagas' disease both in patients and in infected mice (9, 10). Together with apoptosis induction in the immune system (7), other evidence supports that TS constitutes a virulence factor from T. cruzi (11–13). Infection survivor animals and chronic Chagas' disease patients display neutralizing Abs that inhibit TS activity (14–16); therefore, the systemically distributed enzyme may only work early during the infectious process.
During the acute phase of the infection, T. cruzi is known to induce strong although transient cell depletion in thymus, especially in the cortical zone (17–19), a phenomenon described as early as 1955 (20). Deep alterations are found in cell count both in CD4/CD8 double-positive thymocytes (DP) and CD4 or CD8 single-positive thymocytes (SP) thymocyte populations; meanwhile, the CD4/CD8 double-negative (DN) thymocyte precursor population seems unaffected (17–19). Thymocyte depletion seems associated with T. cruzi-induced alterations of the thymic microenvironment, where reduced thymic epithelial cells (TEC) numbers and ultrastructural alterations in these cells are found (21). These findings still lack a suitable explanation based in a pathogenic mechanism, especially because no TEC infection is required for cell depletion (21).
Here, we present in vivo evidence that TS is the virulence factor from T. cruzi responsible for inducing the thymic involution observed during the acute phase of Chagas' disease, and that this effect is mediated by apoptosis induction in the nurse cell complex. We conclude that this effect is transient mainly because of the elicitation of TS-neutralizing Abs in infection survivors.
Materials and Methods
Mice.
BALB/c or C3H/HeN male mice (60 to 90 days old) were used in all of the experiments. Animals were either bred in our facilities or obtained from the SPF (specific pathogen free) colony of the Comisión Nacional de Energía Atómica, Ezeiza, Buenos Aires.
trans-Sialidase.
Enzymatically active TS (6) or inactive TS (iTS; ref. 22) recombinant proteins were expressed in Escherichia coli DH5α. Because 13 units of SAPA repeats allow TS to persist in blood for at least 3 days (23), constructions containing this repeat amount were used throughout. Proteins were induced with isopropyl β-d-thiogalactopyranoside (Sigma) and purified to homogeneity by immobilized metal affinity chromatography through Ni2+-charged Hi-Trap chelating columns (Amersham Pharmacia) followed by ion exchange chromatography through a Mono Q column (Amersham Pharmacia), as described (24). TS or iTS (1 μg/dose) contained in 0.2 ml of PBS were injected i.v.
T. cruzi Infection.
High virulent RA T. cruzi strain maintained by serial passages in mice was used in this work. Mice were inoculated with 50 sanguineous trypomastigote forms by the i.p route.
Thymocyte Isolation and Culture.
Thymi were collected, and a single-cell suspension was prepared in RPMI 1640 medium plus 10% (vol/vol) FBS (both from GIBCO/BRL). RBC were lysed by Tris-buffered ammonium chloride treatment, and viable nucleated cells (trypan blue dye-exclusion test) were counted in a hemocytometer and plated in RPMI medium 1640 plus 10% (vol/vol) FBS at a density of 500,000 per well in 96-well flat-bottom tissue culture plates (Sarstedt). Cells were treated with Con A (2.5 μg/ml; Sigma) and/or recombinant IL-2 (20 ng/ml, 2 × 106 units/mg protein; PharMingen).
To determine the ability of TS to induce apoptosis in vitro, 106 normal thymocytes per well were cultured with 1 μg of enzyme for 24 h in 24-well flat-bottom tissue culture plates in a final volume of 1 ml (Becton Dickinson). Hipoploidy was determined by propidium iodide staining. Fluorescence was analyzed with an Ortho Cytoron Absolute flow cytometer (Ortho Diagnostics).
TS Activity on Thymocytes.
Single-cell suspension was obtained and thoroughly washed in RPMI medium 1640 without FBS, then 500,000 thymocytes were used as sialyl residue donors. Cells were incubated with d-glucose-1-[14C]lactose [400,000 cpm, 55 mCi/mmol (1 Ci = 37 GBq), Amersham Pharmacia] and 0.5 μg of TS in a final volume of 50 μl. After a 10-min reaction at room temperature, cells were pelleted, and supernatant was diluted with 1 ml of water. As control, a similar reaction without TS was performed. Reaction products were recovered by the addition of QAE-Sephadex (Amersham Pharmacia) as described (23), and radioactivity was determined. The reaction product was characterized as α2–3 sialyllactose because treatment with α2–3 neuraminidase from Salmonella typhimurium (New England Biolabs) inhibited radioactive binding to the resin.
Surface Thymocyte Markers Immunofluorescence Analysis.
After RBC lysis, thymocytes were washed with cold staining buffer (PBS plus 2% FBS/0.1% NaN3) and pelleted at 1–2 × 106 viable cells per tube. Cells were resuspended in 50 μl of the same buffer plus 1–2 μg of a rat mAb anti-mouse CD16/CD32 (clone 2.4G2, Fc Block). After a 30-min incubation on ice, 1 μg of each R-phycoerythrin-conjugated rat anti-mouse CD4 (clone H129.19) or anti-mouse CD25 (clone 3C7) or FITC-conjugated rat anti-mouse CD8 (clone 53–6.7) mAbs were added and incubated 30 min more. Isotype-matched labeled mAbs were used as control. All mAbs were from PharMingen. Fluorescence was assayed with an Ortho Cytoron Absolute flow cytometer (Ortho Diagnostics).
Passive TS-Neutralizing Abs Transference.
Rabbits were immunized with three doses of enzyme (50 μg/each) adsorbed onto alumina at 20 day intervals. Animals were bled, and sera were tested in a TS-inhibition assay (TIA; ref. 15). Sera from best responders were pooled and IgG was purified by protein A affinity chromatography (Hi-Trap, Amersham Pharmacia). To deplete from anti-SAPA Abs, IgG was seeded onto an affinity column constructed by coupling TS without SAPA antigen (24) to NHS-HiTrap columns (Amersham Pharmacia). Eluted Abs were tested for their ability to inhibit the enzyme by TIA (15). No remnant anti-SAPA Abs were detected.
T. cruzi-infected mice received 400 μg of Ab i.p. at day 7 postinfection (pi). Infected control animals received the same amount of protein A-purified IgG from normal rabbits. Transferred TS-neutralizing Abs were detected in sera when thymi were collected at day 12 pi (not shown).
Histology.
Organs were collected, washed in PBS, and fixed in 4% (wt/vol) paraformaldehyde in PBS, then dehydrated and embedded in paraffin. Contiguous 5-μm sections were mounted and stained with hematoxylin/eosin (H&E) following standard procedures. The number of apoptotic bodies by field (×400) was determined by optical microscopy. To highlight cells undergoing apoptosis, unstained sections mounted in silanized slides were subjected to terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) by using the ApopTag kit for immunoperoxidase staining (Intergen, Purchase, NY) followed by slight hematoxylin counterstaining.
To label TEC cells, rat anti-mouse CD44 (BioGenex Laboratories, San Ramon, CA) mouse anti-cytokeratin-8 low molecular weight (Dako) and mouse anti-ICAM-1 (clone G-5, Santa Cruz Biotechnology) mAbs were used in unstained contiguous sections. Detection was performed with biotinylated secondary Abs (Multilink; BioGenex Laboratories) and horseradish peroxidase-labeled streptavidin (Label; BioGenex Laboratories). Reaction was developed with 3,3′-diaminobenzidine tetrahydrochloride (DAB Kit, BioGenex Laboratories). Slight counterstaining with hematoxylin then was performed.
Quality control of sections was performed by anti-CD3 immunostaining. Positive and negative controls for each Ab also were performed.
Nurse Cells Purification and TUNEL Assay.
Thymi were collected from normal or TS-injected (1 μg) mice. Nurse cells were obtained after cutting the organs into small pieces; protease digestion and FBS gradient followed essentially the procedure described in (25). TUNEL assay was performed with the Apoptag Fluorescein Direct in Situ Apoptosis Detection Kit from Intergen, following the manufacturer's recommendations.
Thymic Organ Cultures.
Melted 2% agarose in water was mixed 1:1 vol:vol with RPMI medium 1640 without FBS. Twenty-four-well culture plates (Falcon) at 0.2 ml per well were seeded and hardened. Wells then were filled with RPMI medium 1640 plus 10% (vol/vol) FBS and incubated overnight at 37°C in 5% CO2. Medium was aspirated, and small pieces of normal thymus (about 2–3 mm) were incubated at 37°C in 5% CO2 with 1 ml of RPMI medium 1640 plus 10% (vol/vol) FBS with or without 1 μg of TS. Eighteen hours later, pieces were collected in 4% (wt/vol) paraformaldehyde in PBS, processed for histology, and stained with H&E, as above.
Results
T. cruzi Infection Induces Thymocyte Apoptosis That Correlates with Thymic Involution.
Several groups have previously described a reduction in thymocyte number during the acute infection with T. cruzi (18–20). To elucidate if this phenomenon is associated with apoptosis induction, histologic analysis and TUNEL assays were performed in organs from 18 days pi mice. As shown in Fig. 1, profound histoarchitecture alterations compatible with thymic involution (26) were observed in T. cruzi-infected mice that are consistent with previous descriptions (17, 20). As revealed by TUNEL labeling, apoptosis was highly increased and involved defined groups of cells. Cells undergoing apoptosis were located especially in the thymus cortex in coincidence with a strong reduction of this area. Therefore, thymus involution in T. cruzi infection seems to be directly related with apoptosis induction.
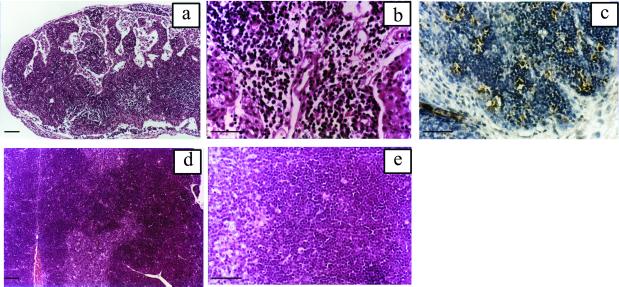
Figure 1.
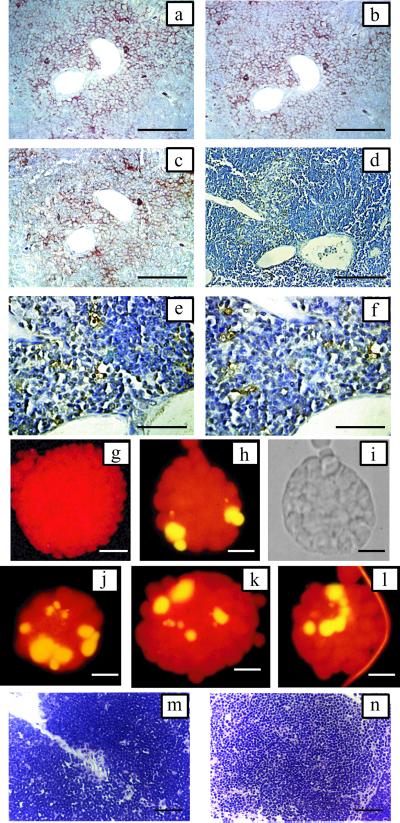
Thymic alterations induced by T. cruzi infection. Animals were injected with 50 bloodstream forms and thymi were collected 19 days pi. (a) Low magnification of the organ displaying strong cortex reduction and highly altered histoarchitecture. (b) Detail of the thymic cortex showing thymocyte depletion. (c) TUNEL labeling of cortex displaying high number of cells with positive staining (dark brown). (d and e) Normal thymus for comparative purposes. (a, b, d, and e) H&E staining. [Bars = 100 μm (a and d) and 50 μm (b, c, and e).]
In Vivo TS Treatment Mimics T. cruzi Acute Infection-Induced Thymocyte Depletion.
The role of T. cruzi TS in thymus involution was evaluated by i.v. injection of recombinant enzyme. Apoptosis pattern in organs taken 18 h after TS injection was indistinguishable from that observed in T. cruzi-infected mice (Fig. 2). The effect of sustained TS administration then was assayed. When a three-dose schedule (days 0, 2, and 4) was used, a strong reduction of about 70% in viable thymocyte suspension count (Fig. 2) and thymic weight (not shown) was achieved at day 7. Histologic findings accompanied these observations (Fig. 2). Strong reduction of thymus cortex that displays about 75% less cellularity, as evaluated by direct count, was found. Therefore, when the thymus is exposed to TS for long periods, it displays histologic images and suffers cell number alteration that mimics findings in T. cruzi-infected animals.
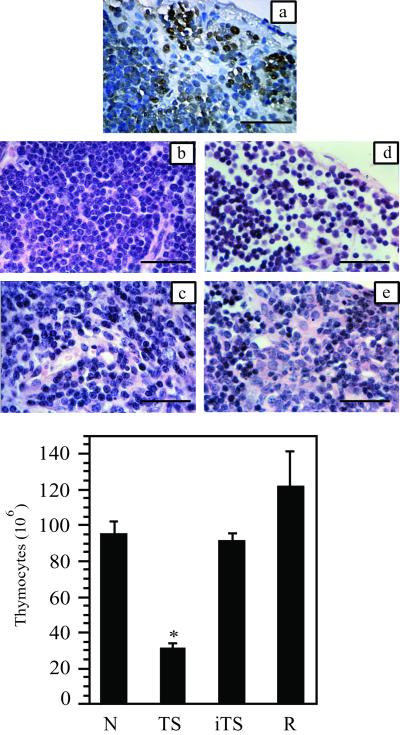
Figure 2.
Thymic alterations induced by TS. (a) TUNEL labeling of thymus cortex 18 h after a single dose of 1 μg of TS. (b and c) Thymus from normal mice cortex (b) and medulla (c). (d and e) Thymus from mouse receiving three doses of TS with thymocyte depletion in cortex (d) and medulla (e). (b–e) H&E staining. (Bar = 25 μm.) Total thymocyte count in organs taken from: N, naive mice; TS, TS-treated animals (three doses of 1 μg per each mouse, given i.v.); iTS, mice treated with Tyr-342→His enzymically inactive TS (three doses of 1 μg per each mouse, given i.v.); R, animals allowed to recover for 10 days after the last TS dose. There were at least four animals in each group. (*, P < 0.01; Student's t test.)
Cell-surface sialyl residue mobilization was required, because no alterations were observed when a recombinant iTS made enzymatically inactive by the natural mutation Tyr-342→His (27) was administered (Fig. 2). The inactive protein still retained the ability to interact with the sugar molecules involved in the sialyl residue transfer reaction (22), being, therefore, properly folded and able to recognize putative cell-surface acceptor molecule(s). Involvement of possible contaminant bacterial products can be ruled out, because both proteins were produced and purified in parallel following exactly the same protocol.
Normal thymocyte count was recovered in animals left 10 days after administration of TS in a three-dose schedule (Fig. 2). These results indicate that the effect of the treatment was transient, which is consistent with the observation that, in survivor animals, thymic cellularity is recovered (18, 19).
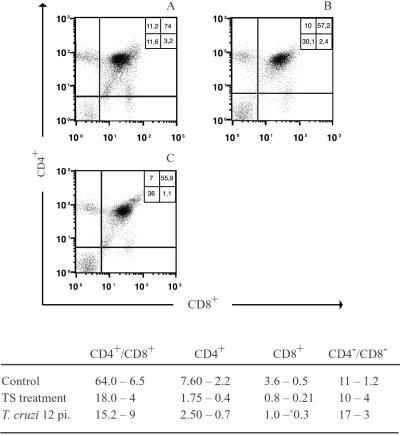
Thymus population subsets were studied in more detail. The viable-cell count and CD4/CD8 surface markers bearing cell percentages were found to be comparable in organs taken either at 12 days after T. cruzi infection or after TS treatment (Fig. 3). The DP thymocyte population and its SP progeny were reduced about 70% in both cases. In contrast, the total cell number in the precursor DN populations remained unaffected or even increased. Similar results also were reported for T. cruzi-infected mice by other authors (17–19), thus supporting the TS involvement in these alterations during infection.
Figure 3.
CD4/CD8 profiles of total thymocytes from T. cruzi-infected or TS-treated mice analyzed by fluorocytometry. (A) Naive mouse. (B) Mouse receiving three TS doses (1 μg per each) i.v. (C) T. cruzi-infected mouse killed at day 12 pi. Numbers indicate relative percentages of cells in each quadrant. (Bottom) Total cell numbers from each subpopulation (× 106). There were at least three animals in each group.
In Vivo TS Treatment Affects in Vitro Thymocyte Proliferation.
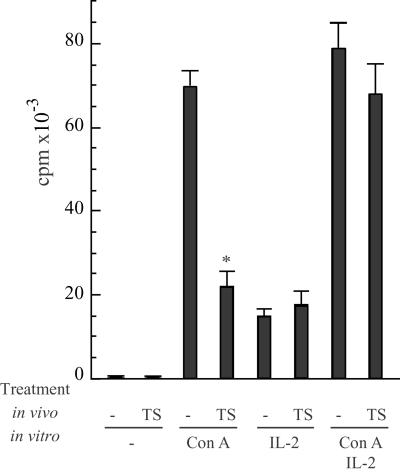
Reduced ConA proliferation of thymocytes was reported during T. cruzi infection (28). The ability of cells from TS-treated mice to respond to in vitro mitogen stimuli was, therefore, tested. Animals were injected with a single dose of TS, and cells were taken 24 h later to assay for ConA proliferation. As depicted in Fig. 4, thymocytes taken from TS-treated mice showed a highly reduced response to ConA. No significant difference to normal proliferation values was found when IL-2 was added either in the absence or presence of ConA, thus suggesting that those cells that do not enter into apoptosis remained able to respond to exogenous IL-2 addition (but not to fully proliferate to single ConA stimulus). After TS treatment, the percentage of CD25+ thymocytes was found to be similar to that in untreated mice (2.2 ± 0.15 control vs. 1.8 ± 0.25 TS treatment; n = 3), thus suggesting that the depleted population can be included beyond the DN CD25+ immature cells.
Figure 4.
Proliferation of thymocytes taken from TS-treated mice. In vivo, cells were collected from naive mice (−) or from animals receiving a single dose of 1 μg of TS 24 h before (TS); in vitro, cells were treated with ConA (2.5 μg/ml) and/or IL-2 (20 units/ml). (−), no in vitro treatment. (*, P < 0.01; Student's t test.)
trans-Sialidase Induces Apoptosis in the Nurse Cell Complex.
It was reported that TEC isolated from T. cruzi-infected mice release thymocytes and are less adherent to substrate (21). Here, we show (Fig. 1) that apoptosis occurs in grouped cells in the thymus either from infected or TS-treated animals leading to deletion of DP cells; meanwhile, the pool of DN cells is not affected either in number or functionality, as evaluated by their proliferation response. Taken together, these findings suggest that, where the immature DP thymocytes suffer selection, the nurse cell complex might constitute the target of the enzyme. To test this hypothesis, thymi were taken from mice that received a single TS dose 18 or 24 h before, and contiguous sections were processed for immunohistochemistry. Areas of coincidence of TEC markers reactivity (CD44, ICAM-1, and cytokeratin 8; ref. 29) displayed more TUNEL-positive cells by direct counting under a 100-μm grid than did unreactive areas (2–3 TUNEL-positive cells per 150 cells vs. 0–1 per 150; Fig. 5). Furthermore, TUNEL assay was performed on nurse cells purified from thymus collected 18 h after TS administration. Cell complexes obtained from treated animals were consistently smaller than those from normal mice (Fig. 5, g vs. h–l). All of the cell complexes contained apoptotic thymocytes (at least two, with an estimated mean of four); meanwhile, only 10% of complexes from normal mice contained TUNEL-positive cells, and, in these cases, it was usually only one. When TS was added to normal thymus organ cultures, strong cell depletion was found (Fig. 5 m and n). By another way, TS was unable to induce a significant apoptosis percentage on thymocytes in culture, as evaluated by propidium iodide staining/FACS analysis (18 ± 1.1 control vs. 18.4 ± 2 TS treatment, n = 9). However, the transference of sialyl residues from the cell surface to [14C]lactose was readily detected (6,181 cpm vs. 680 cpm without TS and 720 cpm after α2–3 neuraminidase treatment). Besides, the ability of the enzyme to act on cellular surfaces was also documented by other authors (30–32). These results strongly support that the nurse cell complex is the target of the apoptosis-mediated thymic cell depletion induced by the TS.
Figure 5.
TS-induced apoptosis of thymocytes occurs in the nurse cell complexes. Detection of TEC markers by immunohistochemistry and TUNEL staining in contiguous sections of cortico-medullar junction at 18 h after i.v. TS administration. (a) CD44 membrane staining in nurse cell complex. (b) Cytokeratin 8 cytoplasmic staining in epithelial-reticular cells and nurse cells. (c) I-CAM 1 membrane staining in nurse cell complex. (d–f) TUNEL staining. Reactive cells and apoptotic bodies located at the same area displaying immunoreactivity in a–c. Blood vessels are included as references. Bars = 50 μm (a–d) and 25 μm (e–f). Fluorescein-TUNEL reactivity of nurse cell complex collected 18 h after in vivo TS administration. Yellow fluorescence highlights TUNEL reactive cells. Counterstaining was done with propidium iodide. (g) Nurse cell complex from normal animals. (Bar = 50 μm.) (h–l) Nurse cell complexes from TS-treated mice. (Bars = 25 μm.) (i) Phase contrast of h. Thymic organ cultures. Small pieces of normal thymus were cultured with TS for 18 h. (m) Control. (n) TS-treatment, H&E staining. (Bar = 50 μm.)
During T. cruzi Infection, TS Is the Virulence Factor That Induces the Transient Thymic Involution.
Evidence reported here strongly suggests that the bloodstream-borne enzyme might be the virulence factor responsible for the observed transient thymus involution. It should be noted that from day 24 pi, TS-neutralizing Abs are detected in sera from survivor animals (10), thus suggesting that they might be involved in the reversion of thymic involution. In looking for direct evidence supporting these hypotheses, purified TS-neutralizing Abs were given to T. cruzi-infected mice on day 7 pi. At day 12 pi, thymi were taken, and the ability of neutralizing Abs to prevent thymocyte depletion was researched. Only slight reduction (20%) in thymus cellularity was observed in mice receiving neutralizing Abs, in contrast to a 70% overall reduction when normal IgG was given (Fig. 6 and Table 1). In animals passively transferred with TS-neutralizing Abs, cellularity was conserved in concert with a marked reduction of the T. cruzi-induced apoptotic rate, a finding that contrasts with that from animals that received normal IgG as control (Fig. 6). These findings provide further support to our working hypothesis that associates the bloodstream-borne TS with the thymocyte depletion observed during T. cruzi infection.
Figure 6.
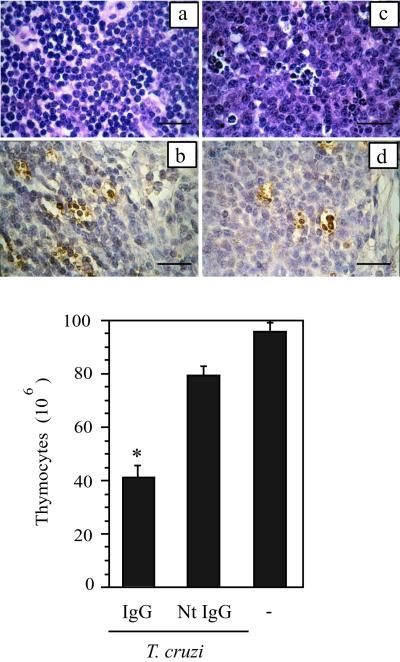
TS-neutralizing Abs prevent T. cruzi-induced apoptosis in thymocytes. (a and b) Thymus cortex from infected mice receiving normal IgG. (c and d) Thymus cortex from an infected mouse receiving TS-neutralizing IgG. (a and c) H&E staining. (b and d) TUNEL staining. Notice in d the reduced number of reactive cells. (Bar = 25 μm.) Total thymocyte count in organs collected from T. cruzi-infected mice at 12 days pi. Animals received 400 μg of either normal rabbit IgG or rabbit TS-neutralizing Abs (NtIgG) i.p. at day 7 pi. (−), naive mouse. There were at least five animals in each group. (*, P < 0.01; Student's t test)
Table 1.
TUNEL positive cells in thymus
| Treatment | Cortex | Medulla |
|---|---|---|
| None | 0 | 0 |
| Normal IgG | 15 ± 4.6 | 6 ± 0.2 |
| TS-neutralizing IgG | 3.5 ± 0.5 | 1.5 ± 0.6 |
Results are from direct counting on tissue sections displayed in Fig. 6.
Discussion
In the host–parasite interplay, the identification of molecules able to interact with the immune system of the host is central to revealing the hidden pathogenic mechanisms of an infectious disease. In this context, those molecules that are shed by the infectious agents being systemically distributed may be crucial in the evolution of phenomena observed during mammalian infections.
During the acute stage of T. cruzi infection, thymocyte depletion (especially in the cortical zone) leading to thymic involution was observed (17, 19, 20). Similar cell depletion was also observed in Peyer's patches, an observation that has been associated with the thymus involution and presumably low T cell output (19).
Apoptosis induction is a frequent pathogenic mechanism used by parasites and other pathogens to avoid immune response (33). An increased apoptosis rate in thymus from T. cruzi-infected mice is reported here as causative for cell depletion. At present, no other parasite molecule than TS (7) has been associated with this phenomenon. Exogenously administered enzyme was able to mimic findings from thymus collected after T. cruzi infection either by cell count, histology, or CD4/CD8 markers analysis. These alterations reverted to normal parameters several days after the last dose of TS. When systemic TS activity in infected mice was abolished by the administration of TS-neutralizing Abs, the reduction in thymus cellularity was prevented (Fig. 6). These results strongly support both that TS is the virulence factor from T. cruzi inducing thymus cell depletion and that the neutralizing Abs allow the replenishing of the organ. These results are consistent with observations from others working with infected mice showing that thymus cellularity recovers after the parasitemia is controlled (18, 19). By coincidence, TS-neutralizing Abs were detected in serum from 24 days pi (10).
The DP cell population was reduced in number after TS treatment (Fig. 3), a finding also consistent with that obtained in T. cruzi infection by others (17, 19). Results from Con A and IL-2 stimuli support that DN CD25+ cells from TS-treated mice were still functional, although Con A-responding cells (mainly DP cells) were impaired. The DN population was not reduced in number, and, therefore, it seems that apoptosis induced by TS happens in those immature single positive cells that are the immediate precursors of the DP cells or in the DP cells themselves (34). It is noteworthy that cells entering into apoptosis were grouped and not necessarily located close to thymic vessels (see Figs. 1 and 2), suggesting that the effect of the enzyme is not just associated with its diffusion from blood. An attractive hypothesis is that TS does not induce apoptosis on immature T cells directly, but acts through the nurse cell complex, because affected cells express the markers of nurse cell-associated DP thymocytes. In fact, TS was unable to induce significant apoptosis on isolated thymocytes in vitro despite the fact that TS was able to mediate sialyl residue transfer from the thymocyte surface. In contrast, cell depletion was readily observed in TS-treated thymic organ cultures (Fig. 5). Serial slices from thymus taken from TS-treated mice allowed us to superimpose TEC-related markers with apoptosis in thymocytes. Finally, TUNEL assay performed on purified complexes from TS-treated animals showed a high number of apoptotic thymocytes. These results support both that TS is able to mobilize surface sialyl residues and that further apoptosis induction requires interaction with another cell/molecule. This interaction seems to occur inside the nurse cell complex. Therefore, the depletion of thymocytes inside the nurse cell complex seems to be the TS-mediated event leading to thymic cellularity reduction induced by T. cruzi. In this sense, Cotta-de-Almeida et al. (21) have provided evidence supporting that the infection strongly affects the thymic nurse cell complex both in number and in behavior.
Different patterns of sialylation were found in different thymocyte subsets as well as the expression of sialyl transferases (1, 2, 35). Peanut agglutinin-binding properties vary from cortical to medullary thymocytes in association with sialic acid acquisition on the cell surface (1). Then, the expression of α2–3 and α2–6 sialyl transferase activities perhaps are among the most important mechanisms to modify the cell surface and then to regulate the intrathymic routing. Therefore, an exogenous α2–3 sialyltransferase-like activity mediated by TS and working at the thymocyte vicinity may modify the cell surface, mimicking the endogenous expression of the equivalent enzyme, although in the wrong place and/or at the wrong time. Exogenous modifications of the surface sialyl-residue pattern might be crucial to decide the final fate of the cells altered by positive/negative selection interference or interaction with thymic lectins (3, 35, 36). The enzyme acts by mobilization of the sialyl residue, leading to either acquisition or loss of the residue by transferring it among glycoconjugates of the same cell, among different cells, or to the milieu. Therefore, using TS as a tool to modify the cell surface sugar motifs on T and/or thymic epithelial cells, T. cruzi seems able to subvert the thymocyte fate by apoptotic induction.
Acknowledgments
We thank Dr. C. A. Buscaglia for critical reading of the manuscript. This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica and the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) from Argentina, and from the World Bank/United Nations Development Programme/World Health Organization Special Program for Research and Training in Tropical Diseases. M.S.L. and O.C. are Researchers from the CONICET.
Abbreviations
- TS
trans-sialidase
- DP
CD4/CD8 double-positive thymocytes
- SP
CD4/CD8 single-positive thymocytes
- DN
CD4/CD8 double-negative thymocytes
- TEC
thymic epithelial cells
- iTS
enzymatically inactive trans-sialidase
- pi
postinfection
- H&E
hematoxylin/eosin
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gillespie W, Paulson J C, Kelm S, Pang M, Baum L G. J Biol Chem. 1993;268:3801–3804. [PubMed] [Google Scholar]
- 2.Baum L G, Derbin K, Perillo N L, Wu T, Pang M, Uittenbogaart C. J Biol Chem. 1996;271:10793–10799. doi: 10.1074/jbc.271.18.10793. [DOI] [PubMed] [Google Scholar]
- 3.Perillo N L, Uittenbogaart C H, Nguyen J T, Baum L G. J Exp Med. 1997;185:1851–1858. doi: 10.1084/jem.185.10.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandekerckhove F, Schenkman S, Pontes de Carvalho L, Tomlinson S, Kiso M, Yoshida M, Hasegawa A, Nussenzweig V. Glycobiology. 1992;2:541–548. doi: 10.1093/glycob/2.6.541. [DOI] [PubMed] [Google Scholar]
- 5.Ferrero-Garcia M A, Trombetta S E, Sanchez D O, Reglero A, Frasch A C, Parodi A J. Eur J Biochem. 1993;213:765–771. doi: 10.1111/j.1432-1033.1993.tb17818.x. [DOI] [PubMed] [Google Scholar]
- 6.Campetella O E, Uttaro A D, Parodi A J, Frasch A C. Mol Biochem Parasitol. 1994;64:337–340. doi: 10.1016/0166-6851(94)00036-0. [DOI] [PubMed] [Google Scholar]
- 7.Leguizamón M S, Mocetti E, García Rivello H, Argibay P, Campetella O. J Infect Dis. 1999;180:1398–1402. doi: 10.1086/315001. [DOI] [PubMed] [Google Scholar]
- 8.Agusti R, Couto A S, Campetella O E, Frasch A C, de Lederkremer R M. Glycobiology. 1997;7:731–735. doi: 10.1093/glycob/7.6.731. [DOI] [PubMed] [Google Scholar]
- 9.de Titto E H, Araujo F G. Clin Immunol Immunopathol. 1988;46:157–161. doi: 10.1016/0090-1229(88)90016-5. [DOI] [PubMed] [Google Scholar]
- 10.Leguizamón M S, Campetella O E, González Cappa S M, Frasch A C. Infect Immun. 1994;62:3441–3446. doi: 10.1128/iai.62.8.3441-3446.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schenkman S, Eichinger D, Pereira M E, Nussenzweig V. Annu Rev Microbiol. 1994;48:499–523. doi: 10.1146/annurev.mi.48.100194.002435. [DOI] [PubMed] [Google Scholar]
- 12.Chuenkova M, Pereira M E. J Exp Med. 1995;181:1693–1703. doi: 10.1084/jem.181.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belen Carrillo M, Gao W, Herrera M, Alroy J, Moore J B, Beverley S M, Pereira M A. Infect Immun. 2000;68:2728–2734. doi: 10.1128/iai.68.5.2728-2734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leguizamón M S, Campetella O, Russomando G, Almiron M, Guillen I, Ganzález Cappa S M, Frasch A C. J Infect Dis. 1994;170:1570–1574. doi: 10.1093/infdis/170.6.1570. [DOI] [PubMed] [Google Scholar]
- 15.Leguizamón M S, Russomando G, Luquetti A, Rassi A, Almiron M, González-Cappa S M, Frasch A C, Campetella O. J Infect Dis. 1997;175:1272–1275. doi: 10.1086/593697. [DOI] [PubMed] [Google Scholar]
- 16.Leguizamón M S, Russomando G, Rojas de Arias A, Samudio M, Cabral M, González-Cappa S M, Frasch A C, Campetella O. Clin Diagn Lab Immunol. 1998;5:254–255. doi: 10.1128/cdli.5.2.254-255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savino W, Leite-de-Moraes M C, Hontebeyrie-Joskowicz M, Dardenne M. Eur J Immunol. 1989;19:1727–1733. doi: 10.1002/eji.1830190930. [DOI] [PubMed] [Google Scholar]
- 18.Leite-de-Moraes M C, Hontebeyrie-Joskowicz M, Dardenne M, Savino W. Immunology. 1992;77:95–98. [PMC free article] [PubMed] [Google Scholar]
- 19.Antunez M I, Feinstein R E, Cardoni R L, Gronvik K O. Exp Parasitol. 1997;87:58–64. doi: 10.1006/expr.1997.4171. [DOI] [PubMed] [Google Scholar]
- 20.Taliaferro W H, Pizzi T. J Infect Dis. 1955;96:199–226. doi: 10.1093/infdis/96.3.199. [DOI] [PubMed] [Google Scholar]
- 21.Cotta-de-Almeida V, Bertho A L, Villa-Verde D M, Savino W. Clin Immunol Immunopathol. 1997;82:125–132. doi: 10.1006/clin.1996.4283. [DOI] [PubMed] [Google Scholar]
- 22.Cremona M L, Campetella O, Sanchez D O, Frasch A C C. Glycobiology. 1999;9:581–587. doi: 10.1093/glycob/9.6.581. [DOI] [PubMed] [Google Scholar]
- 23.Buscaglia C A, Alfonso J, Campetella O, Frasch A C. Blood. 1999;93:2025–2032. [PubMed] [Google Scholar]
- 24.Buschiazzo A, Frasch A C, Campetella O. Cell Mol Biol (Paris) 1996;42:703–710. [PubMed] [Google Scholar]
- 25.Hiramine C, Nakagawa T, Miyauchi A, Hojo K. Lab Invest. 1996;75:185–201. [PubMed] [Google Scholar]
- 26.Bar-Dayan Y, Afek A, Goldberg I, Kopolovic J. Tissue Cell. 1999;31:391–396. doi: 10.1054/tice.1999.0001. [DOI] [PubMed] [Google Scholar]
- 27.Cremona M L, Sanchez D O, Frasch A C, Campetella O. Gene. 1995;160:123–128. doi: 10.1016/0378-1119(95)00175-6. [DOI] [PubMed] [Google Scholar]
- 28.Leite de Moraes M D, Minoprio P, Dy M, Dardenne M, Savino W, Hontebeyrie-Joskowicz M. Scand J Immunol. 1994;39:51–58. doi: 10.1111/j.1365-3083.1994.tb03339.x. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira-dos-Santos A J, Rieker-Geley T, Recheis H, Wick G. J Histochem Cytochem. 1997;45:1293–1297. doi: 10.1177/002215549704500912. [DOI] [PubMed] [Google Scholar]
- 30.Libby P, Alroy J, Pereira M E. J Clin Invest. 1986;77:127–135. doi: 10.1172/JCI112266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomlinson S, Pontes de Carvalho L, Vandekerckhove F, Nussenzweig V. Glycobiology. 1992;2:549–551. doi: 10.1093/glycob/2.6.549. [DOI] [PubMed] [Google Scholar]
- 32.Ciavaglia M d C, de Carvalho T U, de Souza W. Biochem Biophys Res Commun. 1993;193:718–721. doi: 10.1006/bbrc.1993.1684. [DOI] [PubMed] [Google Scholar]
- 33.DosReis G A, Barcinski M A. Adv Parasitol. 2001;49:133–161. doi: 10.1016/s0065-308x(01)49039-7. [DOI] [PubMed] [Google Scholar]
- 34.Di Santo J P, Aifantis I, Rosmaraki E, Garcia C, Feinberg J, Fehling H J, Fischer A, von Boehmer H, Rocha B. J Exp Med. 1999;189:563–574. doi: 10.1084/jem.189.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moody A M, Chui D, Reche P A, Priatel J J, Marth J D, Reinherz E L. Cell. 2001;107:501–512. doi: 10.1016/s0092-8674(01)00577-3. [DOI] [PubMed] [Google Scholar]
- 36.Galvan M, Tsuboi S, Fukuda M, Baum L G. J Biol Chem. 2000;275:16730–16737. doi: 10.1074/jbc.M001117200. [DOI] [PubMed] [Google Scholar]