Abstract
Glial calcium signals play important roles during CNS development. Calcium transients induced by ATP, acting on purinergic receptors, stimulate DNA synthesis, increase astrocytic and neural stem cell proliferation, and are prominent during the differentiation of radial glia. We have shown previously that expression of P2Y receptors in astrocytes is altered when connexin43 (Cx43) is downregulated. To evaluate the consequences of Cx43 deletion on calcium signaling during neural progenitor development, studies were performed on neurospheres derived from embryonic striatum. After adhesion, cells migrating from wild-type (WT) and Cx43-null neurospheres displayed spontaneous calcium oscillations. Such activity was blunted by apyrase, 2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate (MRS-2179), and suramin, suggesting that ATP released by neural cells acts on purinergic receptors to induce calcium oscillations. The amplitudes of Ca2+ transients induced by P2Y but not P2X receptor agonists were larger in WT than in Cx43-null progenitors, suggesting that these two cell populations express different P2 receptors. Suramin, a nonselective P2 receptor antagonist, and MRS-2179, a P2Y1 receptor-selective antagonist, reduced the proliferation rate and the migration of WT progenitor cells to levels similar to those of Cx43-null cells. Conversely, exogenous expression of P2Y1 receptors in Cx43-null cells restored their migration pattern to levels seen in WT progenitors. However, treatment with P2 receptor antagonists did not alter the ratio of nestin to GFAP expression in WT neural progenitors. These data show that altered autocrine-paracrine communication attributable to reduced levels of P2Y1 receptors in neural progenitor cells lacking Cx43 affects proliferation and migration but not cell differentiation during early CNS development.
Keywords: P2 receptors, calcium oscillations, neurospheres, progenitors, gap junction, connexin
Introduction
Calcium oscillations in glial cells play important roles in the physiology and pathology of the CNS (Parri and Crunelli, 2001; Parri et al., 2001; Aguado et al., 2002; Tashiro et al., 2002). These signals can be restricted within one cell or transmitted to adjoining cells as intercellular Ca2+ waves. The propagation of intercellular calcium waves depends both on the diffusion of second messengers through gap junction channels and on ATP released from glial cells activating plasmalemmal P2 receptors (P2Rs) on neighboring cells (Scemes, 2000). The mammalian P2Rs comprise two families: the ligand-gated, cation-permeable, ionotropic P2XR (P2X1-P2X7) and the G-protein-coupled metabotropic P2YR (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11-P2Y14) (Ralevic and Burnstock, 1998; Abbracchio et al., 2003). Although some P2YRs stimulate adenylyl cyclase, thus generating cAMP, all P2YR subtypes are coupled to phospholipase C production, leading to Ca2+ mobilization. Activation of P2R induces DNA synthesis in glial cells (Neary et al., 1994; Milenkovic et al., 2003) and is implicated in the development of gliosis after brain trauma (Neary et al., 2003) as well as in the proliferation of radial glia (Uckermann et al., 2002) and human neural stem cells (Ryu et al., 2003).
Astrocytes are coupled to each other mainly by connexin43 (Cx43) gap junction channels (Spray et al., 1998; Dermietzel et al., 2000; Scemes et al., 2000) and express several P2R subtypes (Ballerini et al., 1996; Jimenez et al., 2000; Suadicani et al., 2003). We have shown previously that the expression of P2Y1R in astrocytes is decreased when Cx43 is downregulated (Scemes et al., 2000; Suadicani et al., 2003), and that the growth rate of Cx43-null astrocytes is decreased compared with that of wild-type (WT) cells (Dermietzel et al., 2000). In an in vitro model of CNS differentiation, neural progenitor cells were found to be coupled by Cx43 channels, and blockade of gap junctional communication strongly reduced proliferation and differentiation of neural progenitors (Duval et al., 2002). Using this same model, we here describe properties of calcium oscillations during the development of neural progenitors derived from striata of embryonic day 14 (E14) WT and Cx43-null mice and provide direct evidence for the participation of P2Y1R in the process of cell migration. Spontaneous calcium oscillations in neural progenitors are shown to depend on the activation of P2R, likely because of released ATP. In WT neural progenitors, the metabotropic P2Y1R is likely to mediate calcium transients, whereas in Cx43-null cells, which are shown to have reduced levels of P2Y1R, the ionotropic P2X7R appears to mediate this activity. Blockade of P2YR did not alter the differentiation of these progenitors, as measured by the ratio of nestin:glial fibrillary acidic protein (GFAP) expression levels, but reduced the distance of emigration of cells from WT neurospheres to distances similar to those observed in Cx43-null cells. Conversely, exogenous expression of P2Y1R in Cx43-null neurospheres restored the migration pattern observed in WT neural progenitors. These data suggest that deletion of the Cx43 gene impacts on other nonjunctional proteins, thus leading to physiological alterations that are not directly controlled by gap junctional proteins.
Materials and Methods
Animals. Heterozygous (Cx43del/+) mice (C57BL/6J) obtained by a Cre-mediated general replacement of a floxed Cx43 coding region by LacZ (Theis et al., 2001a,b) were kindly provided by Dr. K. Willecke (University of Bonn, Bonn, Germany).
Neural progenitors. Neurospheres were prepared as described previously (Duval et al., 2002). Briefly, striata dissected from E14 Cx43-null and WT mice were mechanically dissociated in ice-cold HBSS (Ca2+-and Mg2+-free), and viable cells were transferred to tissue culture dishes containing DMEM-F12 Ham's medium (Invitrogen, Carlsbad, CA) supplemented with 5% B27 (Invitrogen), 1% antibiotics, and 20 ng/ml human recombinant epidermal growth factor (EGF; Sigma, St. Louis, MO). After growth of neural progenitors into floating neurospheres, in vitro cell differentiation was induced by withdrawing EGF from culture medium and plating the neurospheres on polylysine- (10 μg/ml; Sigma) and fibronectin-coated (10 μg/ml; Sigma) glass-bottom microwells (MatTek, Ashland, MA).
Transfection of neurospheres with P2Y1receptor cDNA. Floating Cx43-null neurospheres were transfected with 6 μg of either enhanced green fluorescent protein (eGFP)-P2Y1R or eGFP cDNAs using lipofectamine 2000 (Invitrogen). At 18-19 hr after transfection, neurospheres were centrifuged at 1500 rpm for 5 min, resuspended in DMEM-F12 supplemented with B27 but without EGF, and plated on coated glass-bottom MatTek dishes for outgrowth (migration) assays (see below). The coding region of the mouse P2Y1R DNA was amplified from C57BL/6J Mus musculus genomic DNA (The Jackson Laboratory, Bar Harbor, ME), using reverse transcriptase modification of the PCR (RT-PCR) and the following specific primers designed on the basis of published sequences (National Center for Biotechnology Information, Bethesda, MD): forward primer, 5′-AAAGCTCGAGATGACCGAGGTGCCTTGGTC-3′; reverse primer, 5′-CTCGGGTACCTTCAAACTCGTGTCTCCATTCT-3′. The primers used contained palindromic sequences of XhoI (forward primer) and KpnI (reverse primer) restriction enzymes used for cloning into peGFP-N3 mammalian expression vector (BD Biosciences, Palo Alto, CA).
Semiquantitative RT-PCR. Total RNA was extracted from 7 d adherent neurospheres derived from WT and Cx43-null embryos using Trizol reagent (Invitrogen) followed by treatment with RNase-free DNaseI (Invitrogen) to eliminate the contaminating genomic DNA. Reverse transcription was performed using random hexamer primers. The semiquantitative PCR was performed within the linear range of amplification, as determined previously for the P2Y1R primer after 27 cycles of a PTC-100 Thermocycler (M. J. Research, Watertown, MA). Each cycle consisted of denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec, and extension at 72°C for 30 sec. The last cycle was followed by a final extension cycle at 72°C for 8 min and a soak cycle at 4°C. Reaction products were analyzed by electrophoresis on 2% agarose gels. The ribosomal 18S primer and competimers (Ambion, Austin, TX) at a 1:9 ratio were added to the samples and used as an invariant endogenous control against which the products from the gene of interest were normalized. The following P2Y1R primers were used: 5′-CTGTGTCTTATATCCCTTTCC-3′ (sense) and 5′-CTCCATTCTGCTTGAACTC-3′ (antisense).
Intracellular calcium transients. Spontaneous calcium transients were measured in cells outgrowing from adherent neurospheres loaded for 30 min at 37°C with either fluo-3 AM (5 μm; Molecular Probes, Eugene, OR) or fura-2 AM (2.5 μm; Molecular Probes). For analysis of agonist-induced intracellular Ca2+ transients, cells were loaded with fura-2 AM (10 μm; Molecular Probes) for 45 min at 37°C, as described previously (Scemes et al., 2000; Suadicani et al., 2003). Cells were then washed with Dulbecco's PBS (DPBS; pH 7.4; Cellgro, Herndon, VA) containing 5 mm glucose and imaged on an epifluorescence microscope (Eclipse TE2000-S; Nikon, Tokyo, Japan). Intracellular calcium measurements were performed on cells plated on MatTek dishes and bathed in DPBS (similar results were obtained using HEPES-buffered DMEM-F12 without phenol red). Images were acquired with a CCD camera (Orca-ER; Hamamatsu, Hamamatsu City, Japan). Changes in fluo-3 fluorescence intensity emitted at one excitation wavelength (485 nm) and the ratio of fura-2 fluorescence intensities emitted at two excitation wavelengths (340 and 380 nm) were obtained using combined systems of filters and mirrors (Lambda DG-4 Diaphot; Sutter Instruments, Burlingame, CA) driven by a computer through Metafluor software (Universal Imaging Corporation, West Chester, PA). Fluo-3 and fura-2 fluorescence images were acquired continuously at rates of 1.3 and 0.7 Hz, respectively. At least three independent cultures of neurospheres from three different litters were used for analysis. Measurements were obtained from regions of interest placed on cells (nestin- and GFAP-positive cells; see below) that migrated from the neurospheres. Changes in intracellular calcium levels were monitored before and after bath application of P2R agonists and antagonists, as described previously (Scemes et al., 2000; Suadicani et al., 2003). The P2R agonists ATP (Sigma), UTP (Sigma), 2-methylthio-ATP (2-MeS-ATP) (Calbiochem, La Jolla, CA), and 3′-O-4-bezoylbenzoyl-ATP (BzATP) (Sigma) were dissolved in DPBS (pH 7.4; Cellgro; Sigma) as stock solutions. Stock solutions of the P2R antagonists suramin (Sigma) and MRS-2178 (Tocris Cookson, Ballwin, MO) were prepared in H2O, and the P2X7R antagonist KN62 (Sigma) was dissolved in DMSO. Apyrase (grade III; Sigma) was dissolved in DPBS.
Outgrowth index. To evaluate the contribution of P2R during migration of neural progenitors, floating WT and Cx43-null neurospheres of similar diameters (WT, 277.8 ± 6.97 μm; Cx43-null, 271.3 ± 7.61 μm; n = 50; p > 0.05; t test) plated on coated glass-bottom microwells containing DMEM-F12 supplemented with B27 but without EGF were exposed for 3 d to either 100 μm suramin, a broad-spectrum P2R antagonist, 2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate (MRS-2179), a specific P2Y1R antagonist, or a mixture of the two antagonists. The outgrowth index (OI) of migrating cells was evaluated daily as the ratio of the distance of the foremost cells to the center of the sphere and the diameter of the sphere core. The OI of Cx43-null neurospheres transfected with either eGFP-P2Y1R or eGFP constructs was also analyzed as described above.
Proliferation assay. To determine whether P2R antagonists affected the cell proliferation rate within WT and Cx43-null neurospheres, the Vybrant 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay (Molecular Probes) was used according to the instructions of the manufacturer. Floating WT and Cx43-null neurospheres were centrifuged at 1500 rpm for 5 min, and neurospheres were resuspended in 900 μl of DMEM-F12 without phenol red (Invitrogen). A total of 100 μl/well of WT and Cx43-null neurosphere suspensions were transferred to a 96 well plate, and neurospheres were treated with either 100 μm suramin or MRS-2179 for 3 d. Six replicates of untreated and P2R antagonist-treated WT and Cx43-null neurospheres were performed. Proliferation rates of WT and Cx43-null neurospheres were obtained by measuring the optical density (570-595 nm) of solubilized formazan produced by the reduction of MTT by metabolic active cells. Because the level of formazan produced is directly proportional to the number of viable cells (Mossmann, 1983; Gerlier and Thomasset, 1986), proliferation rate can be estimated. An MRX Revelation microplate reader equipped with Revelation 4.21 software (Dynex Technology, Chantilly, VA) was used.
Western blots. Samples of whole-cell lysates of WT and Cx43-null neural progenitors grown for 3 d in coated MatTek dishes in the presence and absence of P2R antagonists were electrophoresed in 10% SDS-PAGE (Bio-Rad, Hercules, CA) and then transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH). Immunoblots were performed after overnight incubation of membranes with blocking solution (5% dry nonfat milk in 1× PBS) using polyclonal anti-GFAP (1:500; Sigma) and monoclonal anti-nestin (1:1000; Chemicon, Temecula, CA) antibodies. After several washes with 1× PBS containing 0.4% polyoxyethylenesorbitan monolaurate (Tween 20; Sigma), membranes were incubated with HRP-conjugated secondary antibodies (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA). Detection of bands was performed on x-ray films (Kodak, Rochester, NY) after incubation with enhanced chemiluminescence reagents (Amersham Biosciences, Piscataway, NJ). Nestin to GFAP expression ratios were measured by densitometric analysis (Scion-NIH Image software) of the two bands (200-240 kDa:nestin and 56 kDa:GFAP) obtained from each sample lane.
Immunocytochemistry. After 6 d in culture, adherent neurospheres from WT and Cx43-null mice were fixed in 4% paraformaldehyde for 5 min. After several washes in 1× PBS and 30 min of incubation with 1× PBS containing 0.4% Triton X-100 and 10% goat serum, cells plated on glass-bottom microwells were incubated overnight at 4°C with both primary antibodies, monoclonal anti-nestin (1:500; Chemicon) and polyclonal anti-GFAP (1:200; Sigma). Monoclonal and polyclonal secondary antibodies (Alexa Fluor 488 and 594 nm, respectively; Molecular Probes) at 1:2000 dilution were added to the cells for 1 hr at room temperature. Images were acquired using a Nikon inverted epifluorescence microscope equipped with a SPOT-RT digital camera (Diagnostic Instruments, Sterling Heights, MI), 100× oil immersion objective (Nikon), and appropriate filter sets (Nikon).
Results
Spontaneous intracellular calcium oscillations during neural progenitor development
When plated in polylysine-fibronectin-coated dishes containing medium free of growth factors, floating neurospheres readily adhered, and within 30 min, cells started migrating out of the neurospheres. This process of migration proceeds over several days, with migrating cells forming chains that extend from the sphere (Jacques et al., 1998). The cells that have emigrated out of the sphere are referred to here as neural progenitors, given that after 6 d in culture, they express both nestin, an intermediate filament protein of undifferentiated cells, as well as GFAP, a marker for astrocytes (Fig. 1A). Intracellular calcium levels were then monitored daily from fluo-3 AM-loaded WT and Cx43-null neural progenitors and found to spontaneously oscillate (Fig. 1B). After 1 d in culture, 98% (118 of 120) of WT neural progenitors displayed spontaneous calcium activity. This number decreased to 42% (50 of 120 cells) after 4 d in culture, remaining near that level (57-60%) during the next 4 d of measurements (Fig. 1C). The cells that displayed Ca2+ transients had an elongated, fusiform morphology and were radially oriented among the other quiescent cells, most of which had a flat shape resembling mature astrocytes. Similar results were described recently for neurosphere-derived precursors in which the percentage of cells displaying both global and local spontaneous calcium oscillations was greater during the first 2 d of development, decreasing over time as the cells differentiated into neuronal and glial phenotypes (Ciccolini et al., 2003). A different result was obtained in Cx43-null progenitors; at 1 d after plating, only 17% (20 of 120) of cells displayed spontaneous calcium oscillations. Such activity increased over time, attaining 100% Cx43-null cells 5 d after plating and remaining at that level (98-100%) for the following 3 d (Fig. 1C). These results suggest that Ca2+ oscillations may play an important role during development. Therefore, the difference observed between WT and Cx43-null cells may be anticipated to result in differences in cell differentiation, migration, or proliferation. To address these issues, we first evaluated the nature of these calcium transients and then investigated the contribution of these oscillations to neural progenitor development.
Figure 1.
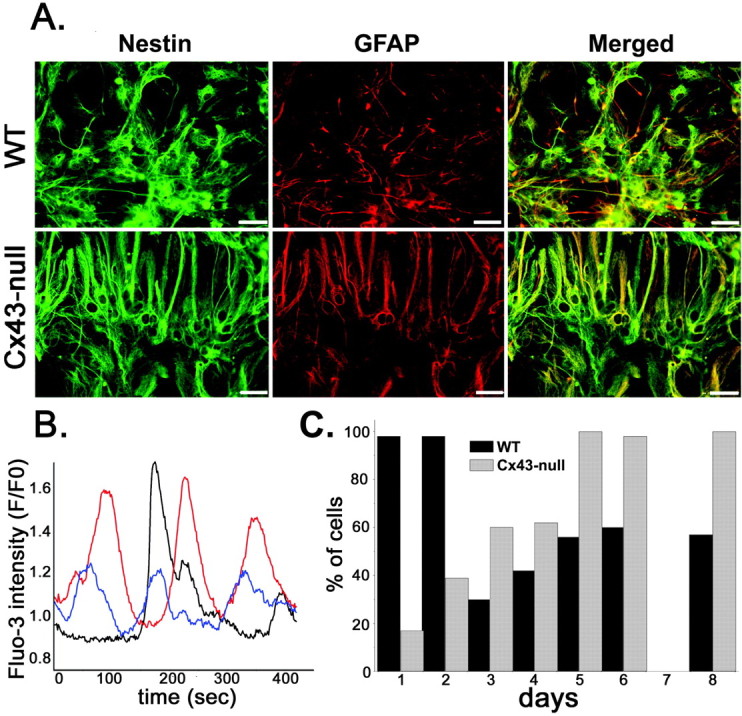
Characteristics of WT and Cx43-null neural progenitors. A, Double immunocytochemistry showing expression of nestin (green) and GFAP (red) in neural progenitors derived from 6 d adherent neurospheres of WT and Cx43-null mice. Note the coexpression of nestin and GFAP in WT and Cx43-null cells, suggesting incomplete astrocytic maturation. Scale bar, 9 μm. B, Example of spontaneous calcium oscillations simultaneously recorded from three WT neural progenitor cells (the oscillations of each cell are represented by a different color) loaded with fluo-3 AM. C, Developmental time course of spontaneous calcium oscillations in adherent neural progenitors that migrated out of WT (black bars) and Cx43-null (gray bars) neurospheres. The percentage of cells displaying intracellular calcium oscillations was obtained from continuous recordings (5 min) of fluo-3 intensity measured in 120 emigrated progenitors from three experiments.
Released ATP mobilizes different calcium sources in WT and Cx43-null neural progenitors
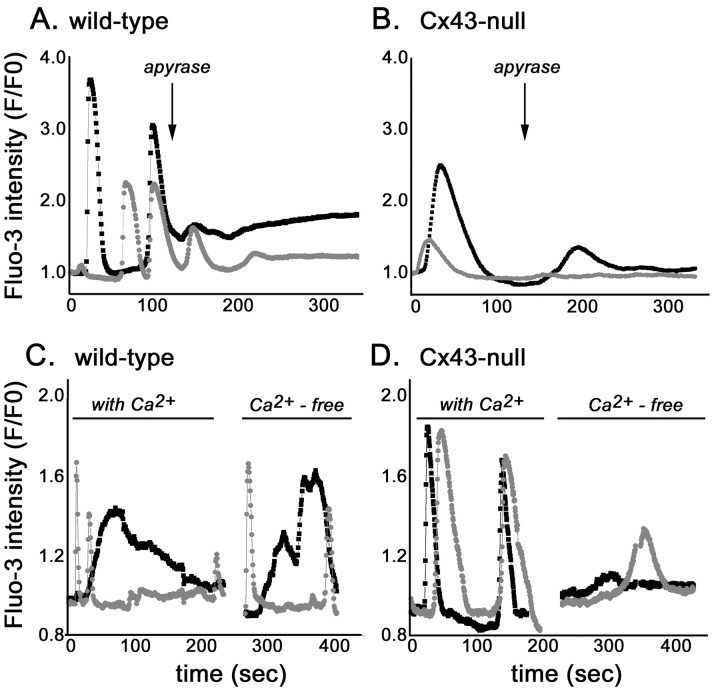
The spontaneous intracellular calcium transients observed in neural progenitor cells had variable durations (20-100 sec) and amplitudes (1.1- to 3.5-fold change in fluo-3 intensity) (Fig. 2). Statistical analysis of data obtained from three independent experiments indicated that the mean amplitude (measured as fold change in fluo-3 intensity) of spontaneous calcium oscillations in WT neural progenitors (2.00 ± 0.09; n = 43 cells) was significantly higher (p < 0.002; t test) than that of Cx43-null cells (1.63 ± 0.06; n = 63 cells). The frequency of spontaneous calcium oscillations recorded from WT cells 2-3 d after adhesion varied from 0.01 to 0.02 Hz, whereas in Cx43-null cells, such activity ranged from 0.002 to 0.006 Hz. Apyrase (5 U in 400 μl) strongly decreased, within 1-2 min of bath application, the amplitude of spontaneous calcium transients in both WT cells (from 2.00 ± 0.09 to 0.90 ± 0.11; n = 43 cells; p < 0.001; t test) and Cx43-null cells (from 1.62 ± 0.06 to 1.39 ± 0.04; n = 58 cells; p < 0.001; t test) (Fig. 2A,B). No spontaneous calcium oscillations were observed 5 min after apyrase application. These results indicate that the observed Ca2+ oscillations are probably mediated by the release of ATP from the progenitor cells acting on P2 receptors. Accordingly, application of 100 μm suramin, a nonselective P2 antagonist, at any time point of neural progenitor development (up to 6 d in culture) completely prevented spontaneous calcium oscillations in both WT and Cx43-null cells (data not shown).
Figure 2.
Spontaneous calcium oscillations in neural progenitors. Migrating neural progenitors derived from WT (A, C) and Cx43-null (B, D) mice exhibit spontaneous intracellular calcium transients that were greatly attenuated by bath application (arrow) of apyrase (A, B). Removal of extracellular calcium did not appreciably affect the amplitude of either long- or short-duration calcium oscillations in WT cells (C) but greatly reduced the amplitude of Ca2+ oscillations in Cx43-null progenitor cells (D). Black and gray traces were simultaneously recorded from two cells.
When bathed in Ca2+-free solution, the amplitudes of spontaneous calcium oscillations were greatly attenuated in Cx43-null progenitors (from 1.87 ± 0.16 to 1.19 ± 0.04-fold fluo-3 intensity; n = 34; p < 0.05) but not in WT cells (from 1.67 ± 0.10 to 1.85 ± 0.14-fold fluo-3 intensity; n = 22; p > 0.05) (Fig. 2C,D). Thus, these data suggest that although both WT and Cx43-null cells seem to release ATP, intracellular calcium elevations were induced by the mobilization of different calcium sources. Therefore, whereas WT neural progenitors mobilize Ca2+ from intracellular stores, an influx of Ca2+ from the extracellular solution occurs in Cx43-null cells.
WT and Cx43-null neural progenitors express different purinergic receptors
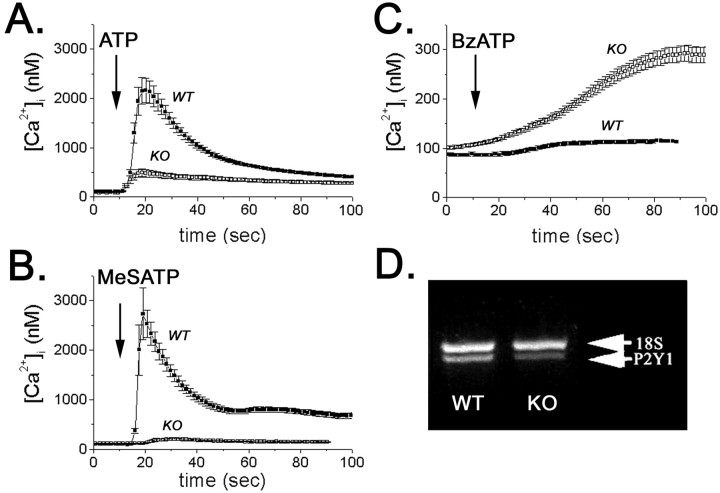
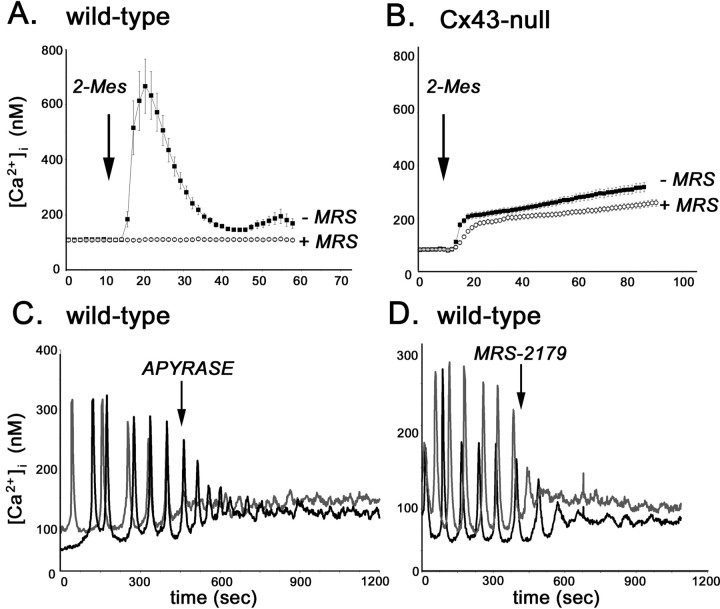
To evaluate the contribution of metabotropic and ionotropic P2Rs to the spontaneous calcium oscillation of WT and Cx43-null neural progenitors, intracellular calcium transients induced by the P2R agonists ATP, UTP, 2-MeS-ATP, and BzATP were measured in fura-2 AM-loaded cells. Except for UTP, all other P2R agonists induced intracellular calcium elevations in WT and Cx43-null neural progenitors (Fig. 3). However, the amplitudes of these Ca2+ transients were different in WT and Cx43-null cells. At 30 μm, the broad-spectrum P2R agonist ATP induced in WT progenitor cells an increase in cytosolic calcium levels (from 108 ± 4 to 2405 ± 322 nm; n = 40 cells) that was five times higher than the amplitude observed in Cx43-null cells (from 90 ± 3 to 490 ± 81 nm; n = 31 cells) (Fig. 3A). Similarly, bath application of 30 μm 2-MeS-ATP, a specific P2Y1R agonist, induced an increase in intracellular calcium that was 35 times larger in WT cells (from 101 ± 2 to 4077 ± 627 nm; n = 40 cells) than in Cx43-null progenitors (from 105 ± 5 to 225 ± 27 nm; n = 49 cells) (Fig. 3B). In contrast, the ionotropic P2X7R agonist BzATP (100 μm) caused an elevation in intracellular calcium that was four times higher in Cx43-null cells (from 102 ± 3 to 291 ± 16 nm; n = 50 cells) than in WT cells (from 87 ± 3 to 116 ± 4 nm; n = 50 cells) (Fig. 3C). Thus, these results suggest that WT and Cx43-null cells express different levels of metabotropic P2YR. Accordingly, semiquantitative RT-PCR amplification of total RNA indicated that P2Y1R mRNA levels were ∼50% lower in Cx43-null than in WT neurospheres (Fig. 3D). Furthermore, 5 μm MRS-2179, a specific P2Y1R antagonist, completely blocked intracellular calcium elevation induced by 30 μm 2-MeS-ATP in WT cells (Fig. 4A), whereas 1 μm KN62, a potent P2X7R antagonist (Baraldi et al., 2000), prevented Ca2+ rises induced by 100 μm BzATP in Cx43-null neural progenitor cells (Fig. 4B). Together, these data indicate that WT cells predominantly express the P2Y1R subtype, whereas in Cx43-null progenitors, the P2X7R is likely the predominant P2R subtype mediating calcium rises. Additional evidence favoring the participation of these P2Rs during spontaneous calcium oscillations in progenitor cells was obtained through the use of P2R antagonists. Addition of 5 μm MRS-2179, similar to the addition of 5 U of apyrase, decreased the amplitudes of spontaneous calcium oscillation in WT neural progenitors (Fig. 4C). Calcium oscillations in Cx43-null cells were attenuated by apyrase (Fig. 2B) and by 1 μm KN62 (Fig. 4D).
Figure 3.
Intracellular calcium transients induced by P2 receptor agonists. Bath application of 30 μm ATP (A) and 2-MeS-ATP (MeSATP; B) induced larger changes in cytosolic calcium levels in fura-2 AM-loaded WT than in Cx43-null (KO) progenitor cells. The P2X7R agonist BzATP (100 μm), however, was more effective in inducing Ca2+ changes in Cx43-null [knock-out (KO)] than in WT cells (C). Semiquantitative RT-PCR (D) showed that P2Y1R mRNA levels in Cx43-null neurospheres (KO) are decreased by 50% compared with WT neurospheres.
Figure 4.
P2Y1 and P2X7 receptors participate in spontaneous calcium oscillations of WT and Cx43-null neural progenitors. A, Wild-type neural progenitor calcium transients induced by bath application of 30 μm 2-MeS-ATP (2-Mes; arrow) were totally prevented by the specific P2Y1R antagonist MRS-2179 (MRS; 5 μm). B, The potent P2X7R antagonist KN62 (1 μm) blocked calcium transient induced by 100 μm BzATP in Cx43-null progenitors. Spontaneous intracellular calcium oscillations recorded from fura-2 AM-loaded (2.5 μm) WT (C) and Cx43-null (D) neural progenitors were prevented by bath application of 5 μm MRS-2179 and 1 μm KN62, respectively. The oscillations of each cell are represented by black and gray traces.
P2Y1 receptors contribute for the migration of neural progenitors
To evaluate the contribution of P2R during the migration of neural progenitors, WT and Cx43-null neurospheres plated on coated glass-bottom microwells were exposed for 3 d to P2R antagonists. At the time of plating, all neurospheres had similar diameters [WT, 277.8 ± 6.97 μm; Cx43-null 271.3 ± 7.61 μm; n = 50 neurospheres from four independent experiments; p > 0.05; t test]. The distances of migration of neural progenitors out of the neurospheres were then evaluated daily and expressed as an OI, which was given by the migration distance of the foremost cells from the center of the sphere normalized by the diameter of the neurospheres at each particular day.
Untreated WT and Cx43-null neural progenitors showed an OI that increased with time during 3 d of culture (Fig. 5); however, the OI of Cx43-null cells was significantly lower (p < 0.001; t test) than that of WT cells (Fig. 5). To assess whether this difference could be accounted for by the different numbers of progenitor cells within the neurospheres, larger Cx43-null neurospheres (diameter, 864 ± 54 μm; n = 11) were plated in parallel with regular-sized WT neurospheres (diameter, 370 ± 11 μm; n = 19). Twenty-four hours after plating, the OI of Cx43-null cells (1.63 ± 0.11; n = 11 neurospheres) was again significantly smaller (p < 0.01; t test) than that of WT progenitors (2.86 ± 0.11; n = 19 neurospheres). These data indicate that Cx43-null progenitors migrate shorter distances per unit time than WT cells, and that this migration is independent of the number of proliferating cells within the neurospheres.
Figure 5.
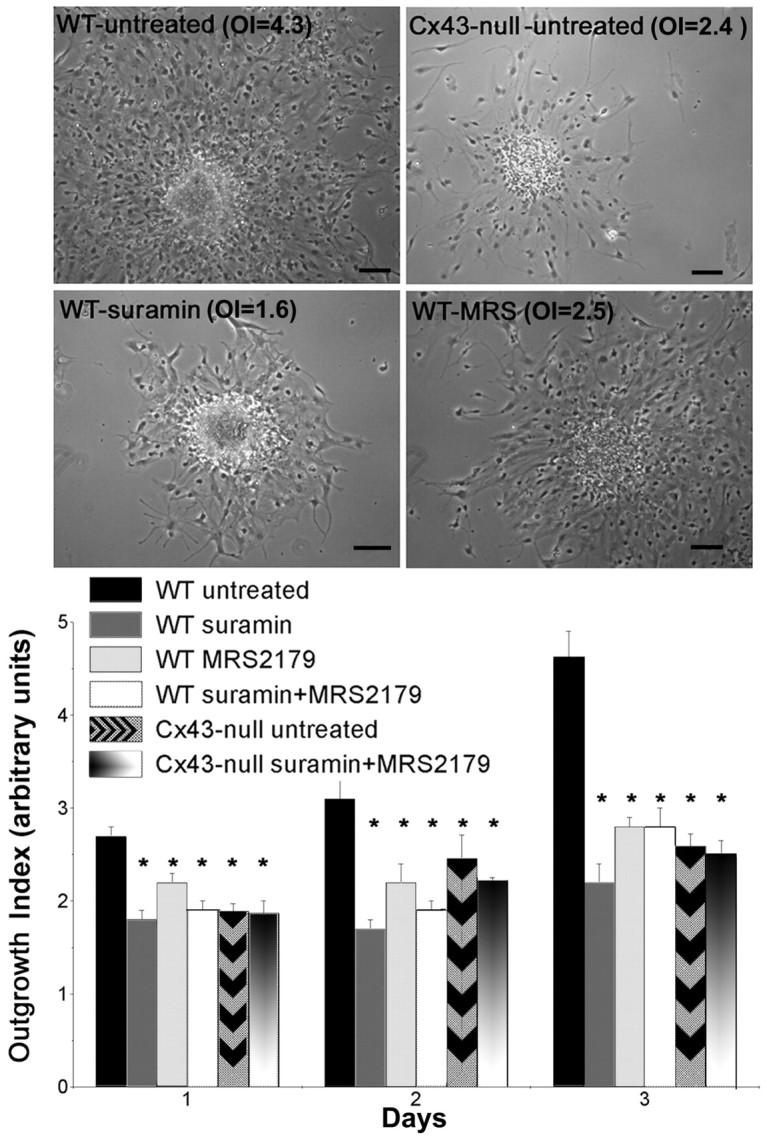
Contribution of P2Y1 receptors during neural progenitor migration. Top, Phase-contrast images of the migration of WT and Cx43-null progenitors after 3 d of culture. The correspondent OI is indicated in each image. Scale bar, 95 μm. Bottom, Time course of this migration as evaluated by the outgrowth index in the absence (untreated) and presence of P2R antagonists (100 μm; suramin, MRS-2179, and suramin plus MRS-2179). Note that both P2R antagonists significantly reduced (*p < 0.0001; ANOVA) the outgrowth index of WT neural progenitors to levels that were similar to those obtained for Cx43-null cells.
Exposure to 100 μm suramin, MRS-2179, or a mixture of the two antagonists significantly reduced the OI of WT. Thus, after 1 d of suramin treatment, the OI of WT neural progenitors decreased from 2.7 ± 0.1 to 1.8 ± 0.13 and decreased to 2.2 ± 0.1 and to 1.9 ± 0.1 when exposed to MRS-2179 and suramin plus MRS-2179, respectively (n = 8 neurospheres; p < 0.0001; ANOVA) (Fig. 5). In contrast, no changes in OI were observed in Cx43-null neurospheres (Fig. 5). As a result of these differential changes, after 3 d of culture, the OI of WT progenitors treated with P2R antagonist was approximately twofold smaller than that of untreated WT cells (OIuntreated, 6.38 ± 0.40; OIsuramin-treated, 2.68 ± 0.25; OIMRS-2179-treated, 2.89 ± 0.16; OIsuramin+MRS-2179, 3.38 ± 0.55; n = 8) and similar to the OI of Cx43-null cells (OIuntreated, 2.59 ± 0.13; OIsuramin+MRS-2179, 2.51 ± 0.14; n = 8) (Fig. 5).
To test whether P2X7Rs also participate in the migration of neural progenitors, Cx43-null neurospheres were treated for 2 d with KN62 (1 and 10 μm). No significant difference (p > 0.05; ANOVA) was observed between the OI of treated (OIKN62(1 μm), 1.45 ± 0.12, n = 4; OIKN62(10 μm), 1.85 ± 0.19, n = 4) or untreated (OIuntreated, 1.85 ± 0.27; n = 4) Cx43-null progenitor cells.
These results showing that P2R antagonists affect the OI of WT cells but not of Cx43-null neural progenitors suggest that the decreased migration of Cx43-null progenitors out of the neurospheres does not involve P2X7R and is likely attributable to the reduced expression of P2Y1R in these cells.
P2Y receptors play an important role during cell proliferation
After 3 d of exposure to 100 μm suramin and MRS-2179, the proliferation rate of cells within floating WT neurospheres decreased, as evidenced by the reduced values of absorbance obtained with the MTT assay (Fig. 6A). In contrast, neither of these P2R antagonists altered the proliferation rate of floating Cx43-null neurospheres (Fig. 6A). These data indicate that blockade of P2Y1R delays proliferation of WT neurospheres.
Figure 6.
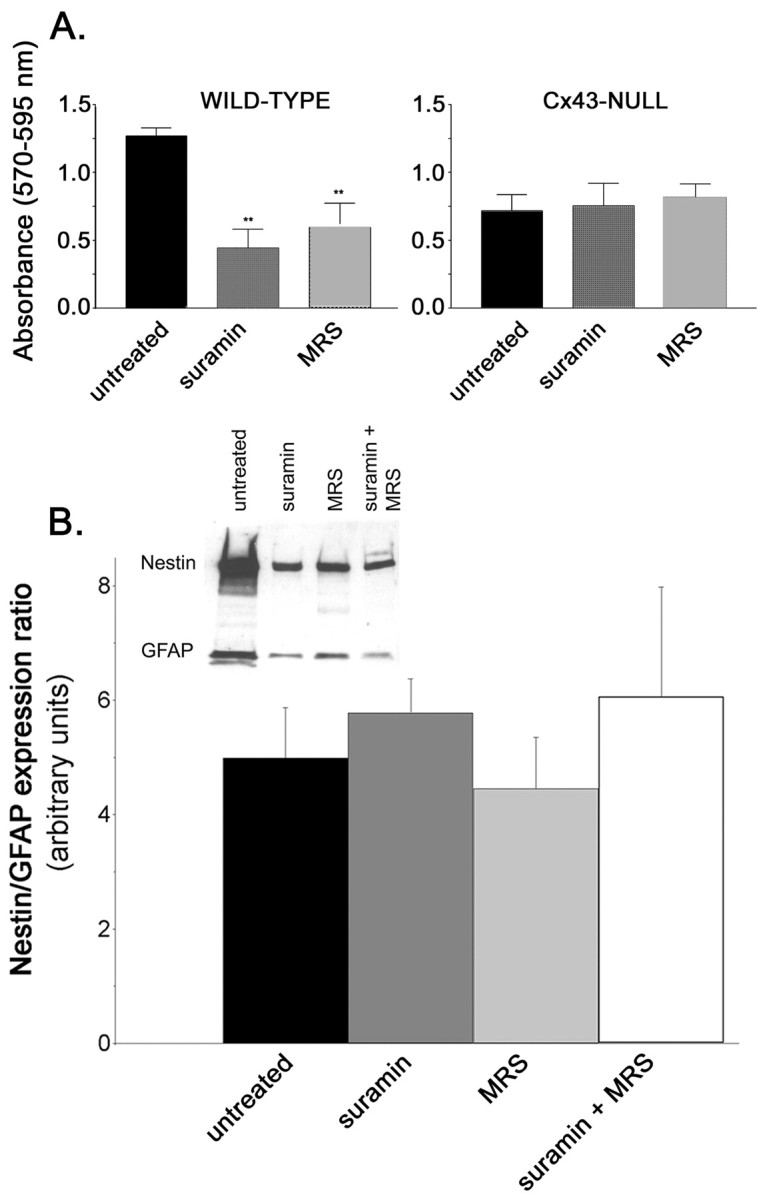
Blockade of P2 receptors affects the proliferation but not differentiation of neural progenitors. A, Optical density measurements obtained for the MTT assay indicate that the proliferation rate of floating WT but not of Cx43-null neurospheres was greatly reduced after 3 d of exposure to 100 μm suramin or MRS-2179 (MRS) (p < 0.001; ANOVA; n = 6 replicas). B, Western blot (inset) showing the expression of nestin and GFAP in WT neural progenitors untreated and treated for 3 d with P2R antagonists. The ratio of nestin to GFAP expression was not affected by 3 d of treatment with the P2R antagonists (p > 0.05; ANOVA; n = 3 experiments).
To evaluate whether P2Rs play a role in cell differentiation, the ratio of expression levels of nestin to GFAP, two proteins of the intermediate filaments, were quantified. Western blot analysis of whole-cell lysates of WT neural progenitors indicated that the ratio of nestin to GFAP expression was not significantly altered by 3 d of exposure to P2R antagonists (p > 0.05; ANOVA) (Fig. 6B).
Together, these results show that P2 receptors, most likely of the P2Y1R subtype, play an important role in the processes of migration and proliferation of neural progenitors but are not involved in their differentiation.
Exogenous expression of P2Y1 receptors restores the outgrowth index of Cx43-null neural progenitors
To confirm the participation of P2Y1R in the migration of neural progenitors, Cx43-null floating neurospheres were transfected with eGFP-P2Y1R cDNA and then plated in coated MatTek dishes for OI measurements. As shown in Figure 7A, eGFP-P2Y1Rs were widely expressed in proliferating cells located at the core of the sphere (Fig. 7A, arrowhead) as well as in the migrating neural progenitors (Fig. 7A). Expression of these receptors in Cx43-null neurospheres led to increased amplitudes of calcium transients in response to 30 μm 2-MeS-ATP (from 123.0 ± 3.0 to 930.0 ± 68.3 nm; n = 120 cells from three independent experiments; Fig. 7B) (compare with Fig. 3C, untransfected Cx43-null neurospheres). These larger transients were greatly attenuated by 5 μm MRS-2179, a P2Y1R antagonist (Fig. 7B, compare Fig. 4A, WT response). Moreover, forced expression of P2Y1R in Cx43-null cells led to a dramatic increase in the frequency of spontaneous calcium oscillation compared with that seen in untransfected cells (Fig. 7C,D). Thus, these data suggest that the low frequency of spontaneous calcium oscillations observed in Cx43-null progenitors is likely related to the decreased P2Y1R expression level in these cells. Regardless of whether Cx43-null progenitor cells release smaller amounts of ATP than WT cells, as suggested recently by studies implicating connexin hemichannel as a route for ATP release (Cotrina et al., 2000; Stout et al., 2002; Braet et al., 2003; Stout and Charles, 2003), this does not seem to contribute to the differences in spontaneous Ca2+ oscillation patterns observed between Cx43-null and WT cells.
Figure 7.
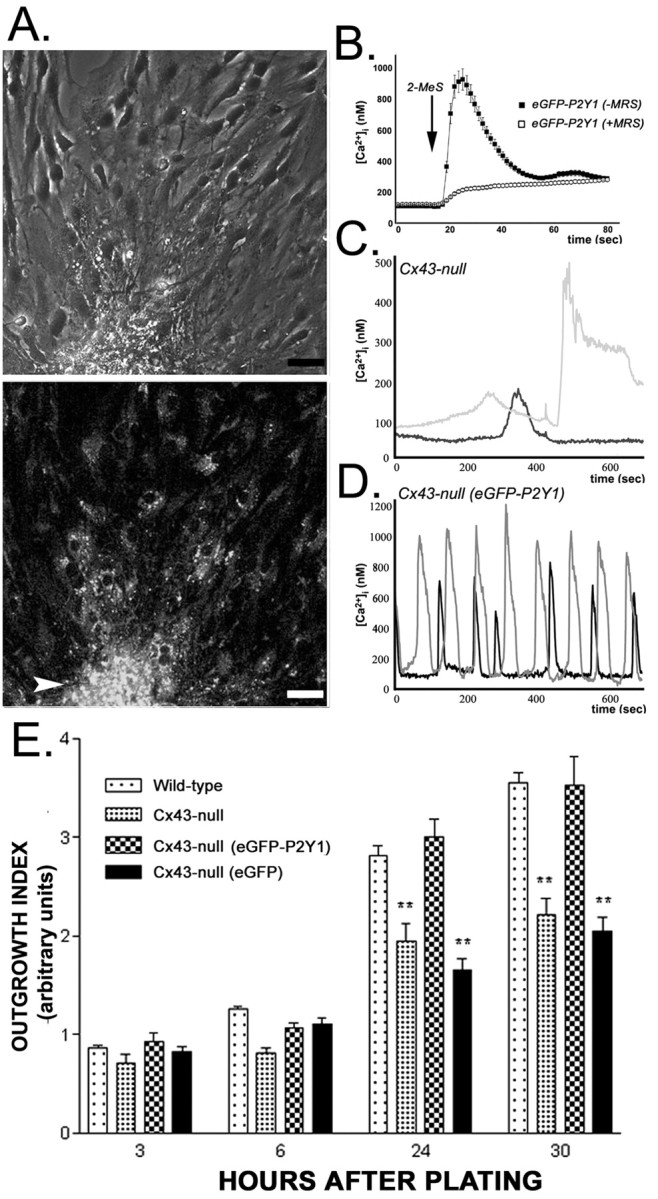
Exogenous expression of P2Y1 receptor increases the outgrowth index of Cx43-null neural progenitors. A, Phase contrast (top) and fluorescence (bottom) images obtained 30 hr after plating Cx43-null neurospheres transfected with eGFP-P2Y1R. The arrowhead points to the core of the neurosphere. Scale bar, 24 μm. B, Calcium transient induced by 30 μm 2-MeS-ATP was mostly blocked by the P2Y1R antagonist MRS-2179 (5 μm; n = 120 cells from 3 independent experiments), indicating proper function of the transfected receptor. C, Note the low frequency of spontaneous calcium oscillations recorded from Cx43-null progenitors and the increased frequency of these events in Cx43-null cells transfected with eGFP-P2Y1R (the oscillations of each cell are represented by black and gray traces) (D). E, Thirty hours after adhesion to fibronectin-polylysine-coated substrate, the outgrowth index values of Cx43-null progenitors transfected with eGFP-P2Y1R were similar to those of WT cells. Note that transfection of Cx43-null neurospheres with eGFP constructs did not increase the outgrowth index of Cx43-null cells, which was lower than that of WT controls. (**p < 0.001; ANOVA; n = 30 neurospheres).
Because functional expression of eGFP-P2Y1R greatly declined 72 hr after transfection, we modified the migration protocol to measure the outgrowth index during the period of time in which P2Y1R expression is maximal (24-48 hr). Thus, the OIs of neural progenitors migrating out of Cx43-null neurospheres that had been transfected with either eGFP-P2Y1R or eGFP cDNAs 18-19 hr before adhesion were measured at 3, 6, 24, and 30 hr after plating in coated dishes; parallel experiments were performed with untransfected WT and Cx43-null neurospheres. As shown in Figure 7B, 30 hr after plating, the OI of Cx43-null neural progenitors expressing eGFP-P2Y1R (3.53 ± 0.30; n = 23 neurospheres) was similar to that of WT cells (3.56 ± 0.10; n = 10 neurospheres). In contrast, the OI of progenitors of untransfected Cx43-null cells (2.22 ± 0.16; n = 15 neurospheres) and of eGFP-transfected Cx43-null cells (2.05 ± 0.14; n = 14 neurospheres) was significantly lower than that of WT progenitors (p < 0.001; ANOVA).
These data showing that exogenous expression of P2Y1R restores the OI of Cx43-null progenitors to levels seen in WT cells strongly support the notion that this receptor subtype plays an important role in the migration of neural progenitors. Furthermore, because forced expression of eGFP-P2Y1R increased the frequency of spontaneous calcium oscillation in Cx43-null cells to levels similar to those of WT cells, it is plausible that the extent of cell migration is related to the frequency of intracellular calcium events generated by the activation of P2Y1R.
Discussion
Spontaneous astrocytic Ca2+ oscillations have been reported to occur during CNS development (Parri and Crunelli, 2001; Parri et al., 2001; Aguado et al., 2002) and under epileptiform conditions (Tashiro et al., 2002). Calcium signals can be transmitted to neighboring astrocytes via gap junctions and via released ATP activating P2 receptors (for review, see Scemes, 2000). The abundance of gap junctions and purinergic receptors in the developing CNS and the evidence implicating their participation in cell physiological events, such as apoptosis (Abbracchio, 1997; Virginio et al., 1999; Lin et al., 2003) and differentiation (Neary and Zhu, 1994; Belluardo et al., 2000), has led to the proposal that gap junctions and P2 receptors not only play important roles in early organization of the CNS but also in the remodeling and repair of neuronal circuits after brain injury.
Very little is known about the respective roles played by these two forms of intercellular communication during early stages of CNS development. Using neurospheres as an in vitro model of CNS differentiation, neural progenitors were shown to be coupled by Cx43 gap junction channels, and blockade of these channels was shown to affect cell viability, proliferation, and differentiation (Duval et al., 2002). Using this same model, we report here on the properties of Ca2+ signaling in neurospheres derived from E14 WT and Cx43-null striata during neural progenitor migration.
Our data show that WT and Cx43-null neural progenitors display spontaneous Ca2+ oscillations that are highly dependent on ATP acting on P2 receptors. These findings open the possibility that spontaneous calcium oscillations are a hallmark feature of migratory progenitor cells with the ability to release ATP. In concert with growth factors, nucleotides and nucleosides contribute to phenotypic changes in both neuronal and non-neuronal cells (Abbracchio, 1997). Spontaneous calcium oscillations in astrocytes are also prominent during epileptic seizures (Manning and Sontheimer, 1997; Tashiro et al., 2002), a condition that stimulates the proliferation of neuronal precursors and astrocytes (Parent et al., 2002).
Our study also shows that although both WT and Cx43-null neural progenitor cells are sensitive to ATP, different P2 receptors mediate calcium elevation in these two cell populations. Thus, whereas P2Y1Rs predominate in WT cells, P2X7Rs are likely to be the prevalent P2R subtype in Cx43-null neural progenitors. These findings are in agreement with previous observations on primary cultures of Cx43-deficient astrocytes indicating reduced levels of P2Y1R (Scemes et al., 2000; Suadicani et al., 2003). Given that UTP did not elicit Ca2+ transients in WT and Cx43-null progenitors, it is likely that the reported upregulation of the uridine-sensitive P2Y4R in Cx43 knock-out astrocytes (Suadicani et al., 2003) occurs in later stages of development.
In the present study, we document for the first time that the change in P2 receptor subtypes that occurs after loss of Cx43 is associated with a decreased migration of cells from embryonic neurospheres. Because we observed that a specific P2Y1R antagonist (MRS-2179) decreased the OI of WT neural progenitors, and that expression of exogenous P2Y1R restored the OI of Cx43-null cells to levels similar to those of WT cells, it is likely that the difference in the development of WT and Cx43-null neural progenitors is related to the decreased expression levels of this type of receptor in Cx43-null cells. Reduced growth rate of astrocytes (Dermietzel et al., 2000), altered neural crest cell migration (Xu et al., 2001; Li et al., 2002), and delayed neuronal migration (Fushiki et al., 2003) have been reported previously to occur in Cx43-null mice. Although the underlying mechanism remains to be fully elucidated, it is of interest that activation of P2Y1R and P2X7R have been reported to stimulate mitogen-activated protein kinases, specifically the extracellular receptor protein kinases ERK1 and ERK2 (Panenka et al., 2001; Gendron et al., 2003; Neary et al., 2003), thus leading to Mueller cell proliferation (Milenkovic et al., 2003), gliosis (Neary et al., 2003), and production of the chemokine monocyte chemoattractant protein-1 (Panenka et al., 2001). Recently, it was also reported that ATP acting on P2YR might augment the proliferation of human neural stem cells through the phosphoinositide 3-kinase-dependent p70 S6 kinase pathway (Ryu et al., 2003).
Evidence favoring the participation of P2Y1 receptors in early stages of neural progenitor development is shown here through the use of the specific P2Y1 antagonist MRS-2179 (Baurand et al., 2001), which greatly reduced the OI of WT neural progenitors. Furthermore, because suramin in combination with MRS-2179 or the P2X7R antagonist KN62 (Baraldi et al., 2000) did not alter the OI of Cx43-null neural progenitors, it is likely that signal-transduction pathways associated with the activation of P2Y1R and not of P2X7R contribute to the migration of the neural progenitors investigated in this study. Although other mechanisms may be involved, it is possible that different patterns (frequencies) of intracellular calcium transients resultant from the action of ATP on these two P2Rs differentially regulate downstream events such as the phosphorylation of MAP kinases.
However, regarding cell differentiation, because blockade of P2Rs with either the broad-spectrum suramin or the specific MRS-2179 antagonists did not affect the nestin to GFAP expression ratio, it is possible that mechanisms other than the ones mediated by purinergic receptors are likely to be involved.
In addition to the proposed contribution of purinergic receptors, gap junctional intercellular communication has long been suggested to play a key role in the control of cell growth, allowing the diffusion between coupled cells of putative factors that are supposed to prevent the maintenance of a clonal (proliferating) state (Loewenstein and Kanno, 1966; for review, see Mesnil, 2002). However, recent studies indicate that connexins may have other roles besides providing the intercellular route for the diffusion of signaling molecules. For instance, forced expression of connexins provides resistance to apoptotic signals (Lin et al., 2003) and alters the expression pattern of several other genes (Naus et al., 2000), and deletion of Cx43 alters the expression levels of astrocytic genes related to several distinct cellular processes (Iacobas et al., 2003). Although the mechanism by which changes in Cx43 expression levels affect the expression of other proteins, including purinergic receptors, is yet unknown, it is possible that they are mediated by Cx43-interacting proteins. Some of these proteins, such as c-Src (Giepmans et al., 2001) and β-catenin (Ai et al., 2000), possess transcriptional activity. Thus, a number of biological functions that have been associated with Cx43-dependent signaling (Meda and Spray, 2000) may rather result from changes in expression of other proteins.
In summary, using an in vitro model of CNS development, we describe the properties of calcium signaling in neural progenitors and provide direct evidence for the participation of P2Y1 receptor-activated pathways in the early development of neural progenitors. The data also provide support for an intriguing interaction between the expression of connexin proteins, notably Cx43, and that of purinergic receptors.
Footnotes
This work was supported by National Institutes of Health Grants NS-41023 to E.S. and DK-63443-01 to P.M., the Association pour la Recherche Contre la Sclérose en Plaques to N.D., Swiss National Foundation Grant 3100-067788.02 to P.M., Juvenile Diabetes Research Foundation International Grant 1-2001-622 to P.M., and European Union Grant QLK3-CT-2002-01777 to P.M. We appreciate the discussions and suggestions provided by Drs. David C. Spray and Peter Mabie and are grateful to M. Beelitz for her technical assistance in maintaining the neurosphere cultures and to C. N. F. Fort and M. Urban-Maldonado for cloning the P2Y1 receptor.
Correspondence should be addressed to Dr. Eliana Scemes, Department of Neuroscience, Kennedy Center, Room 203, Albert Einstein College of Medicine, Bronx, NY 10461. E-mail: scemes@aecom.yu.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/2311444-09$15.00/0
References
- Abbracchio MP ( 1997) ATP and brain function. In: Purinergic approaches in experimental therapeutics (Jacobson KA, Jarvis MF, eds), pp 383-404. New York: Wiley-Liss.
- Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G ( 2003) Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci 24: 62-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado F, Espinosa-Parrilla JF, Carmona MA, Soriano E ( 2002) Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ J Neurosci 22: 9430-9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI ( 2000) Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest 105: 161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballerini P, Rathbone MP, Di Iorio P, Renzetti A, Giuliani P, D'Alimonte I, Trubiani O, Caciagli F, Ciccarelli R ( 1996) Rat astroglia P2Z (P2X7) receptors regulate intracellular calcium and purine release. NeuroReport 7: 2533-2537. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Romagnoli R, Tabrizi MA, Falzoni S, Di Virgilio F ( 2000) Synthesis of conformationally constrained analogues of KN62, a potent antagonist of the P2X7 receptor. Bioorg Med Chem Lett 10: 681-684. [DOI] [PubMed] [Google Scholar]
- Baurand A, Raboisson P, Leon C, Cazenave JB, Bourguingon JJ, Gachet C ( 2001) Inhibition of platelet function by administration of MRS-2179, a P2Y1 receptor antagonist. Eur J Pharmacol 412: 213-221. [DOI] [PubMed] [Google Scholar]
- Belluardo N, Mudo G, Trovato-Salinaro A, Le Gurun S, Charollais A, Serre-Beinier V, Amato G, Haefliger JA, Meda P, Condorelli DF ( 2000) Expression of connexin36 in the adult and developing rat brain. Brain Res 865: 121-138. [DOI] [PubMed] [Google Scholar]
- Braet K, Vandame W, Martin PE, Evans WH, Leybaert L ( 2003) Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap26. Cell Calcium 33: 37-48. [DOI] [PubMed] [Google Scholar]
- Ciccolini F, Collins TJ, Sudhoelter J, Lipp P, Berridge MJ, Bootman MD ( 2003) Local and global spontaneous calcium events regulate neurite outgrowth and onset of GABAergic phenotype during neural precursor differentiation. J Neurosci 23: 103-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Lopez-Garcia JC, Naus CC, Nedergaard M ( 2000) ATP-mediated glia signaling. J Neurosci 20: 2835-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, Bennett MV, Spray DC ( 2000) Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Brain Res Rev 32: 45-56. [DOI] [PubMed] [Google Scholar]
- Duval N, Gomes D, Calaora V, Calabrese A, Meda P, Bruzzone R ( 2002) Cell coupling and Cx43 expression in embryonic mouse neural progenitor cells. J Cell Sci 115: 3241-3251. [DOI] [PubMed] [Google Scholar]
- Gendron F-P, Neary JT, Theiss PM, Sun GY, Gonzalez FA, Weisman GA ( 2003) Mechanisms of P2X7 receptor-mediated ERK1/2 phosphorylation in human astrocytoma cells. Am J Physiol Cell Physiol 284: C571-C581. [DOI] [PubMed] [Google Scholar]
- Gerlier D, Thomasset N ( 1986) Use of MTT colorimetric assay to measure cell activation. J Immunol Methods 94: 57-63. [DOI] [PubMed] [Google Scholar]
- Giepmans BN, Hengeveld T, Postma FR, Moolenaar WH ( 2001) Interaction of c-Src with gap junction protein connexin-43. Role in the regulation of cell-cell communication. J Biol Chem 276: 8544-8549. [DOI] [PubMed] [Google Scholar]
- Fushiki S, Perez Velazquez JL, Zhang L, Bechberger JF, Carlen PL, Naus CC ( 2003) Changes in neuronal migration in neocortex of connexin43 null mutant mice. J Neuropathol Exp Neurol 62: 304-314. [DOI] [PubMed] [Google Scholar]
- Iacobas DA, Urban-Maldonado M, Iacobas S, Scemes E, Spray DC ( 2003) Array analysis of gene expression in connexin-43 null astrocytes. Physiol Genomics 15: 177-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques TS, Relvas JB, Nishimura S, Pytela R, Edwards GM, Streuli OH, ffrench-Constant C ( 1998) Neural precursor cell chain migration and division are regulated through different beta-1 integrins. Development 215: 3167-3177. [DOI] [PubMed] [Google Scholar]
- Jimenez AI, Castro E, Communi D, Boeynaems JM, Delicado EG, Miras-Portugal MT ( 2000) Coexpression of several types of metabotropic nucleotide receptors in a single cerebellar astrocytes. J Neurochem 75: 2071-2079. [DOI] [PubMed] [Google Scholar]
- Li WE, Waldo K, Linask KL, Chen T, Wessels A, Parmacek MS, Kirby ML, Lo CW ( 2002) An essential role for connexin43 gap junctions in mouse coronary artery development. Development 129: 2031-2042. [DOI] [PubMed] [Google Scholar]
- Lin JH, Yang J, Liu S, Takano T, Wang X, Gao Q, Willecke K, Nedergaard M ( 2003) Connexin mediates gap junction-independent resistance to cellular injury. J Neurosci 23: 430-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein WR, Kanno Y ( 1966) Intercellular communication and the control of tissue growth: lack of communication between cancer cells. Nature 209: 1248-1249. [DOI] [PubMed] [Google Scholar]
- Manning TJ, Sontheimer H ( 1997) Spontaneous intracellular calcium oscillation in cortical astrocytes from a patient with intractable childhood epilepsy (Rasmussen's encephalitis). Glia 21: 332-337. [PubMed] [Google Scholar]
- Meda P, Spray DC ( 2000) Gap junction function. In: Advances in molecular cell biology, Vol 30 (Hertzberg EL, ed), pp 263-322. Stamford, CT: JAI. [Google Scholar]
- Mesnil M ( 2002) Connexins and cancer. Biol Cell 94: 493-500. [DOI] [PubMed] [Google Scholar]
- Milenkovic I, Weick M, Wiedemann P, Reichenbach A, Bringmann A ( 2003) P2Y receptor-mediated stimulation of Muller glial cell DNA synthesis: dependence on EGF and PDGF receptor transactivation. Invest Ophthalmol Vis Sci 44: 1211-1220. [DOI] [PubMed] [Google Scholar]
- Mossmann T ( 1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55-63. [DOI] [PubMed] [Google Scholar]
- Naus CC, Bond SL, Bechberger JF, Rushlow W ( 2000) Identification of genes differentially expressed in C6 glioma cells transfected with connexin43. Brain Res Brain Res Rev 32: 259-266. [DOI] [PubMed] [Google Scholar]
- Neary JT, Zhu Q ( 1994) Signaling by ATP receptors in astrocytes. NeuroReport 5: 1617-1620. [DOI] [PubMed] [Google Scholar]
- Neary JT, Whittemore SR, Zhu Q, Norenberg MD ( 1994) Synergistic activation of DNA synthesis in astrocytes by fibroblast growth factor and extracellular ATP. J Neurochem 63: 490-494. [DOI] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Willoughby KA, Ellis EF ( 2003) Activation of extracellular signal regulated-kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci 23: 2348-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panenka W, Jijon H, Herx LM, Armstrong JN, Feighan D, Wei T, Yong VW, Ransohoff RM, MacVicar BA ( 2001) P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J Neurosci 21: 7135-7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Valentin VV, Lowenstein DH ( 2002) Prolonged seizures increase proliferating neuroblasts in the adult rat subventricular zone-olfactory bulb pathway. J Neurosci 22: 3174-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri HR, Crunelli V ( 2001) Pacemaker calcium in thalamic astrocytes in situ NeuroReport 12: 3897-3900. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V ( 2001) Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci 4: 803-812. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G ( 1998) Receptors for purines and pyrimidines. Pharmacol Rev 50: 413-492. [PubMed] [Google Scholar]
- Ryu JK, Choi HB, Hatori K, Heisel RL, Pelech SL, McLamon JG, Kim SU ( 2003) Adenosine triphosphate induces proliferation of human neural stem cells: role of calcium and p70 ribosomal protein SG kinase. J Neurosci Res 72: 352-362. [DOI] [PubMed] [Google Scholar]
- Scemes E ( 2000) Components of astrocytic intercellular calcium signaling. Mol Neurobiol 22: 167-179. [DOI] [PubMed] [Google Scholar]
- Scemes E, Suadicani SO, Spray DC ( 2000) Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J Neurosci 20: 1435-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Vinck MJ, Scemes E, Suadicani SO, Fishman GI, Dermietzel R ( 1998) Characteristics of coupling in cardiac myocytes and CNS astrocytes cultured from wild-type and Cx43-null mice. In: Gap junctions (Werner R, ed), pp 281-285. Amsterdam: IOS.
- Stout CE, Charles AC ( 2003) Modulation of intercellular calcium signaling in astrocytes by extracellular calcium and magnesium. Glia 43: 265-273. [DOI] [PubMed] [Google Scholar]
- Stout CE, Constantin JL, Naus CC, Charles AC ( 2002) Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277: 10482-10488. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, De Pina-Benabou MH, Urban-Maldonado M, Spray DC, Scemes E ( 2003) Acute down-regulation of Cx43 alters P2Y receptor expression levels in mouse spinal cord astrocytes. Glia 42: 160-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Goldberg J, Yuste R ( 2002) Calcium oscillations in neocortical astrocytes under epileptic conditions. J Neurobiol 50: 45-55. [DOI] [PubMed] [Google Scholar]
- Theis M, Mas C, Doring B, Kruger O, Herrera P, Meda P, Willecke K ( 2001a) General and conditional replacement of connexin43-coding DNA by a lacZ reporter gene for cell-autonomous analysis of expression. Cell Commun Adhes 8: 383-386. [DOI] [PubMed] [Google Scholar]
- Theis M, de Wit C, Schlaeger TM, Eckardt D, Kruger O, Doring B, Risau W, Deutsch U, Pohl U, Willecke K ( 2001b) Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis 29: 1-13. [DOI] [PubMed] [Google Scholar]
- Uckermann O, Grosche J, Reichenbach A, Bringmann A ( 2002) ATP-evoked calcium responses of radial glial (Muller) cells in the postnatal rabbit retina. J Neurosci Res 70: 209-218. [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, North RA, Surprenant A ( 1999) Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol (Lond) 519: 335-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Li WE, Huang GY, Meyer R, Chen T, Luo Y, Thomas MP, Radice GL, Lo CW ( 2001) Modulation of mouse neural crest cell motility by N-cadherin and connexin43 gap junctions. J Cell Biol 154: 217-230. [DOI] [PMC free article] [PubMed] [Google Scholar]