Abstract
Invasive lobular carcinoma (ILC) is a common subtype of breast cancer that is defined in part by genetic loss of CDH1 caused by mutation or deletion, leading to loss of cell adhesion protein E-cadherin in >90% of ILC. Genetic loss of CDH1 is an early event in ILC oncogenesis, yet the mechanisms by which CDH1/E-cadherin acts as a tumor suppressor are not well understood. To study how early CDH1 loss drives ILC oncogenesis, we used a series of non-transformed human mammary epithelial cell (HMEC) models to target CDH1/E-cadherin, inhibiting extracellular E-cadherin signaling using antibodies versus modeling genetic CDH1 loss using siRNA or knockout via CRISPR/Cas9. Through transcriptome analyses across four HMEC models, we found that the mode of E-cadherin loss or suppression is critical for the subsequent phenotype. Antibody-mediated inhibition of cell-cell contacts induced gene signatures of epithelial-mesenchymal transition (EMT), consistent with the role of E-cadherin suppression during the EMT process. Conversely, genetic CDH1 loss – as in ILC oncogenesis – repressed EMT signatures, and instead remodeled gene expression toward a luminal epithelial phenotype. Using single cell transcriptomics and flow cytometry analyses of cell lineage markers, we found that genetic loss of CDH1 reprogrammed cells to a luminal progenitor-like phenotype. By isolating luminal versus basal cells prior to CDH1 knockout, we found that CDH1 loss led to remodeling of lineage identity in both populations, converging on a new lineage homeostasis with a luminal progenitor-like phenotype. Consistent with increased progenitor features, CDH1 loss enhanced proliferative capacity over the finite lifespan of the HMECs, highlighting a feature of early CDH1 loss that may contribute to clonal advantage during tumor initiation. Our findings support that inhibition of E-cadherin results in different transcriptional response compared to CDH1 loss, with the latter driving a transcriptional and phenotypic state characteristic of a luminal progenitor-like population, which offers new insight into early events in ILC oncogenesis.
Keywords: Invasive lobular carcinoma, breast cancer, CDH1, E-cadherin, oncogenesis, etiology, EMT, mammary gland
INTRODUCTION
Invasive lobular carcinoma (ILC) as a histological subtype of breast cancer accounts for ~15% of all new diagnoses. ILC is molecularly identified by loss of the cell adhesion protein E-cadherin, encoded by CDH1, which in ILC is typically via loss of heterozygosity of CDH1 (i.e. CDH1 loss-of-function mutation with a 16q22.1 deletion, or similar dual genetic loss-of-function) [1]. CDH1 loss is associated with ILC’s characteristic single file growth pattern and unique metastatic spread [2–4]. Importantly, CDH1 loss is an early event in ILC oncogenesis found in a majority of ILC precursor lesions; ~95% of atypical lobular hyperplasia (ALH) and lobular carcinoma in situ (LCIS) cases are E-cadherin-negative [5–9]. However, clinical disease progression in ILC is poorly understood. Though ALH and LCIS are associated with >4-fold and >10-fold increase in breast cancer risk, respectively, they are not clinically considered obligate precursors, and risk factors for progression to ILC have not been identified [6]. As such, clinical management of non-invasive ILC precursors lacks clear guidelines and patients face uncertainty with their long-term risks. Given the central role of CDH1 in ILC oncogenesis, understanding mechanisms of cancer progression upon CDH1 loss can improve patient risk assessment and identify new diagnostic and treatment opportunities for ALH and LCIS lesions, and may have clinical implications for the management of ILC as well.
CDH1 encodes E-cadherin, a cell surface adhesion protein with critical roles in normal epithelial cell-cell communication, as well as pathologic contexts in epithelial-mesenchymal transition (EMT), invasion, and metastasis. Though CDH1 is a tumor suppressor (e.g. hereditary CDH1 mutations are linked to gastric cancer and ILC [10]), E-cadherin loss or suppression has differing implications based on disease context and the mechanism of E-cadherin loss. In breast cancer of no special type (NST; i.e. invasive ductal carcinoma, IDC), E-cadherin is critical for tumor progression and metastasis [11,12]. Transient loss of E-cadherin occurs in late stages of cancer progression via epigenetic silencing (e.g. hypermethylation) [13], but E-cadherin must be re-activated at metastatic sites to facilitate outgrowth [11,14]. Accordingly, CDH1 loss in malignant cancer cell lines induces EMT and increases invasion [15]. Though E-cadherin suppression is a hallmark of the EMT process, E-cadherin/CDH1 loss in ILC is neither accompanied by other canonical features of EMT [16], nor is it transient or reversible, suggesting that CDH1 loss in ILC is mechanistically and phenotypically distinct from EMT-related E-cadherin suppression. Accordingly, other studies support that E-cadherin loss in transformed settings leads to induction of EMT [15], but not in non-transformed cells [9,17]. Thus, genetic loss of CDH1 in ILC is a distinct phenotype compared to EMT-related silencing of E-cadherin and remains poorly understood.
Within the mammary gland, there are two main epithelial lineages: luminal cells, which make up the inner lining of the duct consisting of hormone-responsive and secretory cells, and basal (or myoepithelial) cells, which line the duct and have contractile capabilities necessary for milk production [18,19]. E-cadherin is expressed in both populations and is critical to maintaining organization in mammary ducts [20,21]. Whether these lineages each differentially support ILC oncogenesis is unclear, however, the restriction of ILC to luminal-type tumors (i.e. >90% of ILC are estrogen receptor α/ER-positive [22]) implicates the importance of mammary lineage in ILC oncogenesis. The potential relevance of lineage is also reflected in genetically engineered mouse models (GEMMs) [23]. Using Cre/loxP recombination systems (i.e. Cdh1flox/flox), not all Cre-driving promoters can drive ILC-like oncogenesis, supporting that cellular lineage is important to permit oncogenesis driven by Cdh1 loss. Cre driven by the whey acidic protein promoter (Wap; mammary secretory/luminal epithelial cells) or by the cytokeratin 14 promoter (Krt14; broadly active in epithelial cells) both facilitate ILC-like mammary oncogenesis in Cdh1flox/flox mice [9,24]. However, Cre driven by the Lgr6 promoter (population of epithelial stem cells that can differentiate into luminal or basal lineage) did not permit ILC-like oncogenesis, instead producing NST/IDC-like mammary tumors with metaplastic squamous features [25]. Taken together, cellular commitment to the epithelial lineage may be important for ILC oncogenesis. These data support that ILC oncogenesis requires CDH1 loss in specific mammary cell types or lineages, and points toward mechanistic explanation for the restriction of ILC to luminal-type tumors.
Mechanistic understanding of how CDH1 loss contributes to ILC oncogenesis continues to develop, in particular toward defining molecular consequences based on how E-cadherin is lost (e.g. loss of cell signaling function vs. genetic loss) within specific contexts that can permit and promote ILC oncogenesis. Here, we take a novel approach to show that different modes of E-cadherin loss have distinct molecular consequences. We used two modes of genetic CDH1 loss (siRNA and CRISPR/Cas9 CDH1-KO), to represent CDH1 loss in ILC, contrasted with functional loss of E-cadherin via extracellular antibody inhibition (α-Ecad). Transcriptomics were used to identify similarities and differences in gene regulation and between the modes of E-cadherin suppression. Since the HMECs are heterogeneous populations, we used single cell transcriptomics to define gene expression changes that may underpin cellular progression toward an ER+ ILC phenotype. Since ILC are predominantly luminal cells (i.e. they do not express classic basal cytokeratins [26,27]), flow analysis was used to determine how CDH1 loss affects lineage using established luminal and basal markers. We further addressed the role of lineage through flow sorting, to determine if CDH1 loss effects were different in luminal vs. basal cells. Together, these findings provide insight into the role of CDH1 loss in ILC oncogenesis.
RESULTS
Genetic loss of E-cadherin represses EMT and shifts cells to a more luminal transcriptional state
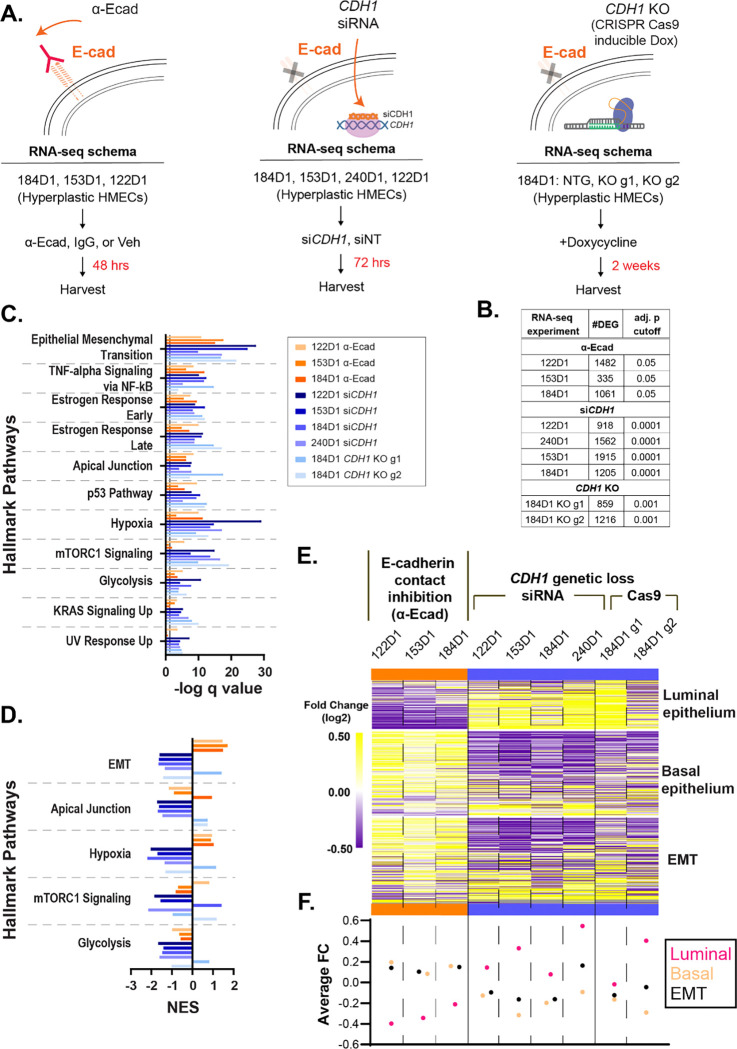
To study the role of CDH1 loss as a driver of ILC oncogenesis, we used non-transformed human mammary epithelial cell (HMEC) models from the Lawrence Berkeley Laboratory series. These HMECs were derived from reduction mammoplasties from different donors, and in culture maintain heterogeneous populations of the luminal and basal cells that make up the normal mammary gland [28]. We used HMECs with CCND1 overexpression, which is a common copy number gain in ILC (~38% of cases [29]), to model early hyperplasia and to facilitate studies targeting CDH1. We used three modes of E-cadherin suppression across 4 HMEC lines: 1) inhibition of E-cadherin using a monoclonal antibody that binds to the extracellular domain of E-cadherin, HECD-1 (α-Ecad), 2) siRNA of CDH1 (siCDH1), and 3) doxycycline-inducible CRISPR/Cas9 knock out of CDH1 (CDH1 KO) (Fig. 1A). Of note, a mixed population workflow was used for the CDH1 KO model (i.e. no clonal selection after Cas9-induction), which was selected to maintain lineage heterogeneity within the HMEC lines. In evaluating the impacts of these modes of E-cadherin suppression, α-Ecad caused cells to have a rounded morphology in which individual cells no longer formed a cohesive monolayer (Supp. Fig. 1A) and reduced the binding efficiency of HMECs to E-cadherin-coated plates (Supp. Fig. 1B). Similarly, siCDH1 reduced binding efficiency and E-cadherin abundance by ~70% as determined by western blot (Supp. Fig. 1C). The efficiency of the CRISPR/Cas9 knock out of CDH1 was confirmed through flow analysis, guide 1 = >30%; guide 2= >40%. (Supp. Fig. 1D).
Figure 1. Genetic loss of E-cadherin represses EMT and shifts cells to a more luminal transcriptional state.
(A) Workflow of RNA-seq using three methods of E-cadherin suppression; extracellular antibody inhibition (α-Ecad), siRNA transfection (siCDH1), and dox-inducible CRISPR/Cas9 (CDH1 KO). (B) Number of differentially expressed genes (DEG) and the adj. p value cutoff used to determine gene lists used for C-E. (C) Overrepresentation analysis of Hallmark pathways in all RNA-seq datasets (q value < 0.0001 across genetic loss model datasets. Color coded by contact inhibition (orange) vs. genetic loss of CDH1 (blue). (D) Fast gene set enrichment analysis (FGSEA) normalized enrichment scores (NES) of Hallmark pathways (q value < 0.05; *EMT in 240D1 q val=0.06) (E) Fold change of gene expression (p<0.05) between E-cadherin inhibition and genetic CDH1 loss within each cell line (HMEC 122D1, 153D1, 184D1, 240D1) in the EMT Hallmark, luminal epithelium, and basal epithelium gene signatures. (F) Average fold change of each gene signature from (E) across cell lines.
E-cadherin loss has been largely studied for its role in cell signaling associated with migration and invasion with less focus on its impact on gene expression changes. To understand the transcriptomic consequences of E-cadherin suppression, we performed RNA-seq using all three modes of E-cadherin loss (Fig. 1A). RNA-seq analyses identified between 335–1,915 differentially expressed genes per cell model (Fig. 1B). We found that differentially expressed genes in all the models were most strongly enriched for the Hallmark [30] signature for EMT and were also universally enriched for Hallmarks for apical junction signaling, estrogen response, hypoxia, and glycolysis pathways, among others (Fig. 1C). We examined these pathways further using fast gene set enrichment analysis (FGSEA) and observed that the genetic models of E-cadherin loss were associated with negative regulation of EMT, whereas contact inhibition of E-cadherin resulted in positive regulation of EMT; the hypoxia Hallmark was similarly differentially regulated (Fig. 1D–F, Supp. Fig. 2–4).
Given the distinct suppression of the EMT signature in genetic CDH1 loss models, and that HMECs are primarily epithelial cells [31], we further examined changes in the epithelial phenotype after CDH1 loss. Using gene signatures for luminal versus basal epithelial phenotypes previously developed through gene expression profiling of these HMEC models (~300 genes total)[32], we found an increase in luminal-associated gene expression and decrease in basal-associated gene expression in the models of genetic loss of E-cadherin, whereas basal gene expression was increased along with the EMT signature with antibody inhibition of E-cadherin (Fig. 1E–F, Supp. Fig. 2). These data show that genetic loss of E-cadherin in non-transformed HMECs is distinct from loss of cell-cell contact inhibition of E-cadherin; the latter activates EMT [15], while the former represses EMT and pushes cells to a more luminal transcriptional phenotype.
Single cell analysis supports CDH1 KO-induced reprogramming in luminal and basal cells
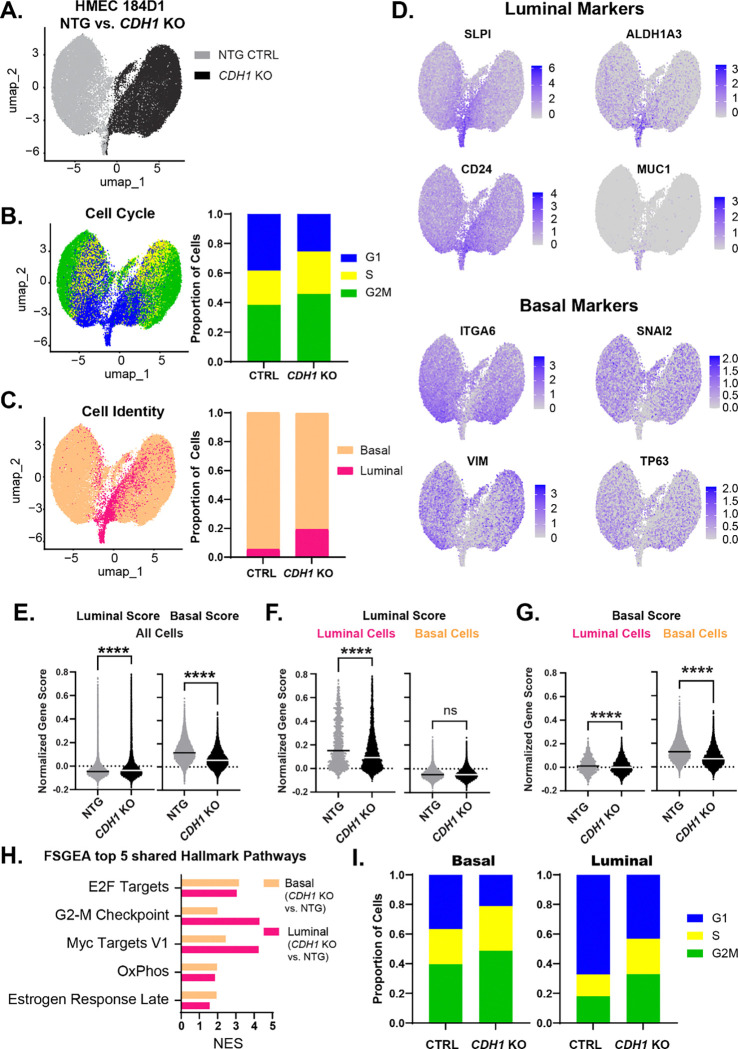
Since these HMEC models are heterogeneous populations of luminal and basal epithelial cells, we used single-cell RNA sequencing (by Particle-templated instant partition sequencing, PIP-seq [33]) to examine gene expression remodeling after CDH1 loss across cellular phenotypes. We compared HMEC 184D1 expressing a non-targeting gRNA (NTG) versus CDH1-targeting gRNA (CDH1 KO g2 from Fig. 1; referred to as CDH1 KO throughout this analysis), two weeks after doxycycline-mediated induction of Cas9 to induce frameshift/knockout mutations in CDH1. In total, we obtained transcriptome data from 36,956 cells (17,306 NTG, 19,650 CDH1 KO), with an average of ~14,000 transcripts per cell covering ~3,800 genes per cell. UMAP analysis showed that NTG vs. CDH1 KO cells were largely independent (Fig. 2A). As observed in bulk RNA-seq analyses (Fig. 1), the Hallmark EMT signature was repressed across CDH1 KO cells versus control cells (NES=−1.81; adj. p=0.038). Cells were further characterized by predicted cell cycle state, which showed an increase in proliferating (S and G2/M) cells in the CDH1 KO population (Fig. 2B).
Figure 2. Single cell analysis supports CDH1 KO-induced reprogramming in luminal and basal cells.
(A) HMEC 184D1 CDH1 KO g2 (CDH1 KO) and HMEC 184D1 NTG UMAP clustering based on sample type. ~17,000 cells per sample. (B) Cells characterized by cell cycle signatures; proportion of cells in each phase by sample type. (C) Using z scores of luminal and basal epithelium gene signatures and the AddModuleScore() function in Seurat, cells were classified as luminal (pink) or basal (orange); proportion of cells within each sample type. (D) Feature plots of representative luminal (SLPI, CD24, ALDH1A3, MUC1) and basal (ITGA6, VIM, SNAI2, TP63) markers. (E-G) The distribution of the normalized gene scores calculated using PIPseeker binning algorithm for both the luminal and basal gene signatures: (E) within all cells (F) luminal scores of the luminal and basal cells (G) basal scores of the luminal and basal cells. (H) FGSEA of the top 5 shared Hallmark pathways for luminal (CDH1 KO vs. NTG) and basal (CDH1 KO vs. NTG). (I) Proportion of cells in each cell cycle phase by cell type. −0.3>logFC >0.3, adj. p <1×10−10 (pathways p<0.05; except the luminal Estrogen Response Late p=0.055).
We then characterized cells as luminal versus basal using the HMEC signatures described above [32] based on relative signature expression within each cell. CDH1 loss drove an increase in the luminal population, which increased from 5.6% of control cells to 19.3% of CDH1 KO cells, a ~3.5-fold increase (Fig. 2C); representative luminal and basal markers highlight the distinction between cell types (Fig. 2D). Overall, basal signature scores decreased across CDH1 KO cells while luminal signature scores increased (Fig. 2E), consistent with the overall increase in the luminal population. However, changes observed in the luminal and basal subpopulations (Fig. 2F–G) suggest that CDH1 loss reduced the fidelity of strict luminal vs basal lineage identity, as indicated by a decreased luminal score in luminal cells and a decreased basal score in basal cells. Similarly, this lineage reprogramming was supported by other cell lineage signatures (e.g. Tabula Muris) (Supp. Fig. 5). CDH1 KO gene expression signatures were coordinately regulated in luminal and basal subpopulations (Fig. 2H); some of this reflected an increase in the predicted proliferative (S and G2/M) cell population in CDH1 KO cells (Fig. 2I). Hallmark signatures for oxidative phosphorylation and estrogen response were also upregulated in both luminal and basal cells after CDH1 loss. This single-cell analysis supports observations from bulk RNA-seq that CDH1 loss drives a population-level shift to a luminal epithelial phenotype, yet both luminal and basal populations may lose strict lineage identity.
To better define these identity-associated changes occurring upon CDH1 loss in luminal versus basal cells, we isolated comparisons within those lineage contexts to use only cells predicted to be in G1 (as otherwise, increased proliferation markers were the predominant output, Fig. 2H). Among the most induced genes were cytokeratin 16 (KRT16) and transcription factor inhibitor of DNA binding 2 (ID2) (Fig. 3A–B), both of which are associated with a progenitor subpopulation of luminal cells [34,35]. To further identify cellular programs affected by CDH1 loss, we conducted a FGSEA of hallmark pathways and found that cholesterol homeostasis was upregulated in both luminal and basal cells (luminal G1 NES= 2.48, adj. p=0.0027; basal G1 NES= 1.71, adj. p= 0.0706) (Fig. 3A).
Figure 3. CDH1 KO induces reprogramming in luminal and basal cells toward a luminal progenitor-like state.
(A) Differentially expressed genes of CDH1 KO vs. NTG cells within the luminal G1 and basal G1 populations relating to progenitor phenotype and cholesterol synthesis; tables on the right show the number of cells included in the analysis and the number of DGE for CDH1 KO vs. NTG within Luminal G1 and Basal G1 cells, respectively (adj. p <1×10−10, −0.3>FC>0.3). (B) Feature plots of two of the top 10 induced genes in CDH1 KO luminal G1 and basal G1cells: KRT16 and ID2 mapped onto original sc-RNAseq feature plot including all NTG vs. CDH1 KO cells (KRT16: avg. logFC= 1.79; pct.1=0.70; adj. p val = 0; ID2: avg. logFC= 2.03; pct.1=0.38; adj. p val = 0). (C) Motifs associated with the luminal G1 and basal G1 DGE using RCisTarget R package and the cisTarget database. Venn diagrams show the overlap of motif-associated genes between the luminal G1 and basal G1 CDH1 KO cells. The expression of overlapping genes are shown in A. (D) Differentially expressed genes of CDH1 KO vs. NTG cells within the luminal G1 and basal G1 populations relating to STAT2 and IRF3 associated genes.
To determine what transcriptional regulators could be implicated in the CDH1 loss-induced remodeling, we further examined the differentially expressed gene lists (luminal G1 CDH1 KO vs. NTG and basal G1 CDH1 KO vs. NTG) for regulatory motif associations. The top three enriched motifs identified in both luminal and basal cell populations corresponded to closely related variants of interferon-stimulated response element (ISRE) binding sites, predominantly associated with IRF3, IRF8, and STAT2 (Fig. 3C). Within the luminal and basal cells, there is substantial overlap of differentially expressed genes relating to the motifs of STAT2 and IRF3 (Fig. 3C). Nearly all these target genes decrease in both the luminal and basal populations (Fig. 3D) in CDH1 KO cells. STAT2 is involved in negative regulation of mitochondrial encoded transcripts [36], so this repression of STAT2 signaling could be correlated with the increase in oxidative phosphorylation identified in Figure 2. Together, these findings support that CDH1 loss remodels luminal and basal cells toward a luminal progenitor-like state with enriched cholesterol synthesis and repressed STAT2/IRF3 signaling.
Cellular lineage markers support a CDH1 loss-induced luminal progenitor-like phenotype
We next examined how the luminal progenitor transcriptional shift was accompanied by changes in cell surface presentation of lineage marker proteins after CDH1 KO. Flow cytometry analysis of EpCAM and CD49f (ITGA6) was used to categorize cells as basal (EpCAMlow/CD49fhi), luminal progenitor (EpCAMhi/CD49fhi), or mature luminal (EpCAMhi/CD49flow) (Fig. 4A). CDH1 was knocked out in the CRISPR/Cas9 CDH1 KO model (via a 2-week induction) in HMEC 184D1 cells prior to flow analyses. One representative experiment shows the change in cell distribution between NTG vs. both CDH1 KO guides (Fig. 4A). To summarize findings across multiple experiments, mean fluorescent intensity (MFI) was calculated based on EpCAM expression within each of the three defined cell populations. Comparison of the fold change of EpCAM MFI between CDH1 KO vs. NTG within each experiment suggests an increase in the luminal progenitor-like phenotype, with a more pronounced luminal progenitor shift in the CDH1 KO g2 line (Fig. 4B). Marker analysis confirmed that lineage-related gene expression changes ultimately resulted in a change in cell phenotype, with an enrichment in EpCAMhi/CD49fhi cells likely indicating an increased luminal progenitor-like population.
Figure 4. Cellular lineage markers support a CDH1 loss-induced luminal progenitor-like phenotype.
(A) Representative plots of flow cytometry analysis of EpCAM/CD49f expression in 184D1 NTG vs. CDH1 KO. Gates were set to designate cell lineage: mature luminal (EpCAMhi CD49flow), luminal progenitor (EpCAMhi CD49fhi), and basal (EpCAMlow CD49fhi). Percentages of cells in each lineage are noted in each quadrant. (B) Changes in cell populations were measured by calculating fold change of the mean fluorescent intensity (MFI) of EpCAM within each quadrant (n=8; n=4 CDH1 KO g1; n=4 CDH1 KO g2. (C) Representative histograms show the changes in CD10 expression within the basal and luminal cell populations defined by EpCAM expression; siNT/NTG (blue) and siCDH1/CDH1 KO (red). (D) Quantification of CD10 expression changes were measured by calculating fold change of CD10 MFI of siCDH1 vs. siNT (n =14; n= 5 siCDH1 184D1, n=3 siCDH1 122D1, n=2 siCDH1 240D1, n=4 siCDH1 153D1) or CDH1 KO vs. NTG (n=8; n=4 CDH1 KO g1; n=4 CDH1 KO g2).
We further examined the putative luminal population using CD10/MME, which marks basal/myoepithelial cells in the normal mammary gland but also indicative of bipotent progenitor capacity in EpCAMhi cells [37–39]. Representative histograms of the CD10 MFIs of siCDH1 vs. siNT or CDH1 KO vs. NTG show an increase in CD10 MFI in both basal (EpCAMlow) and luminal (EpCAMhi: includes luminal progenitor and luminal mature) populations following CDH1 KO (Fig. 4C). Quantification of the fold changes of CD10 MFI between siCDH1 vs. siNT or CDH1 KO vs. NTG shows that CDH1 loss increases CD10 expression in the EpCAMhi luminal population (Fig. 4D). Together these data support that CDH1 KO increases the luminal phenotype while the increase in CD10 expression within the luminal population is further suggestive of a progenitor-like population.
CDH1 KO increases proliferation over a finite lifespan
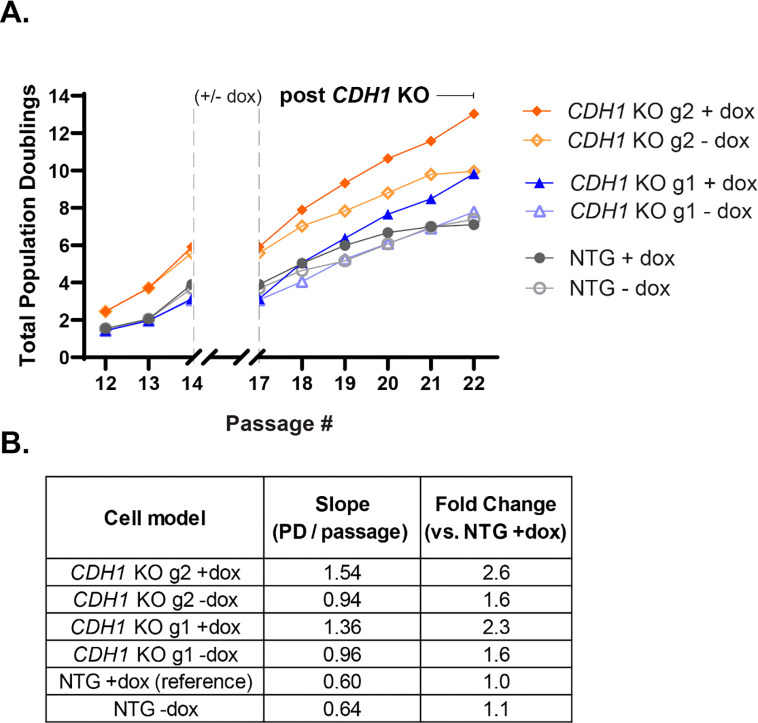
Since HMECs have a finite lifespan, we wanted to determine if CDH1 loss either extended their lifespan or increased the rate of proliferation over their lifespan. We did this by calculating the total population doublings during and after the induction of Cas9 and found that the CDH1 KO populations had more population doublings throughout their lifespan compared to the NTG populations (Fig. 5A). Linear regression analysis of the post-induction phase (passages 17–22) showed that CDH1 KO g1 and g2 +dox populations showed higher proliferation rates than their –dox counterparts, whereas NTG + dox cells had a slightly decreased proliferation rate compared to NTG – dox (Fig. 5B). Moreover, direct comparison of slopes using NTG +dox as a reference shows that CDH1 KO g1/g2 + dox had 2.3-fold and 2.6-fold higher proliferation rates, respectively, compared to NTG +dox (Fig. 5B). These findings support that CDH1 loss allows HMECs to sustain a higher rate of proliferation over a finite lifespan, highlighting a potential feature of early CDH1 loss that may contribute to clonal advantage during tumor initiation.
Figure 5. CDH1 KO HMEC 184D1s increased proliferation over finite lifespan.
(A) Total population doublings over the lifespan of the HMEC 184D1 NTG, CDH1 KO g1, CDH1 KO g2, all +/− dox were calculated by counting cells at the beginning and end of every passage (further described in the methods). The + dox cells were treated from passage 14 through passage 17 with doxycycline (population doublings during this period were excluded from final analysis to remove impact of doxycycline on proliferation). (B) Post-induction proliferation rates were calculated using the following equation: Slope = Δ Population Doublings / 5 passages (17→22). Fold changes of slope using the NTG + dox slope as a reference are shown in the final column.
CDH1 KO induces lineage plasticity in luminal and basal cells
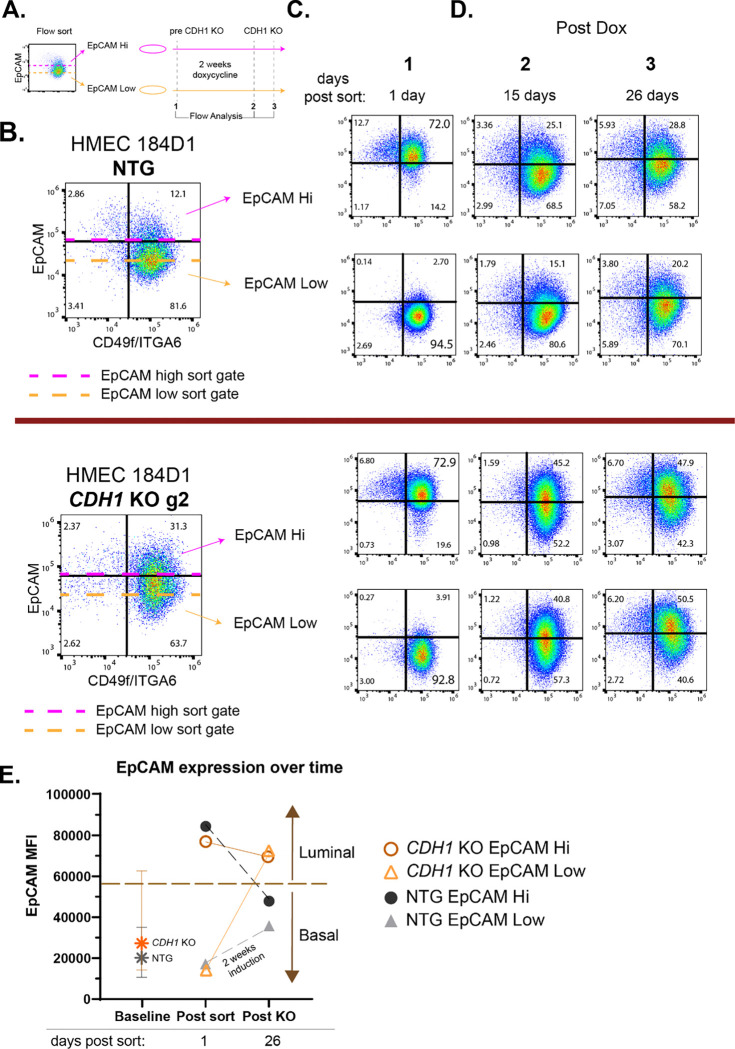
While we identified the population-level luminal progenitor phenotype shift upon CDH1 loss, it was unknown yet whether luminal vs basal cells were each responsible for generating the new post-knockout lineage population. To isolate the effects of CDH1 loss in luminal and basal cells we performed flow sorting of the CDH1 KO HMEC 184D1s before inducing CDH1 loss (Fig. 6A). HMEC 184D1 NTGs and CDH1 KO guide 2 cell lines were sorted into two populations: EpCAM hi and EpCAM low (Fig. 6B). Twenty-four hours after sorting, we confirmed that cells were effectively sorted into luminal (EpCAM hi) and basal (EpCAM low) populations using EpCAM and CD49f (Fig. 6C). After the 1-day post-sort analysis we induced CDH1 KO over the course of 2 weeks with doxycycline within each of the sorted populations (Fig. 6A). Both CDH1 KO analyses (15 days and 26 days post-sort) showed that both of the CDH1 KO sorted populations (EpCAM hi and EpCAM low) had increased luminal progenitor-like populations (as shown by the EpCAMhi CD49fhi; top right quadrant of each flow plot) compared to the NTG control populations (Fig. 6D). Additionally, the CDH1 KO populations all converged on a new homeostasis that was luminal shifted with an increased range of EpCAM expression in comparison to the parental/pre-sorted population (Fig. 6 B and D, CDH1 KO g2). Conversely, the NTG populations re-established a largely basal homeostasis as in the pre-sorted NTG population and parental 184D1 (Fig. 6B and D, NTG). Quantification of the EpCAM mean fluorescent intensity (MFI) within each sorted population over time showed that even though CDH1 KO and NTG cells were sorted similarly in terms of EpCAM expression, the CDH1 KO cells ended with higher EpCAM expression compared to the NTGs (Fig. 6E). Moreover, the CDH1 KO cells from both the luminal and basal post-sort populations converged on the same endpoint, which was substantially more luminal than the baseline population of unsorted HMECs (Fig. 6E). Together these findings support that CDH1 loss induces lineage plasticity in luminal and basal cells leading to a convergent luminal-like endpoint. This plasticity, supported by the progenitor-associated transcriptional changes observed in the sc-RNAseq data, shows that CDH1 loss in both luminal and basal cells induces a luminal progenitor-like phenotype that could be implicated in ILC oncogenesis.
Figure 6. CDH1 KO induces lineage plasticity in luminal and basal cells.
(A) Schematic of flow sorting followed by flow analyses before and after induction of CDH1 KO. Cells were sorted into two populations using EpCAM: EpCAM hi (luminal) and EpCAM low (basal) and were separately treated with doxycycline. (B) Flow sorting and subsequent flow analyses were conducted in two cell lines: HMEC 184D1 NTG and HMEC 184D1 CDH1 KO g2. Dashed lines show where sorting gates were set based off EpCAM expression. (C) 24 hours post sort (before induction of CDH1 KO) the efficiency of the sort was determined by cell distribution based on EpCAM/CD49f expression as described in Fig. 4A (n=1). (D) After CDH1 KO (14 days and 25 days post dox induction) flow analysis of cell distribution based on EpCAM/CD49f was determined (n=3; n=2). (E) EpCAM MFI within parental/pre-sorted population (baseline) and each sorted population before induction of CDH1 KO (post-sort) and after CDH1 KO (post KO). Baseline population error bars show quartile distribution of EpCAM MFI.
DISCUSSION
In this study, we sought out to better understand the transcriptional and phenotypic consequences of early E-cadherin loss to gain a better understanding of ILC oncogenesis. Through transcriptomics and flow cytometry, we demonstrated that CDH1 loss remodels hyperplastic human mammary epithelial cells toward a luminal progenitor-like phenotype. Through transcriptomics we further identified genetic loss of CDH1 as a distinct phenotype in which epithelial mesenchymal transition (EMT) is repressed. Functionally, CDH1 loss increased cell proliferation as analyzed through total population doublings assays and by cell cycle analysis in our single cell transcriptomic analysis. By sorting cells prior to induction of CDH1 loss, we determined that CDH1 loss induces lineage plasticity in both luminal and basal cells toward a similar luminal progenitor-like state that could be implicated in ILC oncogenesis.
Progenitor populations are becoming promising therapeutic targets for treating pre-malignant or primary tumors [40]. There has been significant progress in defining progenitor populations within healthy mammary epithelium using sc-RNA-seq [35]. Cytokeratins are uniquely expressed in specific epithelial types and expression of KRT16 is associated with a luminal progenitor phenotype [35]. The increase in KRT16, KRT6B, and KRT6C is suggestive of a shift in cell identity toward a luminal progenitor-like state (Fig. 3A). Another gene that was strongly induced is inhibitor of DNA binding 2 (ID2) which regulates commitment to luminal lineage and has been shown to be essential for anoikis resistance in multiple models of ILC [34]. The increase in ID2 expression supports a potential mechanism through which CDH1 loss induces a luminal progenitor-like state (Fig. 3B). In addition to identifying cellular markers and potential drivers of this luminal progenitor-like population, it is important to define changes in functional processes related to progenitor phenotypes. Our sc-RNA-seq data showed enrichment for cholesterol homeostasis in both luminal and basal cells. Cholesterol homeostasis has been shown to be associated with cancer stem cell propagation and with luminal progenitors in particular [41,42].
Although CDH1 loss alone does not induce transformation, our findings support that CDH1 loss is associated with changes that support increased tumorigenic potential (e.g. a more proliferative phenotype and a new progenitor-like population). In the case of ILC oncogenesis, we do not yet understand whether CDH1 loss promotes tolerance to additional oncogenic events and/or whether other mutations more specific to luminal tumors (e.g. PIK3CA) have a reciprocal interaction with CDH1 loss in facilitating ILC oncogenesis. Defining how CDH1 loss mediates ILC oncogenesis will yield critical insight into ILC etiology and help us better understand patient risks for ILC development and disease progression.
In breast cancer, loss of E-cadherin expression in late stages of cancer progression induces EMT, which is thought to be required for metastatic spread. However, CDH1 loss in ILC is an early event in oncogenesis, does not induce EMT, and yet ILC metastasizes to typical sites (brain, bone, lung, liver) as well as a few unique sites (GI tract, ovaries, uterus) [43]. The role of E-cadherin loss in EMT has been described in several ways: 1) driver of EMT 2) consequence of EMT or 3) not required for EMT. Our data supports that CDH1 loss represses EMT, as has been shown in other models of CDH1 loss in non-malignant cells [44]. Yet, the molecular and cellular mechanisms responsible for ILC invasion and metastasis are unknown.
Others have found that CDH1 loss leads to exclusion of E-cadherin negative cells from mammary ducts. E-cadherin can either be excluded internally through the lumen or externally beyond the basal lamina [44,45]. E-cadherin negative mammary cells have also been shown to have the single file protrusion pattern as seen in ILC [45]. The combination of a lack of EMT signature and their unique migration pattern suggests that ILC does not require EMT to metastasize and that CDH1 loss in early oncogenesis leads to tissue dysregulation and cell survival depends on the microenvironment. Our data supports that cells with CDH1 loss maintain their epithelial identity; thus, the molecular underpinnings of E-cadherin negative cell migration remain unclear.
In summary, CDH1 loss induces luminal progenitor-like reprogramming in both luminal and basal mammary cells, creating a cellular context that may be permissive to ILC oncogenesis. By identifying potential mechanistic drivers and proliferative processes associated with this reprogramming, our findings highlight new opportunities for targeting early lobular lesions to prevent progression to ILC. This work establishes a foundation for future investigations into the etiology of ILC, including mechanisms underlying its distinct patterns of invasion and metastasis.
MATERIALS AND METHODS
Cell line culture and conditions
Female human mammary epithelial cells (HMEC 184LD1, HMEC 122L-PSD1, HMEC 153D1, HMEC 240D1) (provided by Dr. Martha Stampfer) were maintained in 1:1 MEBM:DMEM/F12. MEBM (Lonza, cat#cc-3151) + 5.0ug/mL Insulin (Thermo Fisher, cat# 41400-045), 70.0ug/mL bovine pituitary extract (Thermo Fisher, CAT # 13028014), 0.5ug/mL hydrocortisone (Sigma-Aldrich, Cat# H4001-1G), 5.0ng/mL EGF (Fisher Scientific, Cat # 236EG200), 5.0ug/mL transferrin (Invitrogen, cat# 11108-016), 10−5M Isoproterenol (IP) (Sigma, CAT# I5627), and 2.0mM glutamine (Sigma, Cat# 59202C). DMEM/F12 (Corning, CAT # 10-092-CV) + 10ug/mL Insulin, 0.1ug/mL hydrocortisone, 2.5mL BSA (Sigma, CAT# A4161), 0.005ug/mL EGF, 2mM glutamine, 10ug/mL Cholera Toxin (Sigma, CAT #C8052), 1uM Oxytocin (Sigma, CAT# O6379-1MG), and 5% Albumax (Invitrogen, CAT # 11020-021). Cells were incubated at 37°C in 5% CO2. All lines were confirmed Mycoplasma negative and authenticated by STR profiling annually; validated cultures were maintained for no more than 3 months before thawing new cultures.
E-cadherin antibody inhibition
E-cadherin inhibition was performed using HECD-1 antibody (Thermo Fisher, CAT# 131700) according to manufacturer’s instructions. For RNA-seq, HMEC 184LD1, 122L-PSD1, 153D1 were plated in 48 well plates and left overnight. Cells were treated with HECD-1 or IgG (whole mouse) at 25ug/mL for 48 hours. Cells were washed twice with PBS and stored in −80°C until RNA extraction.
siRNA knockdown protocol
siRNA was reverse transfected using RNAiMAX (Thermo Fisher) by manufacturer instructions. Constructs are siGENOME SMART pool siRNAs (GE Healthcare Dharmacon): nontargeting pool #2 (D-001206-14-05) and human CDH1 pool (M-003877-02-0005).
Inducible CRISPR/Cas9
CRISPR/Cas9 models were established using Cas9 lentiviral vectors and lentiviral vectors obtained from CU Anschutz Functional Genomics Facility (1 control +2 CDH1-targeting gRNA; lentiCRISPRv2-puro). Mutation found in KO g2 is truncating at exon 2 and KO g1 is truncating at exon 3. These exons make up the propeptide/signal domain, where one of the most commonly recurring CDH1 mutations in ILC can be found [46].
| Clone ID | gene symbol | Sequence | Gene ID | Exon ID |
|---|---|---|---|---|
| 236996584 (KO g1) | CDH1 | AAGATTGCACCGGTCGACAAAGG | ENSG00000039068 | ENSE00003598752 |
| 236987757 (KO g2) | CDH1 | CCGAGAGCTACACGTTCACGGTG | ENSG00000039068 | ENSE00000844392 |
Induction of Cas9 and gRNAs was conducted over a 2-week period with fresh doxycycline (1μg/mL) every three-four days. To preserve HMEC line heterogeneity, a mixed population workflow was followed (i.e. cells were not clonally selected after doxycycline induction).
RNA isolation
RNA extractions were performed using the RNeasy mini kit (Qiagen, cat# 74034); mRNA was converted to cDNA on an Eppendorf Mastercycler Pro (Eppendorf, Hamburg, Germany) and using iSCRIPT cDNA synthesis kit (BioRad, CAT# 1708890).
RNA-seq
Library preparation and sequencing was performed at the U. Colorado Comprehensive Cancer Center Genomics Core Facility. Libraries were generated with Illumina Stranded mRNAPrep, and sequenced on a NovaSEQ6000 with 2150 paired-end reads, targeting 40106 clusters/80106 reads/sample (final mean= 39,019,637 clusters/sample). Data were analyzed with the UCCC Biostatistics and Bioinformatics Shared Resource. Illumina adapters and the first 12 base pairs/read were trimmed using BBDuk (RRID:SCR_016969) and<50 bp reads post-trimming were discarded. Reads were aligned and quantified using STAR (2.6.0a, RRID:SCR_004463) against the Ensembl human transcriptome (hg38.p12 genome, release 96), then normalized to counts per million by edgeR package (RRID:SCR_012802). Differential expression was calculated using limma R package (RRID:SCR_010943) and voom() function. Overrepresentation analysis was performed with cluster Profiler R (RRID:SCR_016884) and Molecular Signatures Database (RRID:SCR_016863) gene sets.
RNA-seq: quantification and statistical analysis
RNAseq was performed using biological replicates, n=3. All cells for each cell line were plated in the same 48 well plate. Biological triplicates are defined as 3 separate wells within the same plate. Reads were aligned and quantified using STAR (2.6.0a, RRID:SCR_004463) against the Ensembl human transcriptome (hg38.p12 genome, release 96), then normalized to counts per million by edgeR package (RRID:SCR_012802). Differential expression was calculated using limma R package (RRID:SCR_010943) and voom() function. Overrepresentation analysis was performed with cluster Profiler R (RRID:SCR_016884) and Molecular Signatures Database (RRID:SCR_016863) gene sets. For antibody inhibition: differential gene expression was analyzed with gene list cutoff using the adjusted p value 0.05. DGE lists were identified following comparison of DGE between HECD-1 vs. CTRL and IgG vs. CTRL, the final list included DGE only from HECD-1. This indirect comparison was performed, rather than direct HECD-1 vs. IgG (negative control) due to a higher than expected immune response induced by the IgG. For all RNA-seq datasets: enriched pathways were identified using Enrichr. Heatmaps were generated using Morpheus with marker selection-signal to noise (α-Ecad vs. siCDH1 and CDH1 KO).
Single cell RNA-seq
Illumina Single Cell 3’ RNA Prep
Following CRISPR editing, single cell suspensions were prepared according to the recommendations of the Illumina Single Cell 3’ RNA Prep, T20 kit (20135692). A detailed workflow of cell processing, mRNA capture, and library preparation can be accessed at https://www.fluentbio.com/resource-category/user-guides/. Samples were pooled and sequenced on a 375Gb lane of the NovaSeq XPlus 10B platform using 150 paired-end base pair reads. Samples were demultiplexed and transcript mapping was performed using PIPseeker software, available at https://www.fluentbio.com/products/pipseeker-software-for-data-analysis/, yielding 1.1 billion barcoded reads between the two samples.
Data Filtering and Processing
The R package Seurat was utilized to process and analyze the scRNA sequencing data[47]. High quality cells were isolated via the criteria: number of transcripts mapped between 3000 and 45000, number of genes detected between 1500 and 8000, percent mitochondrial genes < 10%, and log10 genes per UMI > 0.8. Following filtering, 19,650 CDH1 KO g2 and 17,306 NTG cells remained included for downstream analysis. Feature counts were log normalized to correct for variations in sequencing depth between cells using the “NormalizeData” Seurat function and gene expression was transformed to z-scores using “ScaleData”.
The “FindVariableFeatures” function, using the variance-stabilizing transformation method, was applied to identify the top 3000 variable features. Principal component analysis was run on the variable features, using “RunPCA”, then the “RunUMAP” function was employed to perform the uniform manifold approximation and projection dimensional technique with 15 dimensions. Nearest neighbors were found using the “FindNeighbors” function with 15 dimensions, and clusters were determined using “FindClusters” at a resolution of 0.6.
Cell Annotation
Predicted cell cycle phases were determined using the “CellCycleScoring” function with the Seurat-defined “cc.genes” feature lists. Cells were classified as luminal or basal based on previously defined markers[32]. Using the “AddModuleScore” function, the transcriptome of each cell was compared with the luminal/basal marker lists and given a score for each phenotype. The lineage corresponding with the highest score was assigned to each cell.
Clustering of Luminal Population
The luminal cells were isolated into a separate Seurat object using the “subset” function and data was recentered to account for variability within only the luminal cells by running the “NormalizeData” and “ScaleData” functions. In parallel with the whole-data analysis, the top 3000 variable features were identified, PCA was done using the variable features, and UMAP was run with 15 dimensions. Cell neighbors were found, also using 15 dimensions, and clusters were defined with a resolution of 0.4.
Clustering of Basal Population
The basal cells were isolated into a separate Seurat object using the “subset” function and data was recentered to account for variability within only the luminal cells by running the “NormalizeData” and “ScaleData” functions. In parallel with the whole-data analysis, the top 3000 variable features were identified, PCA was done using the variable features, and UMAP was run with 15 dimensions. Cell neighbors were found, also using 15 dimensions, and clusters were defined with a resolution of 0.4.
Differential Gene Expression Analysis
Differentially expressed genes within each cluster were determined using the Seurat function “FindAllMarkers”, and comparisons between cell groups were calculated using the Wilcoxon rank sum test with the function “FindMarkers”. Fast gene set enrichment analysis (FSGEA) was used to identify Hallmark pathways of genes that passed these filters: −0.3>logFC >0.3, adj. p <1×10−10.
Transcriptional regulator analysis
Differentially expressed genes were used in a transcriptional regulator analysis created by the CU Anschutz Biostatistics and Bioinformatics Shared Resource (BBSR). This tool utilizes the RCisTarget R package using the cisTarget database of gene regulators. This specific dataset labels transcription factor binding sites +/− 10kb for genes that passed these filters: −0.3>logFC >0.3, adj. p <1×10−10.
Flow Cytometry
For extracellular flow cytometry, cells were harvested with 0.05% trypsin/EDTA. Cells were counted, washed with PBS, and resuspended in staining solution (PBS, 1% BSA, 1% EDTA). Cells were plated into Falcon 96-well round bottom plates (Corning # 353077) at 400,000 cells/well. EpCAM-BV421 (Biolegend #324220, clone 9C4, mouse IgG2b, 1:200), CD10-APC/Fire 750 (Biolegend #312230, clone HI10a, mouse IgG1, 1:200), and CD49f-PE (Biolegend #313612, clone GoH3, Rat IgG2a, 1:400) were added to cells in staining solution for 15 minutes on ice. Cells were centrifuged for 3 min at 1400rpm. Staining solution was aspirated and cells were washed with PBS for 10 min on ice. Cells were centrifuged for 4 min at 1400rpm. PBS was aspirated and cells were resuspended in flow solution (PBS, 2% BSA, 0.2% EDTA). Cells were then analyzed in a Novocyte Penteon Flow Cytometer. SpectraComp beads (Bio Slingshot # SSB-05-B), unstained cells, and FMOs were used as controls.
For flow sorting, cells were prepared following the same protocol as the extracellular flow cytometry analysis. Cells were only stained with EpCAM-BV421 (Biolegend #324220, clone 9C4, mouse IgG2b, 1:200). Cells were sorted using the Sony MA900. Gates were set using unstained controls.
For intracellular flow cytometry, cells were harvested with 0.05% trypsin/EDTA. Cells were counted, washed with PBS, and fixed in 4%PFA (100uL/1×106 cells) and incubated at room temperature for 8 minutes. Cells were washed with PBS by centrifugation. Cells were resuspended in 1% Triton-X (100uL/1×106 cells) and incubated at room temperature for 10 minutes. Cells were then washed with PBS by centrifugation and resuspended in staining solution. E-cadherin-AF647 (Cell Signaling #9835, clone 24E10, Rabbit IgG, 1:200) was added to the cells and the staining flow protocol above was followed.
Population Doubling Assay
Total population doubling over a finite lifespan for each culture was calculated using the formula: , where is the number of cells seeded in a dish at each passage and is the number of cells recovered from the dish (adapted from Garbe et al. 2009).
SUPPLEMENTAL METHODS:
Immunoblotting
Whole-cell lysates were obtained by incubating cells in RPPA lysis buffer for 45′ on ice. Cells were centrifuged at ~16,000× g for 15 m at 4 °C and the resulting supernatant was collected for analysis. Protein concentrations were measured and normalized using the Pierce BCA protein assay kit (#23225). Protein loading was kept consistent by mass across matched experiments, and standard methods were used to perform SDS-PAGE. Proteins were transferred onto PVDF membranes. Antibodies were used according to manufacturer’s recommendations: ERα (Leica, Buffalo Grove, IL, USA; 6F11, cat# ER-6F11-L-F) and CDH1 (Cell Signaling, CAT# 3195S, clone #24E10). Secondary antibodies were used according to manufacturer’s instruction and were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA), goat anti-mouse IgG (cat# 115-035-068) and goat anti-rabbit IgG (cat# 111-035-045). Chemiluminescence was used to detect antibodies and the LICOR c-Digit (LI-COR Biosciences, Lincoln, NE, USA) was used to develop the immunoblots. Total protein (Ponceau) served as a loading control.
E-cadherin functional assay
Cells were pretreated with HECD-1 (24 hours) or siCDH1/siNT (48 hours). Plates were coated with a serial dilution of E-cadherin (6μg/mL to 0.375μg/mL) and left overnight at 4°C. Cells were dissociated, blocked with fetal bovine serum (FBS) in Dulbecco’s phosphate-buffered saline (DPBS) (10mg/mL), and stained with Calcein for 30 min at 37°C. Calcein stained cell solution was added to appropriate wells for 4 hours at 37°C. MM134 cells were used as negative controls, as they do not express E-cadherin. Wells with blocking buffer (without an E-cadherin coating) were also included as negative controls. A fluorescent plate reader was used to measure fluorescence after the 4 hour incubation (excitation: 485nm; emission: 538nm). Cells were washed with PBS and then read on the fluorescent plate reader again. Percent adhesion was calculated by the following equation. % adhesion = (OD after wash)/OD before wash)*100. Average OD was used among technical triplicates (n=3).
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT OR RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Estrogen Receptor alpha (6F11) | Leica | Cat # ER-6F11-L-F |
| HECD-1 IgG mouse monoclonal (2mg/mL) | Thermo Fisher | Cat # 13-1700; RRID: AB_2533003 |
| Chrome Pure Mouse, whole IgG | Jackson ImmunoResearch |
Cat # 015-000-003; RRID: AB_2337188 |
| CDH1 IgG rabbit (clone # 24E10) | Cell Signaling | Cat # 3195S |
| Goat anti-rabbit IgG | Jackson ImmunoResearch | Cat # 111-035-045; RRID: AB_2337938 |
| Goat anti-mouse IgG | Jackson ImmunoResearch | Cat # 115-035-068; RRID: AB_2338505 |
| EpCAM-BV421 (clone 9C4, mouse IgG2b, 1:200) | Biolegend | Cat #324220 RRID:AB_2563847 |
| CD10-APC/Fire 750 (clone HI10a, mouse IgG1, 1:200) | Biolegend | Cat #312230 RRID:AB_2616719 |
| CD49f-PE (clone GoH3, Rat IgG2a, 1:400) | Biolegend | Cat #313612 RRID:AB_893373 |
| E-cadherin-AF647 (clone 24E10, Rabbit IgG, 1:200) | Cell Signaling | Cat #9835 RRID:AB_10828228 |
| Experimental models: Cell lines | ||
| HMEC 184LD1 | Dr. Martha Stampfer, LBL | |
| HMEC 153D1 | Dr. Martha Stampfer, LBL | |
| HMEC 122L-PSD1 | Dr. Martha Stampfer, LBL | |
| HMEC 240LD1 | Dr. Martha Stampfer, LBL | |
| Chemicals, Peptides, and Recombinant Proteins | ||
| MEBM | Lonza | Cat #cc-3151 |
| 5.0ug/mL Insulin | Thermo Fisher | Cat # 41400-045 |
| 70.0ug/mL bovine pituitary extract | Thermo Fisher | Cat # 13028014 |
| hydrocortisone | Sigma-Aldrich | Ca t# H4001-1G |
| EGF | Thermo Fisher | Cat # 236EG200 |
| transferrin | Invitrogen | Cat # 11108-016 |
| Isoproterenol (IP) | Sigma-Aldrich | Cat # I5627 |
| glutamine | Sigma-Aldrich | Cat # 59202C |
| DMEM/F12 | Corning | Cat # 10-092-CV |
| BSA | Sigma-Aldrich | Cat # A4161 |
| Cholera Toxin | Sigma-Aldrich | Cat # C8052 |
| Oxytocin | Sigma-Aldrich | Cat # O6379-1MG |
| Albumax | Invitrogen | Cat # 11020-021 |
| Estradiol (E2) was from Sigma | Sigma-Aldrich | Cat # E2758 |
| Hoechst 33258 | Thermo Fisher | Cat # 62249 |
| DNA Extraction Buffer | Cell Signaling | Cat # 42015 |
| Proteinase K | Cell Signaling | Cat # 10012 |
| RNase A | Cell Signaling | Cat # 7013 |
| Critical Commercial Assays | ||
| Illumina Single Cell 3' RNA Prep | Illumina | |
| ATAC-seq Kit | Active Motif | Cat # 53150 |
| ATAC-Seq Spike-In Control | Active Motif | Cat # 53154 |
| DNA Library Prep Kit for Illumina® | Active Motif | Cat # 53220 |
| Dual Index Primers Set 1 for Illumina® | Active Motif | Cat # 53221 |
| PowerUp SYBR Green Master Mix | Life Technologies, Inc. | Cat # 100029284 |
| iSCRIPT cDNA synthesis kit | BioRad | Cat # 1708890 |
| RNeasy mini kit | Qiagen | Cat # 74034 |
| Deposited data | ||
| 230228_HMEC_D1s_HECD-1+E2_RNAseq | This paper | GEO: (hypothetical number) |
| Oligonucleotides | ||
| RPL30 (exon 3) forward primer: 5’ GTC CTG GGG TAC AAG CAG AC 3’ | Sikora Laboratory | N/A |
| RPL30 (exon 3) reverse primer: 5’ CTG GGC AGT TGT TAG CGA GA 3’ | Sikora Laboratory | N/A |
| Software and Algorithms | ||
| Enrichr | Maayan Lab | https://maayanlab.cloud/Enrichr/ RRID:SCR_001575 |
| Morpheus | Broad Institute | https://software.broadinstitute.org/morpheus/ |
| GraphPad Prism 9 | GraphPad | https://www.graphpad.com/ RRID: SCR_002798 |
| BBDuk | R Studio | RRID:SCR_016969 |
| STAR (2.6.0a) | R Studio | RRID:SCR_004463 |
| edgeR package | R Studio | RRID:SCR_012802 |
| limma R package | R Studio | RRID:SCR_010943 |
| Profiler R | R Studio | RRID:SCR_016884 |
| Molecular Signatures Database | R Studio | RRID:SCR_016863 |
| CU Anschutz Bioinformatics RNAseq Analysis tool | CU Anschutz Bioinformatics Core | https://bioinformatics.cuanschutz.edu/UCRNA-sea |
| Venny 2.1 (Venn Diagrams) | CNB | https://bioinfogp.cnb.csic.es/tools/venny/ |
| Others | ||
| Countess 2 Automated Cell Counter | Invitrogen | Cat # AMQAX1000 |
| 4200 TapeStation System | Agilent | Cat # G2991BA |
| Qubit 4 Fluorometer | ThermoFisher | Cat # Q33238 |
| NovaSeq 6000 | Illumina | Cat # 20012850 |
| Magnetic Separation Rack, 0.2 mL Tubes | EpiCypher | Cat # 10-0008 |
ACKNOWLEDGEMENTS
We thank Martha Stampfer, Jim Garbe, and Mark LaBarge for the HMEC cell lines and their invaluable guidance and feedback throughout this project.
This study was partly supported by the National Institutes of Health P30CA046934 by utilizing the Bioinformatics and Biostatistics Shared Resource.
Funding
This work was supported by DoD BCRP awards W81XWH-22-1-0715 (MJS) and W81XWH-22-1-0716 (JHO), ACS TLC-21-163-01-TLC from the American Cancer Society (JHO and MJS), the Lobular Breast Cancer Research Fund at CU Anschutz (MJS), and T32CA190216 from the National Institutes of Health (MM). This work utilized the U. Colorado Cancer Center Genomics Shared Resource, Biostatistics and Bioinformatics Shared Resource, Cell Technologies Shared Resource (RRID: SCR_021982), and Flow Cytometry Shared Resource (RRID: SCR_022035) supported by P30CA046934. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Defense, or other agencies.
REFERENCES
- [1].Grabenstetter A., Mohanty A.S., Rana S., Zehir A., Brannon A.R., D’Alfonso T.M., DeLair D.F., Tan L.K., Ross D.S., E-cadherin immunohistochemical expression in invasive lobular carcinoma of the breast: correlation with morphology and CDH1 somatic alterations, Hum Pathol 102 (2020) 44–53. 10.1016/j.humpath.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mouabbi J.A., Hassan A., Lim B., Hortobagyi G.N., Tripathy D., Layman R.M., Invasive lobular carcinoma: an understudied emergent subtype of breast cancer, Breast Cancer Res Treat 193 (2022) 253–264. 10.1007/s10549-022-06572-w. [DOI] [PubMed] [Google Scholar]
- [3].Arpino G., Bardou V.J., Clark G.M., Elledge R.M., Infiltrating lobular carcinoma of the breast: Tumor characteristics and clinical outcome, Breast Cancer Research 6 (2004). 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mathew A., Rajagopal P.S., Villgran V., Sandhu G.S., Jankowitz R.C., Jacob M., Rosenzweig M., Oesterreich S., Brufsky A., Distinct Pattern of Metastases in Patients with Invasive Lobular Carcinoma of the Breast, Geburtshilfe Frauenheilkd 77 (2017) 660–666. 10.1055/s-0043-109374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vos C., Cleton-Jansen A., Berx G., de Leeuw W., ter Haar N., van Roy F., Cornelisse C., Peterse J., van de Vijver M., E-cadherin inactivation in lobular carcinoma in situ of the breast: an early event in tumorigenesis, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lunt L., Coogan A., Perez C.B., Lobular Neoplasia, Surgical Clinics of North America 102 (2022) 947–963. 10.1016/j.suc.2022.07.001. [DOI] [PubMed] [Google Scholar]
- [7].Mastracci T.L., Tjan S., Bane A.L., O’Malley F.P., Andrulis I.L., E-cadherin alterations in atypical lobular hyperplasia and lobular carcinoma in situ of the breast, Modern Pathology 18 (2005) 741–751. 10.1038/MODPATHOL.3800362. [DOI] [PubMed] [Google Scholar]
- [8].King T.A., Reis-Filho J.S., Lobular neoplasia, Surg Oncol Clin N Am 23 (2014) 487–503. 10.1016/j.soc.2014.03.002. [DOI] [PubMed] [Google Scholar]
- [9].Derksen P.W.B., Liu X., Saridin F., van der Gulden H., Zevenhoven J., Evers B., van Beijnum J.R., Griffioen A.W., Vink J., Krimpenfort P., Peterse J.L., Cardiff R.D., Berns A., Jonkers J., Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis, Cancer Cell 10 (2006) 437–449. 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- [10].Corso G., Intra M., Trentin C., Veronesi P., Galimberti V., CDH1 germline mutations and hereditary lobular breast cancer, Fam Cancer 15 (2016) 215–219. 10.1007/S10689-016-9869-5/FIGURES/1. [DOI] [PubMed] [Google Scholar]
- [11].Padmanaban V., Krol I., Suhail Y., Szczerba B.M., Aceto N., Bader J.S., Ewald A.J., E-cadherin is required for metastasis in multiple models of breast cancer, Nature 573 (2019) 439–444. 10.1038/s41586-019-1526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fearon E.R., Cancer: Context Is Key for E-cadherin in Invasion and Metastasis, Current Biology 29 (2019) R1140–R1142. 10.1016/j.cub.2019.09.054. [DOI] [PubMed] [Google Scholar]
- [13].Caldeira J.R.F., Prando É.C., Quevedo F.C., Moraes Neto F.A., Rainho C.A., Rogatto S.R., CDH1 promoter hypermethylation and E-cadherin protein expression in infiltrating breast cancer, BMC Cancer 6 (2006). 10.1186/1471-2407-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chao Y.L., Shepard C.R., Wells A., Open Access RESEARCH Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition, 2010. http://www.molecular-cancer.com/content/9/1/179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Onder T.T., Gupta P.B., Mani S.A., Yang J., Lander E.S., Weinberg R.A., Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways, Cancer Res 68 (2008) 3645–3654. 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- [16].Bruner H.C., Derksen P.W.B., Loss of E-Cadherin-Dependent Cell–Cell Adhesion and the Development and Progression of Cancer, Cold Spring Harb Perspect Biol 10 (2018). 10.1101/CSHPERSPECT.A029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen A., Beetham H., Black M.A., Priya R., Telford B.J., Guest J., Wiggins G.A.R., Godwin T.D., Yap A.S., Guilford P.J., E-cadherin loss alters cytoskeletal organization and adhesion in non-malignant breast cells but is insufficient to induce an epithelial-mesenchymal transition, BMC Cancer 14 (2014) 552. 10.1186/1471-2407-14-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Biswas S.K., Banerjee S., Baker G.W., Kuo C.Y., Chowdhury I., The Mammary Gland: Basic Structure and Molecular Signaling during Development, Int J Mol Sci 23 (2022). 10.3390/ijms23073883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Samocha A., Doh H., Kessenbrock K., Roose J.P., Unraveling heterogeneity in epithelial cell fates of the mammary gland and breast cancer, Cancers (Basel) 11 (2019). 10.3390/cancers11101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chanson L., Brownfield D., Garbe J.C., Kuhn I., Stampfer M.R., Bissell M.J., LaBarge M.A., Self-organization is a dynamic and lineage-intrinsic property of mammary epithelial cells, Proceedings of the National Academy of Sciences 108 (2011) 3264–3269. 10.1073/pnas.1019556108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shamir E.R., Ewald A.J., Adhesion in mammary development: Novel roles for E-cadherin in individual and collective cell migration, in: Curr Top Dev Biol, Academic Press Inc., 2015: pp. 353–382. 10.1016/bs.ctdb.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Clelland E.N., Rothschild H.T., Patterson A., Molina-Vega J., Kaur M., Symmans W.F., Schwartz C.J., Chien A.J., Benz C.C., Mukhtar R.A., Quantifying hormone receptor status in lobular breast cancer in an institutional series: the relationship between estrogen and progesterone receptor status and outcomes, Breast Cancer Res Treat 202 (2023) 367. 10.1007/S10549-023-07059-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sflomos G., Schipper K., Koorman T., Fitzpatrick A., Oesterreich S., Lee A. V., Jonkers J., Brunton V.G., Christgen M., Isacke C., Derksen P.W.B., Brisken C., Atlas of lobular breast cancer models: Challenges and strategic directions, Cancers (Basel) 13 (2021). 10.3390/cancers13215396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Boelens M.C., Nethe M., Klarenbeek S., de Ruiter J.R., Schut E., Bonzanni N., Zeeman A.L., Wientjens E., van der Burg E., Wessels L., van Amerongen R., Jonkers J., PTEN Loss in E-Cadherin-Deficient Mouse Mammary Epithelial Cells Rescues Apoptosis and Results in Development of Classical Invasive Lobular Carcinoma, Cell Rep 16 (2016) 2087–2101. 10.1016/j.celrep.2016.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].ter Steege E.J., Sijnesael T., Enserink L., Klarenbeek S., Haakma W.E., Bakker E.R.M., Derksen P.W.B., LGR6-dependent conditional inactivation of E-cadherin and p53 leads to invasive skin and mammary carcinomas in mice, Neoplasia 35 (2023) 100844. 10.1016/j.neo.2022.100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khilko N., Wang J., Wei B., Hicks D.G., Tang P., Invasive Lobular Carcinomas Do Not Express Basal Cytokeratin Markers CK5/6, CK14 and CK17, Breast Cancer (Auckl) 4 (2010) 49. 10.4137/BCBCR.S5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Christgen M., Cserni G., Floris G., Marchio C., Djerroudi L., Kreipe H., Derksen P.W.B., Vincent-Salomon A., Lobular Breast Cancer: Histomorphology and Different Concepts of a Special Spectrum of Tumors, Cancers 2021, Vol. 13, Page 3695 13 (2021) 3695. 10.3390/CANCERS13153695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stampfer M.R., La Barge M.A., Garbe J.C., An integrated human mammary epithelial cell culture system for studying carcinogenesis and aging, in: Cell and Molecular Biology of Breast Cancer, Humana Press Inc., 2013: pp. 323–361. 10.1007/978-1-62703-634-4_15. [DOI] [Google Scholar]
- [29].Desmedt C., Zoppoli G., Gundem G., Pruneri G., Larsimont D., Fornili M., Fumagalli D., Brown D., Rothé F., Vincent D., Kheddoumi N., Rouas G., Majjaj S., Brohée S., Van Loo P., Maisonneuve P., Salgado R., Van Brussel T., Lambrechts D., Bose R., Metzger O., Galant C., Bertucci F., Piccart-Gebhart M., Viale G., Biganzoli E., Campbell P.J., Sotiriou C., Genomic Characterization of Primary Invasive Lobular Breast Cancer, in: Journal of Clinical Oncology, American Society of Clinical Oncology, 2016: pp. 1872–1880. 10.1200/JCO.2015.64.0334. [DOI] [PubMed] [Google Scholar]
- [30].Liberzon A., Birger C., Thorvaldsdóttir H., Ghandi M., Mesirov J.P., Tamayo P., The Molecular Signatures Database (MSigDB) hallmark gene set collection, Cell Syst 1 (2015) 417. 10.1016/J.CELS.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Labarge M.A., Garbe J.C., Stampfer M.R., Processing of human reduction mammoplasty and mastectomy tissues for cell culture., J Vis Exp (2013). 10.3791/50011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee J.K., Garbe J.C., Vrba L., Miyano M., Futscher B.W., Stampfer M.R., LaBarge M.A., Age and the means of bypassing stasis influence the intrinsic subtype of immortalized human mammary epithelial cells, Front Cell Dev Biol 3 (2015). 10.3389/fcell.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Clark I.C., Fontanez K.M., Meltzer R.H., Xue Y., Hayford C., May-Zhang A., D’Amato C., Osman A., Zhang J.Q., Hettige P., Ishibashi J.S.A., Delley C.L., Weisgerber D.W., Replogle J.M., Jost M., Phong K.T., Kennedy V.E., Peretz C.A.C., Kim E.A., Song S., Karlon W., Weissman J.S., Smith C.C., Gartner Z.J., Abate A.R., Microfluidics-free single-cell genomics with templated emulsification, Nat Biotechnol 41 (2023) 1557–1566. 10.1038/S41587-023-01685-Z; SUBJMETA=1647,191,2017,514,61,631; KWRD=FUNCTIONAL+GENOMICS, GENE+EXPRESSION+ANALYSIS, SEQUENCING. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rätze M.A.K., Koorman T., Sijnesael T., Bassey-Archibong B., van de Ven R., Enserink L., Visser D., Jaksani S., Viciano I., Bakker E.R.M., Richard F., Tutt A., O’Leary L., Fitzpatrick A., Roca-Cusachs P., van Diest P.J., Desmedt C., Daniel J.M., Isacke C.M., Derksen P.W.B., Loss of E-cadherin leads to Id2-dependent inhibition of cell cycle progression in metastatic lobular breast cancer, Oncogene 41 (2022) 2932–2944. 10.1038/s41388-022-02314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bach K., Pensa S., Grzelak M., Hadfield J., Adams D.J., Marioni J.C., Khaled W.T., Differentiation dynamics of mammary epithelial cells revealed by single-cell RNA sequencing, Nature Communications 2017 8:1 8 (2017) 1–11. 10.1038/s41467-017-02001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Meier J.A., Larner A.C., Toward a new STATe: The role of STATs in mitochondrial function, Semin Immunol 26 (2014) 20. 10.1016/J.SMIM.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Maguer-Satta V., Chapellier M., Delay E., Bachelard-Cascales E., CD10: A tool to crack the role of stem cells in breast cancer, Proceedings of the National Academy of Sciences 108 (2011) E1264–E1264. 10.1073/PNAS.1116567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kenny A.J., O’Hare M.J., Gusterson B.A., CELL-SURFACE PEPTIDASES AS MODULATORS OF GROWTH AND DIFFERENTIATION, The Lancet 334 (1989) 785–787. 10.1016/S0140-6736(89)90841-6. [DOI] [PubMed] [Google Scholar]
- [39].Keller P.J., Arendt L.M., Skibinski A., Logvinenko T., Klebba I., Dong S., Smith A.E., Prat A., Perou C.M., Gilmore H., Schnitt S., Naber S.P., Garlick J.A., Kuperwasser C., Defining the cellular precursors to human breast cancer, Proc Natl Acad Sci U S A 109 (2012) 2772–2777. 10.1073/PNAS.1017626108/SUPPL_FILE/SD04.XLS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tharmapalan P., Mahendralingam M., Berman H.K., Khokha R., Mammary stem cells and progenitors: targeting the roots of breast cancer for prevention, EMBO J 38 (2019). 10.15252/EMBJ.2018100852/ASSET/095CC093-CCCF-4F37-A6AE-46CF01128F30/ASSETS/GRAPHIC/EMBJ2018100852-FIG-0004-M.PNG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ehmsen S., Pedersen M.H., Wang G., Terp M.G., Arslanagic A., Hood B.L., Conrads T.P., Leth-Larsen R., Ditzel H.J., Increased Cholesterol Biosynthesis Is a Key Characteristic of Breast Cancer Stem Cells Influencing Patient Outcome, Cell Rep 27 (2019) 3927–3938.e6. 10.1016/J.CELREP.2019.05.104/ATTACHMENT/87B0A6C1-487E-4DE0-865F-4EDB17BD1B69/MMC5.PDF. [DOI] [PubMed] [Google Scholar]
- [42].Mahendralingam M.J., Kim H., McCloskey C.W., Aliar K., Casey A.E., Tharmapalan P., Pellacani D., Ignatchenko V., Garcia-Valero M., Palomero L., Sinha A., Cruickshank J., Shetty R., Vellanki R.N., Koritzinsky M., Stambolic V., Alam M., Schimmer A.D., Berman H.K., Eaves C.J., Pujana M.A., Kislinger T., Khokha R., Mammary epithelial cells have lineage-rooted metabolic identities, Nat Metab 3 (2021) 665–681. 10.1038/s42255-021-00388-6. [DOI] [PubMed] [Google Scholar]
- [43].Bullock E., Brunton V.G., E-Cadherin-Mediated Cell–Cell Adhesion and Invasive Lobular Breast Cancer, Adv Exp Med Biol 1464 (2025) 259–275. 10.1007/978-3-031-70875-6_14. [DOI] [PubMed] [Google Scholar]
- [44].Schipper K., Seinstra D., Paulien Drenth A., van der Burg E., Ramovs V., Sonnenberg A., van Rheenen J., Nethe M., Jonkers J., Rebalancing of actomyosin contractility enables mammary tumor formation upon loss of E-cadherin, Nature Communications 2019 10:1 10 (2019) 1–14. 10.1038/s41467-019-11716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shamir E.R., Pappalardo E., Jorgens D.M., Coutinho K., Tsai W.T., Aziz K., Auer M., Tran P.T., Bader J.S., Ewald A.J., Twist1-induced dissemination preserves epithelial identity and requires E-cadherin, Journal of Cell Biology 204 (2014) 839–856. 10.1083/jcb.201306088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Djerroudi L., Bendali A., Fuhrmann L., Benoist C., Pierron G., Masliah-Planchon J., Kieffer Y., Carton M., Tille J.C., Cyrta J., Ramtohul T., Bonneau C., Caly M., Renault V., Bidard F.C., Mechta-Grigoriou F., Vincent-Salomon A., E-Cadherin Mutational Landscape and Outcomes in Breast Invasive Lobular Carcinoma, Modern Pathology 37 (2024) 100570. 10.1016/J.MODPAT.2024.100570. [DOI] [PubMed] [Google Scholar]
- [47].Satija R., Farrell J.A., Gennert D., Schier A.F., Regev A., Spatial reconstruction of single-cell gene expression data, Nature Biotechnology 2015 33:5 33 (2015) 495–502. 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.