Abstract
Long-term potentiation (LTP) is a candidate synaptic mechanism underlying learning and memory that has been studied extensively at the cellular and molecular level in laboratory animals. To date, LTP has only been directly demonstrated in humans in isolated cortical tissue obtained from patients undergoing surgery, where it displays properties identical to those seen in non-human preparations. Inquiry into the functional significance of LTP has been hindered by the absence of a human model. Here we give the first demonstration that the rapid repetitive presentation of a visual checkerboard (a photic ‘tetanus’) leads to a persistent enhancement of one of the early components of the visual evoked potential in normal humans. The potentiated response is largest in the hemisphere contralateral to the tetanized visual hemifield and is limited to one component of the visual evoked response (the N1b). The selective potentiation of only the N1b component makes overall brain excitability changes unlikely and suggests that the effect is due instead to an LTP process. While LTP is known to exist in the human brain, the ability to elicit LTP from non-surgical patients will provide a human model system allowing the detailed examination of synaptic plasticity in normal subjects and may have future clinical applications in the assessment of cognitive disorders.
Keywords: ERP, LTP, synaptic plasticity, VEP, visual cortex
Abbreviations: EEG, electroencephalography; ICA, independent component analysis; LTP, long-term potentiation; VEP, visual evoked potential
Introduction
Long-term potentiation (LTP) refers to the process whereby the efficacy of communication between brain cells can be rapidly increased. LTP is the principal candidate synaptic mechanism underlying learning and memory formation (Bliss & Lomo, 1973; Bliss & Collingridge, 1993; Martin et al., 2000; Teyler, 2000) and has been studied extensively at the cellular and molecular level in laboratory animals (Teyler & DiScenna, 1987; Malenka & Nicoll, 1999; Abraham et al., 2002). To date, there have been no direct demonstrations of LTP of evoked potentials in the intact human brain, although LTP (and long-term depression) is widely thought to be the basis of declarative memory (memory for specific facts or episodes) in humans (Squire, 1986) and has been demonstrated in isolated slices of human cortical tissue, where it displays properties identical to those seen in non-human preparations (Chen et al., 1996). The blink reflex has also been modified in humans after electrical stimulation of afferent fibres in the trigeminal nerve and LTP (and long-term depression) in sensorimotor pathways is thought to underlie these effects (Mao & Evinger, 2001; Schorr & Ellrich, 2002). Although studied most often in hippocampal preparations, LTP has also been regularly demonstrated in slices of neocortex (Kirkwood & Bear, 1994) and, albeit less regularly, in the neocortex of developing (Tsumoto & Suda, 1979; Komatsu et al., 1988) and adult (Heynen & Bear, 2001) animals.
In this study, we explored the possibility that, in contrast to LTP in deep brain structures such as the hippocampus, LTP of synapses in the neocortex may be observable as changes in amplitude of event-related potentials recorded from the scalp of adult humans. Further, we investigated whether it was possible to induce LTP non-invasively by rapid presentation of visual stimuli. LTP of potentials evoked by external sensory stimuli has been demonstrated in the visual cortex of rats (Heynen & Bear, 2001). In that study, the potentiating stimulus was invasive electrical stimulation of visual pathways but this resulted in LTP of visual potentials evoked by natural visual stimuli. Repetitive visual sensory stimulation itself has been shown to result in LTP in the visual system of the developing tadpole (Zhang et al., 2000).
Materials and methods
Subjects
Six right-handed males (aged 23–38 years) participated in the present study. All subjects had normal or corrected to normal vision. Informed consent was obtained from each participant. All procedures were approved by the University of Auckland Human Subjects Ethics Committee.
Apparatus
Electroencephalography (EEG) recordings were carried out in an electrically shielded room using 128-channel Ag/AgCl electrode nets (Electrical Geodesics Inc., Eugene, OR, USA) (Tucker, 1993). EEG was recorded continuously (250 Hz sampling rate; 0.1–100 Hz analogue bandpass) with amplifiers (200 MΩ input impedance; Electrical Geodesics Inc.). Electrode impedances were kept below 30 kΩ, an acceptable level for this system (Ferree et al., 2001). A common vertex (Cz) reference was used in the EEG acquisition.
Stimuli
Visual stimuli consisted of checkerboards presented to the right or left visual field (see Fig. 1C). The stimuli subtended 4° of visual angle from the vertical and horizontal midline (check size subtended 0.3° of visual angle). The stimuli were presented on a computer monitor (15-inch flat-screen SVGA monitor with a resolution of 640 × 480 pixels) 57 cm from the subjects and checkerboard luminance was under experimental control. Checkerboards were generated by custom software on a Pentium II/200 computer. TTL pulses generated via the parallel port of the display computer provided synchronization of stimulus events with EEG acquisition.
Fig. 1.
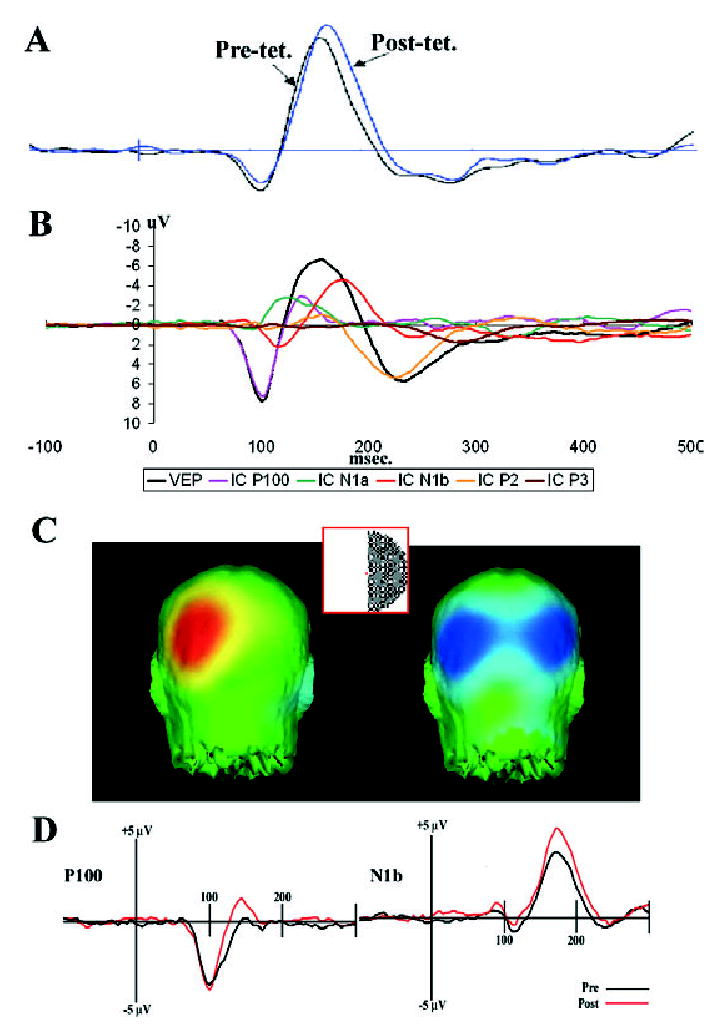
(A) Pre- and post-tetanus average evoked potentials recorded over the occipital cortex contralateral to the visual stimulus. (B) An independent components analysis identified five components of the visual evoked response (VEP) to checkerboard stimuli. (C) Checkerboard stimuli (subtending 4° visual angle with three checks per degree) to the left or right visual hemifield elicited a contralateral P100 response (C, left panel) and a bilateral N1b response (C, right panel) in occipital cortex. (D) Repetitive presentation of the checkerboard (at 9 Hz) led to a significant change in only the N1b component (D, right panel). The amplitude of the P100 (D, left panel), for example, did not change significantly. See Fig. 2A.
Procedure
Subjects were required to fixate on a red circular dot in the centre of the screen during data collection. During the baseline period, the checkerboards were presented at an average rate of approximately 1 Hz (stimulus-onset-asynchrony (SOA) range 950–1120 ms, duration 33 ms). A test frequency of 1 Hz was believed to be low enough not to have a physiological effect and not so long as to unnecessarily prolong the test blocks (see Discussion). The order of stimulus presentation was random, with the constraint that 50% of the checkerboards were presented to the left visual field with the other half presented to the right visual field. Baseline testing consisted of 420 presentations within a block (7 min). In the photic tetanus condition, subjects were again required to maintain fixation while the same checkerboard stimulus was presented at a frequency of 9 Hz to either the left or right visual field for 120 s (1000 presentations). A tetanic-stimulation frequency of 9 Hz was chosen as it is a relatively high flicker rate but below perceptual fusion rate. Subsequently, subjects were given a 2-min period with their eyes closed to allow any visual after-effects to disappear. After this block of tetanic stimulation, visual evoked potentials (VEPs) were again collected at the baseline presentation rate at intervals of 15 min. Thus, test blocks were collected at 2–9, 15–21, 30–37 and 45–52 min after the end of the tetanic stimulation.
Three separate testing sessions per subject were conducted, with a minimum of 3 days between sessions. In each session subjects were exposed to one of three conditions. In two conditions the subjects were presented with the photic tetanus to either the right or left visual field. In the third condition the photic tetanus was omitted and subjects fixated on the red dot for 2 min. Experimental condition (left/right/control) was counterbalanced across subjects.
Analysis
After completion of data collection, EEG files were segmented with respect to event markers into 600-ms epochs (including a 100-ms pre-stimulus baseline). Ocular artefacts were removed from individual trial epochs using procedures from Gratton (1998). Any trials that had horizontal or vertical eye movement (fixation errors) were rejected and were not included in the averaging process.
The VEPs from individual subjects were combined to produce grand-averaged VEPs for each condition. These VEPs were then re-referenced to an average reference for all analysis. The VEPs were subjected to independent component analysis (ICA; Makeig et al., 1999) to help to identify the components underlying the evoked potential complex. ICAs were used to help to identify the time-points of interest, while the amplitude calculations were performed on the raw VEP.
The four electrodes at the location of the peak amplitude of the ICA components were then selected for further analysis. Mean amplitudes were calculated from the individual grand average VEPs at these electrode locations. As many of the components of the VEP are lateralized, based upon the field of simulation, this results in an average of eight electrodes. For example, the P100 is left lateralized with right field stimulation and right lateralized for left field stimulation.
Data were statistically analysed for responses both ipsi- and contralateral to the tetanized visual field and for the control no-tetanus condition. Data were analysed as within-session, post-tetanus minus pre-tetanus difference scores to minimize between-session variables due to slightly different electrode placement and impedance measures. The mean post- minus pre-voltage difference for each component of the ICA was analysed across the four post-tetanus blocks in separate two-way anovas. Linear trend analysis was conducted over blocks. Planned contrasts were used to compare the tetanus with the non-tetanus conditions for ipsi- and contralateral hemispheres.
Source localizations were performed using low-resolution electromagnetic tomography (Pascual-Marqui et al., 1994). Source estimations were performed, using all 129 electrodes, on the time-point at which the ICA was maximal for a particular component. The low-resolution electromagnetic tomography solutions were obtained using a minimally regularized version that guarantees stable solutions.
Results
Post-tetanus, there was an apparent increase in the amplitude of the average VEP recorded over the occipital cortex (Fig. 1A). The ICA identified five components of the VEP (P100, N1a, N1b, P2 and P3; Fig. 1B), only one of which, the N1b negativity peaking at 176 ms post-stimulus, was found to be increased in amplitude after the photic tetanus (Figs 1D and 2A).
Fig. 2.
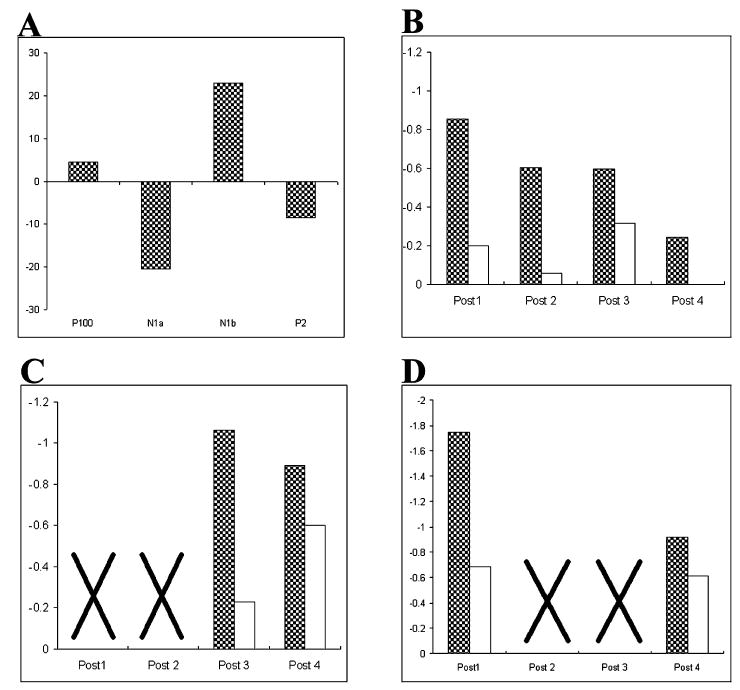
(A) Repetitive presentation of the checkerboard led to a significant amplitude change in only the N1b component. The amplitude of the P100, N1a, P2 and P3 (not shown) components did not change significantly. Data in percentage change from pre-tetanus. (B) The N1b response was potentiated (128%, P < 0.03) during the first post-tetanus average but declined subsequently (B–D, filled bars, responses in contralateral hemisphere after tetanic stimulation; unfilled bars, responses when no tetanic stimulation was delivered). (C) Withholding the initial post-tetanus baseline stimulation resulted in a non-decremented long-term potentiation (142% at 60 min, P < 0.05), suggesting that the baseline stimulation depotentiated the response in B. (D) Withholding post-tetanus blocks 2 and 3 again resulted in significant potentiation in block 4 (relative to baseline; F1,10 = 6.58; P < 0.05). However, it was also significantly reduced in amplitude relative to the post-1 block; F1,10 = 5.4; P < 0.05).
The analysis of the P100, N1a, P2 and P3 produced no reliable changes (P100) or tended to reduce their amplitude over the course of the experimental session independent of condition (N1a, P2 and P3).
After the photic tetanus, the N1b component in the hemisphere contralateral to the tetanus was significantly potentiated (128%, t5 = 2.95; P < 0.03). The N1b in the hemisphere ipsilateral to the tetanus also increased in amplitude but this change did not reach significance. The potentiation of the N1b was significant in the first 7-min block but declined in subsequent blocks, as indicated by a significant linear trend across blocks (F1,15 = 7.11, P < 0.05; Fig. 2B).
Two follow-up studies were conducted to provide both a replication of the increase in the N1b response and to determine whether the return towards baseline over blocks was due to depotentiation induced by repeated post-tetanus baseline stimulation. Different blocks of baseline stimulation were omitted in experiments 2 and 3 to ensure that any effect seen was not due to temporal factors. In experiment 2, baseline stimulation during post-tetanus blocks 1 and 2 was omitted. In this experiment the N1b at electrodes contralateral to the field of stimulation resulted in an increased response relative to the pre-tetanus at blocks 3 and 4 (F1,10 = 4.79, P < 0.05; see Fig. 2C). This supports the hypothesis that the decline seen in experiment 1 was due to the depotentiating effects of baseline stimulation. The increase in the N1b potential in the hemisphere ipsilateral to the tetanus also reached significance (F1,10 = 7.01, P < 0.05), a trend which is apparent in the initial experiment but was not found to be statistically reliable. No reliable change was found during the no-tetanus condition (F < 1.0).
In experiment 3, baseline stimulation during post-tetanus blocks 2 and 3 was omitted. A significant (F1,10 = 18.52, P < 0.05) increase in N1b amplitude was found in post-tetanus blocks 1 and 4 relative to the baseline, with no significant change in the no-tetanus condition (F < 1.0). However, although the N1b amplitude in the post-4 block was still of significantly larger amplitude than the baseline (F1,10 = 6.58; P < 0.05), it was also significantly reduced in amplitude relative to the post-1 block (F1,10 = 5.4; P < 0.05). These data suggest that the repetitive baseline stimulation at low rates, used to measure the VEP amplitudes, is in itself enough to depotentiate the response.
As in experiment 2, the N1b in the hemisphere ipsilateral to the tetanus was also significantly potentiated (F1,10 = 6.17; P < 0.05. Overall, these data suggest that, although seen most reliably in the contralateral hemisphere, the lateralized photic tetanus can potentiate the N1b across both hemispheres.
Finally, it was thought that the significant increase in N1b amplitude post-tetanus might be due to changes in the distribution of the generating sources relative to the pre-tetanus condition. Thus, low-resolution electromagnetic tomography source estimation for the P100, N1a and N1b components of the ERP (identified by ICA) were performed for the pre- and post-tetanus conditions. The estimated sources for left visual field stimulation, pre- and post-tetanus (left visual field tetanus), and right visual field stimulation, pre- and post-tetanus (right visual field tetanus), are shown for the pre-tetanus and first post-tetanus blocks of experiment 1 (see Fig. 3). It can be seen that the P100 and N1a components both have a source on the midline, in the region of the striate visual cortex (approx BA17), and additional contralateral extrastriate sources. The N1b component again has sources in the midline striate region, with additional sources in both the ipsi- and contralateral extrastriate regions. Thus, the distribution of sources underlying the P100, N1a and N1b did not appear to change significantly between the pre- and post-tetanic test blocks.
Fig. 3.
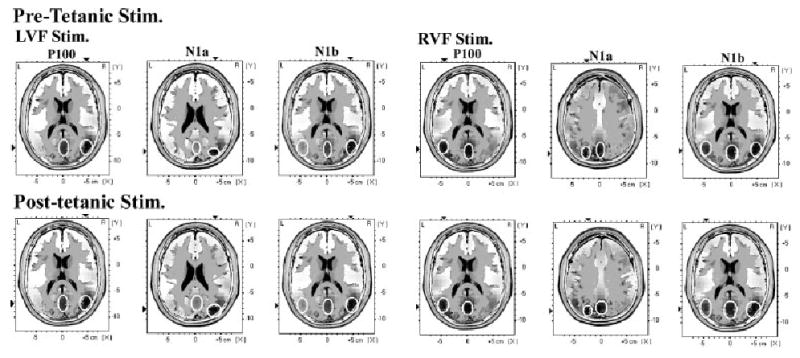
Results of low-resolution electromagnetic tomography source estimation for the P100, N1a and N1b components of the ERP (identified by independent component analysis) performed before and after the application of high-frequency stimulation (pre- and post-tetanic stimulation). Sources obtained with left and right visual field tetanic and test stimulation are shown (LVF Stim. and RVF Stim.). The regions of maximal current density are outlined with white circles. It can be seen that the P100 and N1a components both have sources on the midline, in the region of striate visual cortex (approx. BA17), and additional contralateral extrastriate sources. The N1b component again has sources in the midline striate region, with additional sources in ipsi- and contralateral extrastriate regions. The distribution of sources does not appear to change significantly after the tetanic stimulation.
Discussion
We report here the first demonstration that a component of a visually evoked cortical response, recorded non-invasively from humans, can be potentiated after exposure to a repetitively presented visual stimulus. After the photic tetanus, the N1b component in the contralateral and also the ipsilateral hemisphere was significantly potentiated. The bilateral potentiation of the N1b response is consistent with the bilaterality of this component (Jeffreys & Axford, 1972a,b) and the responses which we observed to baseline stimulation (Fig. 1C). There was no change when the photic tetanus was not delivered.
As noted above, it has been shown previously that VEPs can be potentiated by induction of LTP (Heynen & Bear, 2001). In that study, however, invasive electrical stimulation was used to induce LTP. In the current study, rapid, repetitive visual sensory stimulation itself was demonstrated to result in an LTP-like response of the VEP.
Whether the LTP-like phenomenon which we have demonstrated here in human VEPs is the same phenomenon that has been extensively studied in cellular preparations remains to be determined. Studies investigating the parameters that induce (and those that do not induce) LTP are required. Further, pharmacological investigations to determine whether the potentiation described here is N-methyl-d-aspartate- or calcium channel-dependant are also necessary. However, LTP has been demonstrated in the visual cortex of developing (Tsumoto & Suda, 1979; Komatsu et al., 1988) and adult (Heynen & Bear, 2001) animals. In addition, using a similar sensory stimulation paradigm, Zhang et al. 2000 induced LTP in the visual system of the developing tadpole that was shown to be N-methyl-d-aspartate dependent. It is suggested therefore that the most parsimonious explanation of the potentiation of the VEP demonstrated here must invoke mechanisms very similar to those known to underlie LTP as studied in experimental animals.
It is possible that the potentiation of the VEP observed here is due to differing levels of overall cortical excitability (due perhaps to differing levels of attention) that was not controlled for. However, the selective potentiation of only the N1b component of the VEP suggests that this is unlikely. In addition, the systematic changes in the N1b amplitude demonstrated across all three experiments are difficult to attribute to variations in levels of attention.
The selective potentiation of the N1b component also suggests that the potentiated synapses are due to activity in the neocortex rather than in the ascending visual system. The generators of the N1b component have been suggested to be outside the primary visual cortex and thus not in direct receipt of ascending input (Jeffreys & Axford, 1972a,b; Di Russo et al., 2001). Certainly, the results of the low resolution source estimation performed here (low-resolution electromagnetic tomography; see Fig. 3) implicate bilateral extrastriate areas (as well as striate cortex) in the generation of the N1b. The maximal voxel in sources in both striate and bilateral extrastriate regions showed increases in activation of the order of 5% after tetanic stimulation. However, it must be emphasized that the source analysis employed here was qualitative. Further work with source localization (preferably with confirmatory fMRI studies) is necessary before a conclusion can be reached regarding the location of the potentiated synapses in this paradigm. Nevertheless, it is perhaps most likely that potentiation of the N1b is due to recurrent activity or reverberation within or across the hemispheres of the neocortex (Hebb, 1949). Consistent with this view, the N1b component has a bilateral distribution (see Fig. 1C and Jeffreys & Axford, 1972a,b), its sources are bilateral (see Fig. 3) and its potentiation was also observed bilaterally, even though the inducing stimulus was unilaterally presented. In addition, it is possible that some interaction between the tetanizing stimulus, which is in the alpha frequency range, and ongoing alpha may contribute to the potentiation of the N1b. Phase resetting of ongoing alpha has been proposed to contribute to the VEP (Basar, 1980; Makeig et al., 2002).
The N1b component of the VEP was significantly potentiated in the initial post-tetanus block (1–15 min, see Fig. 2B) but the degree of potentiation decreased thereafter. To investigate the possibility that the post-tetanus baseline stimulation depotentiated the response, we replicated the experiment but withheld baseline stimulation for the first two blocks in experiment 2 (Fig. 2C) and for the middle two blocks in experiment 3 (Fig. 2D). In experiments 2 and 3, significant potentiation was again observed at the first post-tetanus test, with subsequent test sessions resulting in decreased amplitudes relative to the first test. Thus, removing either the first two or the middle two post-tetanus blocks suggested that an active depotentiation, rather than a simple waning of the effect, was the cause of the decrease in the potentiated response. Overall, these results suggest that the potentiated N1b response can be depotentiated by a low-frequency stimulation, analogous to that seen in animal studies. Although only tested to 1 h after the tetanus, it seems likely that the potentiation of the VEP amplitude would last considerably longer if testing was withheld.
It should be noted that, in contrast to LTP in the hippocampus (which has a well-established role in mnemonic processes), LTP in the visual cortex is unlikely to subserve memory formation per se. However, visual system LTP has been inferred as the basis for increases in contrast sensitivity after practice in adult humans (Adini et al., 2002). Changes in the topography of VEPs have also been reported after perceptual learning (Fahl & Skrandies, 1994; Skrandies, 1995) and it is possible that a potentiation of components of the VEP as reported here may contribute to these changes. Similar neuroplastic changes are also believed to underlie long-lasting visual after-effects (Favreau & Corballis, 1976; Humphrey et al., 1999). In any case, it is likely that the process of LTP induction is mechanistically very similar across most brain regions, including the hippocampus and neocortex (see, e.g. Heynen & Bear, 2001).
In summary, our results suggest that it is possible to non-invasively induce and measure LTP in humans. Further work is required to confirm that the potentiation of the N1b component of the VEP observed here is indeed due to mechanisms akin to those underlying LTP induction in experimental animals. If this is so, then the ability to assess cortical plasticity non-invasively in humans will allow, for the first time, a detailed examination of LTP and its behavioural consequences in a variety of human sensory and cognitive processes. This approach may also have future clinical applications in the diagnosis and assessment of a variety of cognitive disorders, including Alzheimer’s disease.
Acknowledgments
This work was supported in part by NIH grant R01 MH064508 and by grant 0311-SPG from the NZ Neurological Foundation.
References
- Abraham WC, Logan B, Greenwood JM, Dragunow M. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J Neurosci. 2002;22:9626–9634. doi: 10.1523/JNEUROSCI.22-21-09626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adini Y, Sagi D, Tsodyks M. Context-enabled learning in the human visual system. Nature. 2002;415:790–793. doi: 10.1038/415790a. [DOI] [PubMed] [Google Scholar]
- Basar, E. (1980) EEG Brain Dynamics: Relation between EEG and Brain Evoked Potentials Elsevier, New York.
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WR, Lee S, Kato K, Spenser DD, Shepherd GM, Williamson A. Long-term modifications of synaptic efficacy in the human inferior and middle temporal cortex. PNAS. 1996;23:8011–8015. doi: 10.1073/pnas.93.15.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Hum Brain Mapp. 2001;15:95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahl M, Skrandies W. An electrophysiological correlate of learning in motion perception. Germ J Ophthalmol. 1994;3:427–432. [PubMed] [Google Scholar]
- Favreau OE, Corballis MC. Negative aftereffects in visual perception. Sci Am. 1976;12:42–48. doi: 10.1038/scientificamerican1276-42. [DOI] [PubMed] [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Electroenceph Clin Neurophysiol. 2001;112:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Gratton G. Dealing with artifacts: The EOG contamination of the event-related brain potential. Behav Res Meth, Instr Comp. 1998;30:44–53. [Google Scholar]
- Hebb, D.O. (1949) The organization of behaviour: A neuropsychological theory Wiley, New York.
- Heynen AJ, Bear MF. Long-term potentiation of thalamo-cortical transmission in the adult visual cortex in vivo. J Neurosci. 2001;21:9801–9813. doi: 10.1523/JNEUROSCI.21-24-09801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey GK, James TW, Gati JS, Menon RS, Goodale MA. Perception of the McCollough effect correlates with activity in extrastriate cortex. Psychol Sci. 1999;10:444–448. [Google Scholar]
- Jeffreys DA, Axford JG. Source locations of pattern-specific components of human visual evoked potentials. I Component of striate cortical origin. Exp Brain Res. 1972a;16:1–21. doi: 10.1007/BF00233371. [DOI] [PubMed] [Google Scholar]
- Jeffreys DA, Axford JG. Source locations of pattern-specific components of human visual evoked potentials. II Component of extrastriate cortical origin. Exp Brain Res. 1972b;16:22–40. doi: 10.1007/BF00233372. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, Fujii K, Maeda J, Sakaguchi H, Toyama K. Long-term potentiation of synaptic transmission in kitten visual cortex. J Neurophysiol. 1988;59:124–141. doi: 10.1152/jn.1988.59.1.124. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Covington J, Townsend J, Sejnowski TJ, Courchesne E. Functionally independent components of the late positive event-related potential during visual spatial attention. J Neurosci. 1999;19:2665–2680. doi: 10.1523/JNEUROSCI.19-07-02665.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung T-P, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation — a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Mao JB, Evinger C. Long-term potentiation of the human blink reflex. J. Neurosci. 2001;21:RC151. doi: 10.1523/JNEUROSCI.21-12-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RGM. Synaptic plasticity and memory: an evaluation of the hypothesis. Ann Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Schorr A, Ellrich J. Long-term depression of the human blink reflex. Exp Brain Res. 2002;147:549–553. doi: 10.1007/s00221-002-1248-9. [DOI] [PubMed] [Google Scholar]
- Skrandies W. Visual information processing: topography of brain electrical activity. Biol Psych. 1995;40:1–15. doi: 10.1016/0301-0511(95)05111-2. [DOI] [PubMed] [Google Scholar]
- Squire LR. Mechanisms of memory. Science. 1986;232:1612–1619. doi: 10.1126/science.3086978. [DOI] [PubMed] [Google Scholar]
- Teyler, T.J. (2000) Long-term potentiation and memory. In Adelman, G. & Smith, B. (Eds), Encyclopedia of Neuroscience Elsevier, New York.
- Teyler TJ, DiScenna P. Long-term potentiation. Annu Rev Neurosci. 1987;10:131–161. doi: 10.1146/annurev.ne.10.030187.001023. [DOI] [PubMed] [Google Scholar]
- Tsumoto T, Suda K. Postnatal development of corticotectal neurons in the kitten striate cortex: an electrophysiological study. Brain Res. 1979;168:190–194. doi: 10.1016/0165-3806(83)90199-2. [DOI] [PubMed] [Google Scholar]
- Tucker DM. Spatial sampling of head electrical fields: the geodesic sensor net. Electroenceph Clin Neurophysiol. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Poo M. Visual input induces long-term potentiation of developing retinotectal synapses. Nat Neurosci. 2000;3:708–715. doi: 10.1038/76665. [DOI] [PubMed] [Google Scholar]


