Abstract
A question of critical importance confronting neuroscientists today is how biochemical signals initiated at a synapse are conveyed to the nucleus. This problem is particularly relevant to the generation of the late phases of long-term potentiation (LTP). Here we provide evidence that some signaling pathways previously associated with late-LTP can be activated in hippocampal CA1 neurons without synaptic activity; somatic action potentials, induced by backfiring the cells, were found to be sufficient for phosphorylation of extracellular signal-regulated kinase-1/2 and cAMP response element-binding protein, as well as for induction of zif268. Furthermore, such antidromic stimulation was adequate to rescue “tagged” synapses (early-LTP) from decay. These results show that a synapse-to-nucleus signal is not necessary for late-phase LTP-associated signaling cascades in the regulation of gene expression.
Keywords: CREB‖ERK1/2‖MAPKinase‖zif268
One of the features that hippocampal long-term potentiation (LTP) shares with long-term memory is its sensitivity to protein synthesis inhibitors (1–4). Several lines of evidence have shown that the key protein synthetic events involved in the late phases of LTP occur very early after the induction process (within 15–30 min) (4, 5), and that if new RNA synthesis occurs, it occurs nearly coincident with the timing of induction (6, 7). Although the mRNA of some proteins can be found in the dendrites of CA1 pyramidal cells (8), protein synthesis from the preexisting mRNA does not seem to be sufficient to support late-phase LTP; LTP induced in dendrites severed from their somata decays with a time course similar to that of LTP induced in the presence of protein synthesis inhibitors (9). Thus, an understanding of how signals initiated at the synapse reach the nucleus to induce this essential gene transcription and protein translation within a limited time window is crucial.
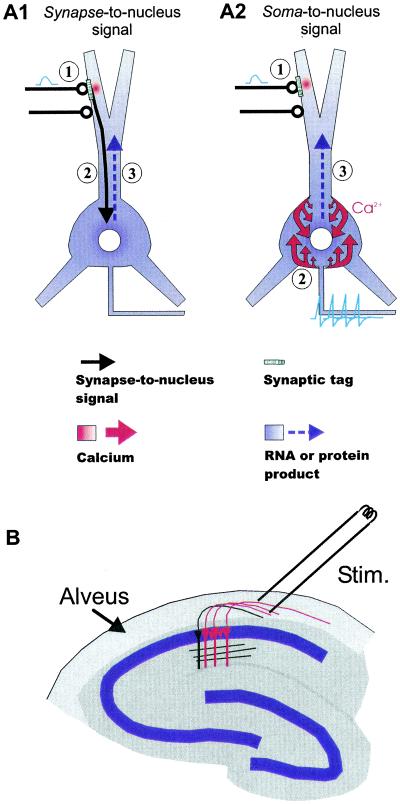
Several synapse-to-nucleus signaling molecules have been proposed, including calcium (10), NFκB (11, 12), and calmodulin (13), but before investigating a specific molecule, the question of whether such a synapse-to-nucleus signal is essential for late LTP-related gene expression needs to be addressed (Fig. 1A1). An alternative possibility is that action potential firing in the postsynaptic neuron might suffice to activate gene expression by influx of calcium through voltage-sensitive calcium channels in the cell membrane (Fig. 1A2). Work by Tsien and colleagues, however, suggests that synaptic stimuli, but not action potential firing, are required for cAMP response element-binding protein (CREB)-dependent gene transcription associated with LTP (14). This work in cell culture showed that the transcription factor CREB was not phosphorylated when hippocampal cultures were electrically stimulated, while synaptic activity was blocked with glutamate receptor antagonists (14). Further work demonstrated that activation of subsynaptic calmodulin and its subsequent translocation to the nucleus is required for inducing phosphorylation of CREB (13). In apparent contrast are experiments from dorsal root ganglion cultures showing that phosphorylation of CREB and subsequent expression of Fos protein could be induced with electrical stimulation, even though the preparation was devoid of synapses (15).
Figure 1.
Schematic diagram outlining two contrasting scenarios for LTP-related nuclear signaling. (A1) Synapse-to-nucleus signal (①): Activation of NMDA receptors that induces LTP creates a “synaptic tag” (for retrieval of gene product, or synthesis of protein from dendritic RNA), and (②) initiates a synapse-to-nucleus signal (black arrow) that triggers nuclear RNA synthesis and (③) distribution of protein or RNA throughout the neuron (blue arrow). (A2) Soma-to-nucleus signal (①). Activation of NMDA receptors and creation of the “tag” proceed as in (A1); (②) the induction of the gene product, in contrast to the case in A1, is induced by a rise in somatic and/or nuclear calcium by action potentials, possibly requiring activation of L-type calcium channels (③). The gene product is distributed in the same way as in A1. Note that the tag is essential for conferring synapse specificity of LTP. (B) Diagram showing how hippocampal CA1 neurons were activated nonsynaptically. Neurons were backfired through their own axons in the alveus, the white-matter structure lining the outside surface of CA1. Most neurons (red) in a restricted region of CA1, albeit not all of them (black), would be antidromically activated. LTP experiments could still be performed in the traditional way, synaptically, by stimulating the stratum radiatum.
We therefore set out to examine this apparent contradiction in a more intact preparation by testing whether action potentials alone are sufficient to stimulate the signaling pathways proposed to be involved in late LTP in hippocampal neurons. In the hippocampal slice, neuronal activation is possible by using antidromic stimulation to backfire the CA1 neurons from the alveus (Fig. 1B). We measured the phosphorylation of extracellular signal-regulated kinase-1/2 (ERK1/2) and CREB by using antibodies to the phosphorylated forms of the proteins. We also tested for the protein encoded by an immediate early gene, zif268, which has been closely linked with late-phase LTP in the dentate gyrus (16).
Furthermore, we sought to test whether such antidromic stimulation could substitute for synaptic stimulation in its ability to rescue early LTP by using a Frey-and-Morris-type paradigm (17). Early-LTP, induced either with high-frequency stimulation in the presence of protein synthesis inhibitors or by using a shortened LTP induction protocol, decays within 3–5 h (4, 17). Frey and Morris found that late-phase LTP induced at one set of synapses could rescue the early-LTP induced at a second pathway from decay, presumably by activating the protein synthetic pathways involved in late-phase LTP. The synapses, having undergone early-LTP (“tagged synapses”), could take advantage of the gene product induced by the late-LTP. With this “tagging” protocol (17), we induced early-LTP and determined whether signals and genes induced with prior antidromic stimulation were sufficient to rescue the early-LTP from decay.
Here we present evidence that antidromic stimulation in a theta-burst pattern, although not producing LTP itself, is sufficient for inducing ERK and CREB phosphorylation, for inducing Zif268 protein, and for rescuing early-LTP from decay. These data suggest that a synapse-to-nucleus signal is not required for induction or expression of late-LTP, and they emphasize the role of action potentials in late-LTP- related gene expression.
Methods
Slice Preparation and Physiology.
Hippocampal slices (400 μm) were prepared from hooded or albino Sprague–Dawley rats between the ages of 5 of 12 weeks. Slices were cut on a vibraslicer in ice-cold artificial cerebrospinal fluid containing (in mill-molar) NaCl, 124; KCl, 4; NaH2PO4, 1.25; NaHCO2, 26; CaCl2, 1.5; MgCl2, 2.5; glucose, 10; kynurenic acid, 3; and bubbled with 95/5% O2/CO2. Slices were perfused at 1 ml/min in an interface chamber (Medical Systems/Haas top; Medical Systems, Greenvale, NY) with artificial cerebrospinal fluid with 2.5 mM CaCl2/1.5 mM MgCl2 and were maintained at 32–34°C. Concentric bipolar stimulating electrodes (Frederick Haer & Co., Bowdoinham, ME) were positioned with their tips in either the alveus or the stratum radiatum, and for recording of population spikes and synaptic potentials, an artificial cerebrospinal fluid-containing glass recording electrode was positioned in stratum pyramidale or radiatum, respectively. Unless stated otherwise, stimulation was delivered with a duration of 50 μs. Theta-burst stimulation (18) for induction of early-LTP consisted of 5–10 bursts of 4 pulses at 100 Hz, delivered at 5 Hz (20–40 pulses total, 100 μs duration). The stimulus duration during LTP induction was increased to evoke spikes. Preliminary studies showed that more than 10 bursts (40 pulses) increased the likelihood of inducing late-LTP, indicating that a threshold number of pulses necessary for late-LTP was not exceeded in the early-LTP induction protocol. Stimulation intensity for the antidromic stimulation was set to evoke a population spike of ≈1 mV recorded in the cell bodies (stratum pyramidale) and present in the dendritic (stratum radiatum) recordings. The same intensity range was used for synaptic stimulation. For pERK and pCREB staining, 10 bursts were given twice, with a 30-s interval, and for Zif268 staining and antidromic rescue experiments, this sequence was repeated two more times with an 8- to 10-min interval. Because ERK and CREB can be rapidly dephosphorylated, slices were fixed in 4% paraformaldehyde 1–5 min after the last pulse of stimulation. In Zif268, slices were maintained for 90–120 min after the last stimulation before fixation to allow for the protein to be expressed. Results were evaluated for statistical significance by ANOVA (both immunocytochemical and electrophysiological experiments) and are presented as mean ± SE.
Immunocytochemistry.
Slices were fixed overnight and then cryoprotected with a 24-h incubation in 10% sucrose/4% paraformaldehyde. Frozen slices were recut at 30 μm, and the resulting sections processed as described by Matthies (19), modified for diaminobenzidine with a Vectastain Elite kit (Vector Laboratories) (20). Sections were incubated for 48 h in polyclonal antibodies to dually phosphorylated ERK I/II (Promega, 1:7,000–1:10,000), CREB phosphorylated at Ser-133 (New England Biolabs, 1:5,000), or Zif268 (Santa Cruz Biotechnology, 1:10,000–1:30,000). To avoid possible saturation of the diaminobenzidine reaction product, the antibody concentration was chosen to be sufficiently dilute to allow the reaction to proceed at a rate slow enough to permit cessation of the reaction before the point of maximum intensity of stain. Images (×4 or ×20) were acquired digitally on a microscope equipped with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI), and the staining intensity was quantified by imaging densitometry with metamorph software (Universal Imaging, Media, PA). The three most representative and/or most complete sections from each slice were chosen for analysis, and the average intensity determined in either a 20 × 20 pixel area in the stratum pyramidale region (pERK) or in thresholded regions of the stratum pyramidale (pCREB and Zif268). Image intensities from stimulated regions were subtracted from unstimulated regions of the same slice to give the difference in immunoreactivity, thus correcting for slice-to-slice variation in staining intensity. In some cases the location of the stimulating electrode was marked with crystals of DiI.
Results
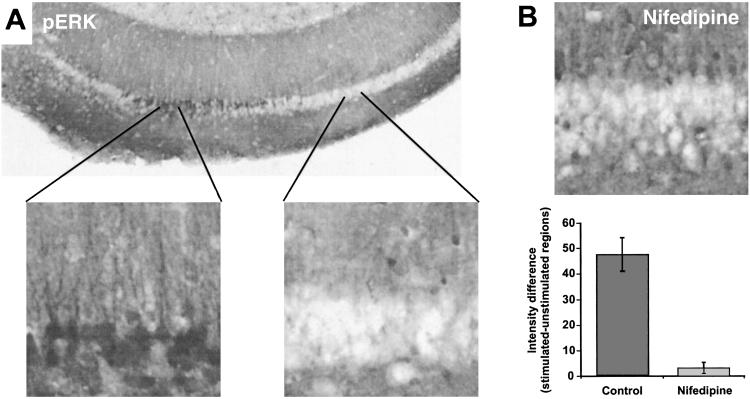
The ERK types of mitogen-activated protein kinases play a major role in stimulus-dependent transcription. ERKs respond to LTP-inducing stimulation of hippocampal neurons (21) and have been proposed to be involved in late-phase LTP (22). As a first step in assessing the effectiveness of antidromic stimulation on late-phase LTP-related signaling, we sought to determine whether such stimulation could activate the ERK pathway. Voltage-sensitive calcium channels (VSCCs) are strongly implicated both in ERK activation and activity-dependent gene expression (23, 24), and because studies have shown that depolarization in the form of single-action potentials does not support activation of VSCCs (14, 25), we used a theta-burst pattern of stimulation. Theta-burst stimulation (TBS; ref. 18) has been used successfully to induce synaptically ERK activation in hippocampal slices, and further, it uniquely recruits VSCCs to activate ERK when delivered at intensities evoking action potentials (20). We found that antidromic TBS induced ERK phosphorylation severalfold over unstimulated regions of the slices (Fig. 2A), even when possible activation of excitatory synapses from axon collaterals was blocked by 2-amino-5-phosphonovaleric acid, 6-cyano-7-nitroquinoxaline-2,3-dione, and α-methyl-4-carboxyphenylglycine. Also consistent with the idea that VSCCs are recruited with TBS is that the staining for phospho-ERK induced with antidromic stimulation was largely blocked with nifedipine (Fig. 2B), an inhibitor of the L-type VSCCs.
Figure 2.
Action potentials are sufficient to induce the phosphorylation of ERK1/2. (A) Antidromic stimulation (theta-burst) from the alveus induced staining for pERK in a restricted region of CA1 (n = 9). 6-Cyano-7-nitroquinoxaline-2,3-dione (20 μM), 2-amino-5-phosphonovaleric acid (50 mμM), and α-methyl-4-carboxyphenylglycine (300 μM) were used to block a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, NMDA, and mGluR receptors, respectively, which could have been activated through axon collaterals. Immunoreactive cells areas appear dark. Higher magnification of the indicated areas (Left, stimulated; Right, unstimulated) are shown to illustrate that pERK staining was limited to proximal dendrites and the cell bodies. (B) Effect of 20 μM nifedipine on antidromically stimulated phospho-ERK staining (n = 8).
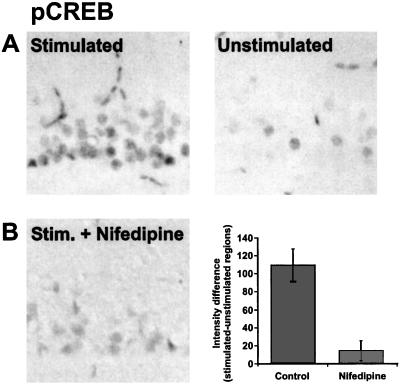
One transcription factor downstream from the ERK pathway is CREB (reviewed in ref. 26). CREB has been implicated in the regulation of a wide variety of genes, some of which may be involved in hippocampal LTP (27, 28) and long-term memory in rats, Drosophila, and Aplysia (29–31). CREB, when phosphorylated at Ser-133, can recruit coactivators such as CREB-binding protein to then promote expression of genes containing cAMP response elements (CREs; 32). Because the phosphorylation of CREB represents a key step in the regulation of CRE-mediated gene expression, we decided to test whether TBS delivered antidromically could induce phosphorylation of CREB (pCREB). This experiment was particularly important because previous studies not using TBS had failed to observe CREB phosphorylation with action potentials alone (14; but see ref. 15). Consistent with our observations that ERK could be phosphorylated with antidromic stimulation was that immunoreactivity for pCREB was increased after backfiring CA1 neurons (Fig. 3A). Furthermore, the increase in pCREB staining was blocked with nifedipine (Fig. 3B), indicating that in this experimental paradigm, L-type calcium channels are necessary for the phosphorylation of CREB at Ser-133.
Figure 3.
Action potentials are sufficient to induce the phosphorylation of CREB. (A) Antidromic stimulation (theta-burst) induces pCREB-like immunoreactivity in the stimulated region of CA1. Kynurenic acid (3 mM) was present during the stimulation to block activity at collaterals (n = 10). (B). Effect of 20 μM nifedipine on antidromically stimulated pCREB staining (n = 8).
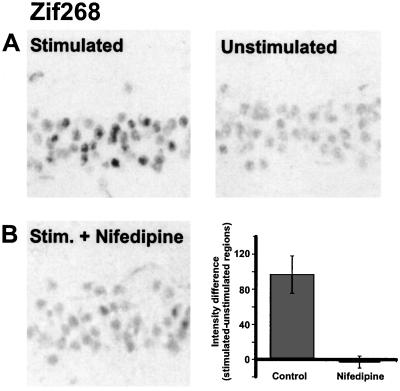
Because CREB phosphorylation alone is not entirely sufficient for CRE-mediated gene expression (27), we next examined whether an LTP-related gene could be induced with antidromic stimulation. Zif268 (Krox-24, EGR-1, or NGFI-A) is an immediate-early gene that contains CREs in addition to its other regulatory elements (33). Among immediate-early genes that are induced with neuronal activity, zif268 mRNA expression is the one most closely associated with the induction of late-phase LTP in the dentate gyrus (16, 34). Moreover, zif268 is apparently necessary for the expression of late-phase LTP and long-term memories, because both are impaired in mutant mice with a targeted inactivation of the zif268 gene (35). By using an antibody against the Zif268 protein, we detected an increase in immunoreactivity in the antidromically stimulated regions of slices, and this increase was blocked with nifedipine (Fig. 4 A and B).
Figure 4.
Action potentials are sufficient to induce the expression of Zif268. (A) Antidromic stimulation (theta-burst) induces Zif268-like immunoreactivity in the stimulated region of CA1. Kynurenic acid (3 mM) was present during the stimulation (n = 9). (B) Effect of 20 μM nifedipine on antidromically stimulated Zif268 staining (n = 4).
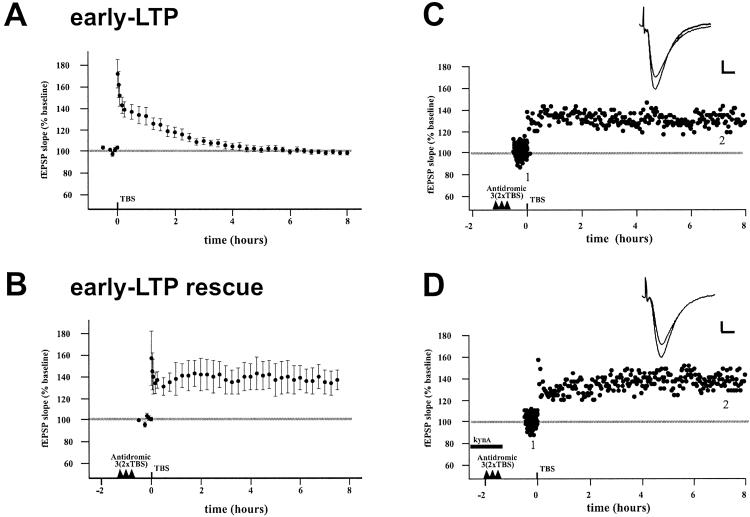
Although the induction of many genes has been correlated with late-LTP induction and/or maintenance, the gene(s) actually necessary for late-phase LTP are unknown. Therefore, because we found that antidromic stimulation could induce signaling associated with late-LTP, we then tested whether this stimulation could actually rescue early-LTP from decay as assessed by LTP maintenance. Five to ten bursts of 4 pulses (20–40 pulses total) delivered synaptically induced a modest potentiation similar to the early-LTP described (17), lasting about 3 h (Fig. 5A). This early LTP is postulated to induce a “synaptic tag”. Once tagged, a synapse is theoretically able to make use of the (unknown) RNA or protein product initiated by late-LTP induction elsewhere in the same cell to then maintain the potentiated state (17). Hence, we reasoned that antidromic stimulation should rescue the early-LTP from decay if signals originating from the synapse were unnecessary. We found this rescue indeed to be the case (Fig. 5 B and C): Antidromic stimulation in a theta-burst pattern was capable of preventing early-LTP from decay when the early-LTP was induced 1–2 h after the antidromic stimulation (105.9% of the LTP at 30 min remaining at 6 h). This difference was significantly different from the early-LTP alone after 4 h (P < 0.05). The result cannot be explained by greater induction in the rescues because the amount of potentiation measured at 1 min in the two cases were not statistically different (early-LTP, 172 ± 50.2 vs. rescue, 157 ± 57.2, P = 0.59). Furthermore, the result is not due to antidromic stimulation itself inducing LTP, because we found that our stimulation has no effect on the size of synaptic responses (n = 5, 100.1 ± 2.1% control at 5 h, not shown), consistent with classic studies in hippocampus showing that antidromic stimulation has no effect on synaptic responses (or induces a depression; 36). Also, the stimulation does not induce a slowly developing potentiation apparent only at later time points. One might propose that the rescue was simply a replication of the synaptic tagging result (17) through the activation of axon collateral synapses. This proposal was not likely to be the case, however, because the rescue was observed in two experiments in which kynurenic acid was applied during the backfiring (Fig. 5D, average of 98.4% of potentiation at 30 min remaining at 6 h for two cases).
Figure 5.
Action potentials are sufficient to rescue early-LTP from decay. (A) TBS (20–40 pulses in 100-Hz bursts) induces early-LTP, which decays to baseline levels by 3 h (n = 14). (B) Antidromic stimulation (3 × 2 theta-burst) rescues early-LTP from decay (n = 5). Induction was not significantly different between early-LTP and early-LTP rescues. (C) Example of rescue experiment. Traces (Inset) are the averages of 10 individual sweeps at the indicated times during the baseline period and at 7.5 h; scale bars represent 0.2 mV and 5 mS. (D) In two cases, 3 mM kynurenic acid was present during the stimulation to block activation of synapses from axon collaterals. One example experiment is shown. EPSP, excitatory postsynaptic potential
Discussion
Synapse-to-nucleus signals have been shown to exist in the context of synaptic plasticity in invertebrate neurons (37–40), but demonstration of synapse-to-nucleus signaling in vertebrate neurons has been difficult. These two models of synaptic plasticity differ in many anatomical and physiological respects, and the essential studies have not yet been performed to directly link such signaling to hippocampal LTP. One such example, involving a translocation of ERK from the synapse to the nucleus, has been demonstrated to occur in Aplysia neurons (38). Such translocation from synapses is now ruled out as a necessary mechanism in hippocampal neurons by our observation that action potentials alone are sufficient for nuclear staining of the phosphorylated enzyme. Any translocation of ERK to the nucleus could come from the somatic cytoplasm.
Our results show that calcium entry through VSCCs is sufficient for ERK activation, CREB phosphorylation, expression of at least one immediate-early gene related to late-phase LTP (zif268), and conversion of early-LTP to late-LTP. Together with studies showing that antidromic stimulation effectively induces a nifedipine-sensitive calcium influx into pyramidal neurons (41), our observations are consistent with the idea that synaptic activity is not required for L-channel activation or calcium rises in somatic compartments. Although signaling molecules originating from the synapse may cooperate in the process of LTP, they are not essential; calcium influx initiated by postsynaptic action potentials are sufficient to trigger the signaling events that induce gene transcription associated with late-LTP. One scenario for the induction of late-phase LTP-related genes suggested by our study is that synaptic activity sufficient for inducing bursts of action potentials recruits calcium from several sources. Increased calcium levels, if sufficient for the translocation of ERK to the nucleus, or for activation of nuclear enzymes such as CaMKinase IV, for example, could lead to phosphorylation of CREB (42, 43). No signal directly from the synapse seems to be necessary.
One proposed synapse-to-nucleus signal in LTP has been calmodulin. Synaptic activity has been reported to induce translocation of calmodulin to the nucleus within 1 min, resulting in CREB phosphorylation (13). Recently, however, Hardingham et al. showed that translocation of calmodulin is not necessary for CREB phosphorylation, and that calcium entry into the nucleus is instead the necessary factor (10). Hardingham further showed that activity-induced CREB phosphorylation was unaffected after blockade of the nuclear pore complex, which prevents import of proteins into the nucleus (10). Our studies do not rule out that calmodulin translocates from the cytoplasm to the nucleus, but it does show that synaptic activity is not necessary for the downstream events such as CREB phosphorylation. An interesting new finding has shown that calmodulin-binding sites on L-channels are critical for conveying the calcium signal to the nucleus for activation of the ERK pathway (44), indicating that calmodulin can modulate CREB phosphorylation independent of nuclear translocation.
The bulk of evidence in favor of a synapse-to-nucleus signal in vertebrate neurons lies in the observation that N-methyl-D-aspartate (NMDA) receptor blockade inhibits immediate early gene expression in most cases (45, 46; but see ref. 47). Can the blockade of immediate-early gene expression by inhibitors of NMDA receptors be explained if there were no synapse-to-nucleus signal? One explanation is that calcium influx through NMDA receptors, alone or together with other sources of calcium, contributes to the induction of immediate-early genes. Hardingham recently showed that in a culture preparation, 2-amino-5-phosphonovaleric acid prevented the rise in nuclear calcium without influencing the number of spikes in a burst (10). Although, 5-Hz and 100-Hz but not TBS-induced ERK activation is blocked by 2-amino-5-phosphonovaleric acid (20, 21). That TBS was shown to uniquely recruit L-channels, which may substitute for NMDA receptor activation in the phosphorylation of ERK (20), illustrates how different kinds of neuronal activation can lead to NMDA receptor-sensitive or -insensitive signaling. Our data presented here do not rule out that during synaptic activation, as contrasted to antidromic stimulation, calcium from NMDA receptors and/or metabotropic glutamate receptor-dependent release of calcium from stores is a primary mode of signaling. L-channels, while necessary for activation of ERK, phosphorylation of CREB and zif268 expression in this study, are clearly not required for synaptically induced ERK activation (20; but see ref. 23). Therefore, during normal synaptic activation of neurons, L-channels may be involved in late-LTP (22), but their role would likely be together with NMDA- and/or mGluR-receptor-dependent release of calcium from internal stores. Although both L-channel- and NMDA receptor-mediated calcium influx can activate serum response element-mediated transcription, only L-channels play an important role in stimulating CRE-mediated gene expression (48). The present experiments using antidromically fired spikes isolate the effects of L-channels and suggest that they are likely to be an important component in the recruitment of sufficient levels of calcium for LTP-related gene expression. Further experiments with inhibitors of RNA and protein synthesis and L-channels in our “tagging” protocol will be necessary to show definitively whether L-channels play a major role in late-LTP signaling regulating the expression of new RNA and proteins.
CRE-mediated gene expression may be a critical step in late-phase LTP-related gene expression, but it is unlikely that genes with large numbers of CREs, such as fos, are required. Expression of zif268, not fos, is most closely tied to LTP induction; many times the number of stimuli required for late-LTP are required for fos induction (16). However, Hardingham showed that a MAPK/ERK kinase inhibitor did not block induction of fos; and it may be that the CaMKIV pathway is required for fos-type expression, whereas the ERK pathway may be sufficient for SRE-mediated gene expression (10). Our experiments do not directly address these issues, however, but with regard to LTP, a promoter for a gene such as zif268, having both CREs and several SREs, would provide a regulation of LTP-related genes that would be distinct from others such as fos (33). Unfortunately, the individual genes critical to late-phase LTP are unknown, and it remains unproven whether SRE-mediated gene expression, without expression of genes regulated by CREB, is sufficient for late-LTP. Nevertheless, many candidate genes are thought to be involved in late-LTP including glutamate receptors, Homer, Arc/Arg-3.1, and brain-derived neurotrophic factor (49, 50–52). Among these, the mRNA-encoding zif268 and another immediate-early gene Arc can be detected as early as 5 min after a rat explores a novel environment (53), consistent with a model of very rapid RNA synthesis induced by neuronal activity. Analysis of the promoters of LTP-related genes, as they become known, may provide answers about what kinds of signals are required.
One possible explanation for the results observed in our rescue experiments is that antidromic stimulation induces release of neuromodulatory neurotransmitters such as norepinephrine or acetylcholine, thus priming the cells for late-LTP in an unknown way. Because both of these compounds can make pyramidal cells more excitable (54, 55), they may allow for greater induction of signaling cascades. Although a possibility, this explanation is unlikely given evidence to the contrary: antidromic stimulation can induce synaptic depression (36), and synaptic “priming” if anything reduces the likelihood of LTP induction (56). Also, excitability increases would have been expected to have decayed to baseline levels by the time the early-LTP (rescue) was induced up to 2 h later. Finally, no significant differences were observed between early-LTP and the rescues at 1 or 30 min, indicating that the induction was not greater in the rescued cases. Future experiments with the “tagging” done first, before the antidromic stimulation, will be required to rule out the possibility of priming, however.
Electrophysiological experiments have, for the most part, largely confirmed Hebb's postulate that a connection between a presynaptic neuron participating in firing a postsynaptic neuron will strengthen; postsynaptic depolarization, not necessarily postsynaptic spiking, is sufficient for LTP induction in hippocampal neurons (57). Recent work, however, has reemphasized the role of the spike in the induction of LTP in that the timing of presynaptic activity in relation to postsynaptic spiking determines whether a synapse will weaken, strengthen, or remain the same (58, 59; reviewed by ref. 60). More recently, the role of postsynaptic bursts is also gaining recognition as an important factor in LTP induction (61). Because action potentials represent powerful integrators of synaptic activity, they are ideally suited to regulate gene expression according to a cell's pattern of firing, singly or in bursts. Our work extends the role of postsynaptic spiking to include regulation of gene expression and demonstrates that LTP maintenance does not require a chemical synapse-to-nucleus signal.
Acknowledgments
We thank Drs. Marc Sommer and Laure Haak for careful reading of the manuscript.
Abbreviations
- LTP
long-term potentiation
- CREB
cAMP response element-binding protein
- ERK
extracellular signal-regulated kinase
- VSCC
voltage-sensitive calcium channel
- NMDA
N-methyl-d-aspartate
- CRE
cAMP response element
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Squire L R, Barondes S H. Brain Res. 1973;56:215–225. doi: 10.1016/0006-8993(73)90336-3. [DOI] [PubMed] [Google Scholar]
- 2.Meiri N, Rosenblum K. Brain Res. 1998;789:48–55. doi: 10.1016/s0006-8993(97)01528-x. [DOI] [PubMed] [Google Scholar]
- 3.Stanton P K, Sarvey J M. J Neurosci. 1984;4:3080–3088. doi: 10.1523/JNEUROSCI.04-12-03080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frey U, Krug M, Reymann K G, Matthies H. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- 5.Otani S, Marshall C J, Tate W P, Goddard G V, Abraham W C. Neuroscience. 1989;28:519–526. doi: 10.1016/0306-4522(89)90001-8. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen P V, Abel T, Kandel E R. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- 7.Frey U, Frey S, Schollmeier F, Krug M. J Physiol (London) 1996;490:703–711. doi: 10.1113/jphysiol.1996.sp021179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steward O, Schuman E M. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- 9.Frey U, Krug M, Brodeman R, Reymann K, Matthies H. Neurosci Lett. 1989;97:135–139. doi: 10.1016/0304-3940(89)90152-3. [DOI] [PubMed] [Google Scholar]
- 10.Hardingham G E, Arnold F J L, Bading H. Nat Neurosci. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- 11.Lerner-Natoli M, Montpied P, Rousset M C, Bockaert J, Rondouin G. Epilepsy Res. 2000;41:141–154. doi: 10.1016/s0920-1211(00)00132-7. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T. Neurosci Res. 1996;25:1–6. doi: 10.1016/0168-0102(96)01023-1. [DOI] [PubMed] [Google Scholar]
- 13.Deisseroth K, Heist E K, Tsien R W. Nature (London) 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- 14.Diesseroth K, Bito H, Tsien R W. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 15.Fields R D, Eshete F, Stevens B, Itoh K I. J Neurosci. 1997;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worley P F, Bhat R V, Baraban J M, Erickson C A, McNaughton B L, Barnes C A. J Neurosci. 1993;13:4776–4786. doi: 10.1523/JNEUROSCI.13-11-04776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frey U, Morris R G M. Nature (London) 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 18.Larson J, Wong D, Lynch G. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 19.Matthies H, Schulz S, Thiemann W, Siemer H, Schmidt H, Krug M, Hollt V. J Neurosci Methods. 1997;78:173–179. doi: 10.1016/s0165-0270(97)00149-0. [DOI] [PubMed] [Google Scholar]
- 20.Dudek S M, Fields R D. J Neurosci. 2001;21:RC122. doi: 10.1523/JNEUROSCI.21-02-j0002.2001. , 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.English J D, Sweatt J D. J Biol Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- 22.Impey S, Obrietan K, Wong S T, Poser S, Yano S, Wayman G, Deloume J C, Storm D R. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 23.Murphy T H, Worley P F, Baraban J M. Neuron. 1991;7:625–635. doi: 10.1016/0896-6273(91)90375-a. [DOI] [PubMed] [Google Scholar]
- 24.Rosen L B, Ginty D D, Weber M J, Greenberg M E. Neuron. 1994;12:1207–1221. doi: 10.1016/0896-6273(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 25.Mermelstein P G, Bito H, Deisseroth K, Tsien R W. J Neurosci. 2000;20:266–273. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayr B, Montminy M. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 27.Impey S, Mark M, Villacres E C, Poser S, Chavkin C, Storm D R. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- 28.Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schultz G, Silva A J. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 30.Yin J C P, Wallach J S, Del Vecchio M, Wilder E L, Zhou H, Quinn W G, Tully T. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 31.Dash P K, Hochner B, Kandel E R. Nature (London) 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- 32.Chrivia J C, Kwok R P S, Lamb N, Hagiwara M, Montiminy M R. Nature (London) 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 33.Alexandre C, Charnay P, Verrier B. Oncogene. 1991;6:1851–1857. [PubMed] [Google Scholar]
- 34.Abraham W C, Dragunow M, Tate W P. Mol Neurobiol. 1991;5:297–314. doi: 10.1007/BF02935553. [DOI] [PubMed] [Google Scholar]
- 35.Jones M W, Errington M L, French P J, Fine A, Bliss T V, Garel S, Charnay P, Bozon B, Laroche S, Davis S. Nat Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- 36.Pockett S, Brookes N H, Bindman L J. Exp Brain Res. 1990;80:196–200. doi: 10.1007/BF00228861. [DOI] [PubMed] [Google Scholar]
- 37.Bailey C H, Chen M, Keller F, Kandel E R. Science. 1992;256:645–649. doi: 10.1126/science.1585177. [DOI] [PubMed] [Google Scholar]
- 38.Martin K C, Michael D, Rose J C, Barad M, Casadio A, Zhu H, Kandel E R. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- 39.Carew T J, Sherff C M. Science. 1999;285:1911–1914. doi: 10.1126/science.285.5435.1911. [DOI] [PubMed] [Google Scholar]
- 40.Freudenthal R, Romano A. Brain Res. 2000;855:274–281. doi: 10.1016/s0006-8993(99)02358-6. [DOI] [PubMed] [Google Scholar]
- 41.Regehr W G, Tank D W. J Neurosci. 1992;12:4202–4223. doi: 10.1523/JNEUROSCI.12-11-04202.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chawla S, Hardingham G E, Quinn D R, Bading H. Science. 1998;281:1505–1509. doi: 10.1126/science.281.5382.1505. [DOI] [PubMed] [Google Scholar]
- 43.Kasahara J, Fukunaga K, Miamoto E. Biol Chem. 2001;276:24044–24050. doi: 10.1074/jbc.M100247200. [DOI] [PubMed] [Google Scholar]
- 44.Dolmetsch R E, Pajvani U, Fife K, Spots J M, Greenberg M E. Science. 2001;294:333–338. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 45.Cole A J, Saffen D W, Baraban J M, Worley P F. Nature (London) 1989;340:474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- 46.Berretta S, Parthasarathy H B, Graybiel A M. J Neurosci. 1997;17:4752–4763. doi: 10.1523/JNEUROSCI.17-12-04752.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Douglas R M, Dragunow M, Robertson H A. Brain Res. 1988;464:259–262. doi: 10.1016/0169-328x(88)90033-2. [DOI] [PubMed] [Google Scholar]
- 48.Bading H, Greenberg M E. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- 49.Nayak A, Zastrow D J, Lickteig R, Zahniser N R, Browning M D. Nature (London) 1998;394:680–683. doi: 10.1038/29305. [DOI] [PubMed] [Google Scholar]
- 50.Brakeman P R, Lanahan A A, O'Brien R, Roche K, Barnes C A, Huganir R L, Worley P F. Nature (London) 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 51.Steward O, Wallace C S, Lyford G L, Worley P F. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 52.Patterson S L, Grover L M, Schwartzkroin P A, Bothwell M. Neuron. 1996;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 53.Guzowski J F, McNaughton B L, Barnes C A, Worley P F. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 54.Cole A E, Nicoll R A. Science. 1983;221:1299–1301. doi: 10.1126/science.6612345. [DOI] [PubMed] [Google Scholar]
- 55.Madison D V, Nicoll R A. Nature (London) 1982;299:636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- 56.Huang Y-Y, Colino A, Selig D K, Malenka R C. Science. 1992;255:730–733. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- 57.Gustafsson B, Wigstrom H, Abraham W C, Huang Y Y. J Neurosci. 1987;7:774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magee J C, Johnston D. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- 59.Markram H, Lubke J, Frotscher M, Sakmann B. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- 60.Linden D J. Neuron. 1999;22:661–666. doi: 10.1016/s0896-6273(00)80726-6. [DOI] [PubMed] [Google Scholar]
- 61.Pike F G, Meredith R M, Olding A W A, Paulsen O. J Physiol (London) 1999;518:571–576. doi: 10.1111/j.1469-7793.1999.0571p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]