Abstract
Thyroid hormone (T3) controls critical aspects of cerebellar development, such as migration of postmitotic granule cells and terminal differentiation of Purkinje cells. T3 acts through nuclear receptors (TR) of two types, TRα1 and TRβ, that either repress or activate gene expression. We have analyzed the cerebellar structure of developing mice lacking the TRα1 isoform, which normally accounts for about 80% of T3 receptors in the cerebellum. Contrary to what was expected, granule cell migration and Purkinje cell differentiation were normal in the mutant mice. Even more striking was the fact that when neonatal hypothyroidism was induced, no alterations in cerebellar structure were observed in the mutant mice, whereas the wild-type mice showed delayed granule cell migration and arrested Purkinje cell growth. The results support the idea that repression by the TRα1 aporeceptor, and not the lack of thyroid hormone, is responsible for the hypothyroid phenotype. This conclusion was supported by experiments with the TRβ-selective compound GC-1. Treatment of hypothyroid animals with T3, which binds to TRα1 and TRβ, prevents any defect in cerebellar structure. In contrast, treatment with GC-1, which binds to TRβ but not TRα1, partially corrects Purkinje cell differentiation but has no effect on granule cell migration. Our data indicate that thyroid hormone has a permissive effect on cerebellar granule cell migration through derepression by the TRα1 isoform.
Keywords: cerebellar granule cells‖Purkinje cells‖cretinism‖development‖GC-1
The physiological actions of thyroid hormone (3,5,3′-triiodo-l-thyronine, T3), including the control of central nervous system (CNS) development, are mediated through interaction with nuclear receptors, which are ligand-modulated transcription factors (1). There are several T3 receptor proteins (TR), which are encoded by two distinct genes, TRα and TRβ. The TRα gene encodes three proteins, TRα1, TRvα2, and TRvα3 that differ in their carboxyl terminus. From these, only TRα1 is a bona fide receptor because it binds T3 and activates or represses target genes, whereas the two variants, TRvα2 and TRvα3, do not bind T3 and may antagonize T3 action (2–4). In addition to these protein isoforms, there are also truncated protein products of the α gene known as Δα1 and Δα2, which may have a role in intestinal development (5). Several amino terminal protein variants are produced from the TRβ gene: the two classical receptors, TRβ1 and TRβ2, and two newly identified, T3-binding proteins, TRβ3 and the truncated protein ΔTRβ3 (6).
Although the role of thyroid hormone in health and disease is well known, the physiological roles and specific functions of the T3 receptor isoforms still remain largely unidentified. Mutant mice have been generated that lack the expression of single or multiple products of the TR genes (7–9), and some discrete specific functions could be assigned to individual receptor isoforms. As a consequence of these studies, it is known that TRβ is involved in the regulation of thyroid stimulating hormone (TSH) secretion, liver metabolism, and hearing (10, 11), whereas TRα1 controls myocardial activity (12, 13) and intestinal development (14). Mice devoid of all known forms of T3 receptors display highly increased TSH, thyroid hormone secretion, growth retardation affecting long bones, and intolerance to cold (15, 16). Otherwise, they are viable and display no obvious signs of CNS defects, except for subtle alterations in expression of some neuropeptides (17). Thus, only in some respects, lack of expression of T3 receptors mimics thyroid hormone deficiency.
A strong discrepancy exists between the phenotypes observed when hypothyroidism is induced during development and those observed in T3 receptor knock-out mice, which is most evident in the CNS. Severe neonatal hypothyroidism is associated with structural and functional alterations, which are most evident in the cerebellum (18). In this organ, thyroid hormone controls the differentiation of Purkinje cells and the migration of granular cells. In the absence of thyroid hormone, Purkinje cells do not develop the characteristic highly elaborated dendritic tree, which remains severely hypoplastic. Also, migration of granular cells from the external germinal layer to the internal granular layer is strongly retarded.
In the present work, we have analyzed the role of TRα1 in granular cell migration and Purkinje cell differentiation. To this end, we have used a combined genetic and pharmacological approach. First, we analyzed euthyroid and hypothyroid mice deficient for TRα1. Our results show that in the absence of TRα1, hypothyroidism is not associated with deficient Purkinje cell differentiation or retarded migration of granule cells as occurs in wild-type mice. Second, we compared the effect of GC-1, a TRβ-selective T3 analog, with that of thyroid hormone, after administration to hypothyroid animals. In contrast to T3, GC-1 had no effect on granule cell migration but it partially enhanced Purkinje cell maturation. Taken together, the data suggest, therefore, that granule cell migration is under TRα1 control, and that the effect of hypothyroidism is likely caused by the repressor activity of the unliganded receptor.
Materials and Methods
Animals and Treatments.
Adult male BALB/c wild-type and TRα1-deficient mice (TRα1−/−) were used. TRα1−/− mice were generated as described (13). The wild-type mice were generated from crosses of the heterozygotes to have the same genetic background as mutant mice. Rats from the Wistar strain were produced in our animal facilities. Animal care procedures were conducted in accordance with the guidelines set by the European Community Council Directives (86/609/EEC). Animals were under temperature- (22 ± 2°C) and light-controlled (12 h light/12 h dark cycle; lights on at 7 a.m.) conditions and had free access to food and water in the colony room. Hypothyroidism was induced by administering 0.02% 2-mercapto-1-methylimidazole (Sigma) and 1% sodium perchlorate in the drinking water to lactating dams from delivery and throughout the experiment. Hormonal treatments were started on postnatal day 2 and consisted of daily single i.p. injections of 15 ng of T3 per g of body weight (Sigma) or 10 ng/g of the TRβ-selective compound, GC-1 (19). These doses are equivalent to about 5× the physiological replacement dose of T3. Similar schedules of GC-1 treatment are effective in end-effects of thyroid hormone mediated through TRβ (20, 21). In our hands, this treatment schedule was able to suppress plasma TSH and normalize pituitary growth hormone (GH) in hypothyroid rats (not shown). T4 also was administered in some experiments at a physiological replacement dose of 20 ng/g of body weight, and the results were identical to those obtained with T3. The animals were killed as indicated for each experiment. Thyroid hormone concentrations in cerebral cortices were determined as described (22) and were less than 90% lower in hypothyroid animals than in control animals (Table 1).
Table 1.
Thyroid hormone concentrations in cerebral cortex
| Thyroid hormone | TRα1+/+ | TRα1−/− | TRα1+/+ hypothyroid | TRα1−/− hypothyroid | Control rats | Hypothyroid rats |
|---|---|---|---|---|---|---|
| T4 | 4.10 ± 0.85 | 4.37 ± 0.96 | 0.33 ± 0.05* | 0.31 ± 0.06* | 1.96 ± 0.63 | 0.20 ± 0.06* |
| T3 | 3.73 ± 1.03 | 4.86 ± 0.18 | 0.28 ± 0.08* | 0.25 ± 0.10* | 1.69 ± 0.23 | 0.10 ± 0.02* |
T4 and T3 concentrations (ng/g wet weight) were measured in extracts of cerebral cortices of P21 mice and P25 rats. All measurements were from five animals per group (means ± SD).
, P < 0.01. Statistical comparisons were made between the euthyroid and hypothyroid animals for each type of animal, using the Student's t-test.
RNA Extraction and Northern Analysis.
Total RNA was prepared from pooled cerebella of five animals with the Trizol procedure (GIBCO/BRL). Northern blotting was performed following standard methods (23) by using 20 μg of total RNA. The filter was hybridized with a 440-bp EcoR1-PstI fragment from the cDNA encoding the Purkinje cell-specific protein PCP-2 (a gift from Cary N. Mariash, Univ. of Minnesota, Minneapolis) and with a cDNA encoding the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The radioactive probes were prepared by using the Ready-To-Go DNA Labeling Beads (-dCTP; Amersham Pharmacia).
Histological Methods.
Animals were perfused transcardially with buffered 4% (wt/vol) paraformaldehyde, the brains were cryoprotected, and 25-μm sagittal sections were obtained in a cryostat and processed as described (24). Cerebellar structure was examined by staining the sections with toluidine blue. Immunohistochemistry was performed on free-floating sections, which were incubated overnight with a monoclonal anti-calbindin D-28K antibody at a 1:400 dilution (Santa Cruz Biotechnology) at 4°C. The sections then were incubated with a biotinylated horse anti-mouse secondary antibody at a 1:200 dilution (Vector Laboratories) followed by avidin-biotin complex (Vector Laboratories). Peroxidase was then visualized with diaminobenzidine (0.05%) and H2O2.
Results
Cerebellar Structure in Mice Deficient of the TRα1 Isoform.
Among the most classical and dramatic effects of thyroid hormone on brain development are those exerted on cerebellar granule cell migration and Purkinje cell differentiation. Cerebellar granule cells originate from the external germinal layer, a secondary neurogenesis center formed from precursors originating in the rhombic lip (25). After proliferation, the cells migrate through the molecular layer, along the fibers of the Bergmann glia, to form the internal granular layer. Neonatal hypothyroidism is known to delay strongly granule cell migration, which is evidenced by the persistence of the external germinal layer. Terminal differentiation of Purkinje cells is another classical end point of T3 action in the cerebellum. Absence of thyroid hormone during the critical neonatal period is associated with severely arrested growth of the apical dendrite and other structural abnormalities (18).
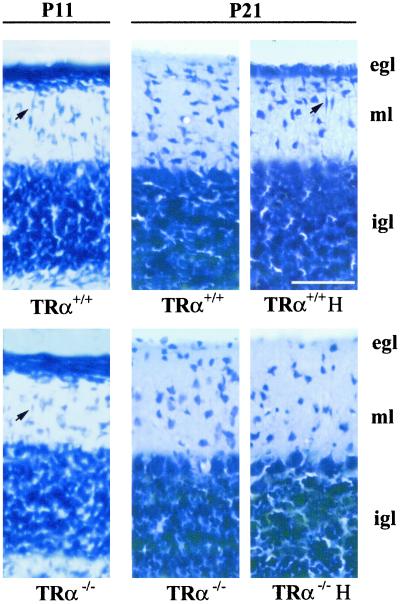
Given the essential role played by thyroid hormone in cerebellar development, we analyzed the cerebellar structure of TRα1−/− mice. The presence or absence of the external germinal layer was examined by toluidine blue staining, and the Purkinje cells were examined by calbindin immunohistochemistry. By these criteria, the structure of the cerebella of intact TRα1−/− mice was apparently normal. Therefore, we decided to study the effect of hypothyroidism in these mice. Groups of wild-type and TRα1−/− mice were made hypothyroid from birth, and the structure of the cerebellum was analyzed. Nissl-stained slices from the different groups are shown in Fig. 1. At P11, the cerebella of TRα1−/− and wild-type mice were similar, with an external germinal layer (egl), a molecular layer (ml), and an internal granular layer (igl). Spindle-shaped migrating cells were identified in these preparations (Fig. 1, black arrows at Upper Left and Lower Left). At P21 migration of cells from the egl to the igl was completed in both kinds of animals. When hypothyroidism was induced in the wild-type mice, granule cell migration was impaired, as assessed by the persistence of the external germinal layer. As a further sign of immaturity, migrating cells were still present. Significantly, and contrary to what could be expected, migration of granule cells had taken place normally in hypothyroid, TRα1−/− mice.
Figure 1.
Cerebellar structure in mice. Toluidine blue-stained sagittal sections of P11 and P21 mice cerebella. The figure shows sections through lobule VII of intact wild-type mice (TRα+/+), hypothyroid wild-type mice (TRα+/+ H), intact TRα1-deficient mice (TRα−/−), and hypothyroid TRα1-deficient mice (TRα−/−H). The arrows point to the presence of spindle-shaped, migrating cells. egl, external germinal layer; ml, molecular layer; and igl, internal granule cell layer. (Bar = 50 μm.)
The morphological appearance of Purkinje cells also was examined. Preparations from lobules V, VII, and X of each group of animals are displayed in Fig. 2. As was the case for granule cells, there were no apparent differences in the morphological appearance of Purkinje cells between the normal wild-type and the intact TRα1−/− mice. Hypothyroidism in the wild-type mice was associated with known classical alterations which included a stunted growth of the Purkinje cell dendrites (Fig. 2, lobule VII), a lack of alignment of the Purkinje cell bodies, indicative of a migration defect (lobules V and X), persistence of the perisomatic processes that establish transient connections with the climbing fibers (black arrows in Fig. 2), and elongated primary dendrites (lobule X). Again, there were no obvious morphological signs of arrested differentiation of the Purkinje cells in hypothyroid TRα1−/− mice.
Figure 2.
Morphology of the Purkinje cells in mice. Purkinje cells were immunostained for calbindin in sagittal sections of lobules V, VII, and X of P11 mice cerebella. Symbols are the same as in Fig. 1. The arrows show the presence of perisomatic terminals in Purkinje cells of hypothyroid wild-type mice. Arrowheads show elongated primary dendrites. (Bar = 50 μm.)
The results of these experiments suggest that TRα1 is dispensable for cerebellar development under normal euthyroid conditions. However, they also show that thyroid hormone deficiency induces developmental alterations that are prevented by TRα1 deletion. This paradox may be explained by assuming that unliganded TRα1 interferes with cerebellar development. To substantiate this hypothesis, we have used a pharmacological approach to study cerebellar development in the presence of liganded TRβ and unliganded TRα1. This result was achieved by using the TRβ-selective, T3 analog GC-1.
Comparative Effects of Thyroid Hormone and GC-1 on Cerebellar Development.
GC-1 is a TRβ-selective, T3 analog that has an affinity for TRβ equal to that of T3 and one order of magnitude lower affinity for TRα1 (19, 26). We aimed to examine whether a comparison of the effects of GC-1 with those of T3 on cerebellar development would allow discrimination between the developmental effects mediated through TRα1 and TRβ. Accordingly, different groups of hypothyroid animals were treated with single daily doses of T3 or GC-1. The effects on granule cell migration and Purkinje cell differentiation then were examined.
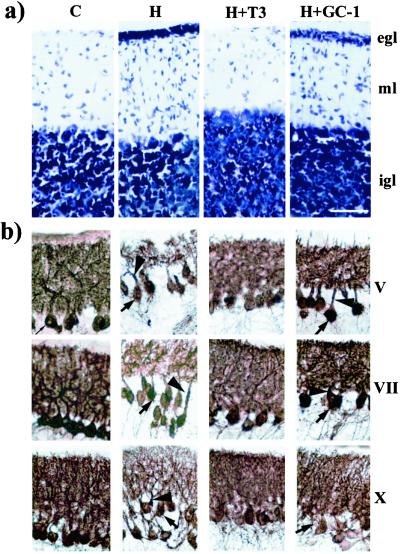
Fig. 3 shows the effects of hypothyroidism (H), T3 (H + T3) and GC-1 (H + GC-1) treatment on the structure of the cerebellum in wild-type mice. In P21 mice, the egl was clearly present in hypothyroid but not control (C) mice (Fig. 3a). Treatment with T3 prevented the delayed migration caused by hypothyroidism. However, treatment with GC-1 was of no effect. Slices from hypothyroid and GC-1-treated mice were very similar, and, in addition to the presence of the egl, spindle-shaped migrating cells were observed in the molecular layer in both preparations. This result was consistent with the idea that the effect of T3 on granule cell migration is exerted through TRα1. Fig. 3b shows the Purkinje cells stained with calbindin. In the hypothyroid mice, alterations of Purkinje cell morphology similar to those described above were observed. Treatment with T3 prevented the alterations induced by hypothyroidism. Treatment with GC-1 induced a clear increase in the density of dendrites, but the effect was not as complete as with T3. Also, other signs of immaturity persisted, such as the presence of perisomatic processes and elongated primary dendrites. Treatment with GC-1 was, however, as effective as T3 in increasing PCP-2 mRNA after administration to hypothyroid mice (Fig. 3c).
Figure 3.
Effects of hypothyroidism and T3 or GC-1 treatment on granular cell migration and Purkinje cell differentiation in mice. (a) Toluidine blue-stained sagittal sections of lobule VII from P21 mice cerebella. Arrows point to spindle-shaped migrating cells in hypothyroid and GC-1-treated mice. egl, external germinal layer; ml, molecular layer; and igl, internal granule cell layer. (b) Purkinje cells immunostained for calbindin in sagittal sections of lobules V, VII, and X of P11 mice cerebella. The arrows show the presence of perisomatic terminals in Purkinje cells from hypothyroid and GC-1-treated mice. Arrowheads show elongated primary dendrites. (c Left) Northern blot of cerebellum RNA probed with PCP-2 and GAPDH cDNA probes. (Right) Densitometric measurements of the autoradiographs. C, Control mice; H, hypothyroid mice; H + T3, hypothyroid mice treated with T3; H + GC-1, hypothyroid mice treated with GC-1. (Bar = 50 μm.)
Similar results were obtained in rats, as shown in Fig. 4. Hypothyroidism was associated with a persistence of the egl, as in mice. The effects on the Purkinje cells were more severe, probably because of species differences, as the degree of hypothyroidism achieved in rats and in mice was similar (Table 1). As in mice and in contrast to T3, GC-1 was not effective in preventing the delayed migration of granule cells (Fig. 4a) and was only partially effective in enhancing Purkinje cell maturation (Fig. 4b). Although an increase in the density of dendrites was evident, for example, in lobule V, the effect was not as complete as with T3. Other signs of immaturity that were more evident in rats than in mice, such as the elongated primary dendrites and the presence of perisomatic processes, were not influenced by GC-1 treatment.
Figure 4.
Effects of hypothyroidism, and T3 or GC-1 treatment on granular cell migration and Purkinje cell differentiation in rats. (a) Toluidine blue-stained sagittal sections of lobule VII from P25 rat cerebella. The arrows show the presence of migrating cells from hypothyroid and GC-1-treated mice. egl, external germinal layer; ml, molecular layer; and igl, internal granule cell layer. (b) Purkinje cells immunostained for calbindin in sagittal sections of lobules V, VII, and X of P16 mice cerebella. Arrows show the presence of perisomatic terminals in Purkinje cells from hypothyroid and GC-1-treated rats. Arrowheads show elongated primary dendrites. (Bar = 25 μm.)
Discussion
The main goal of the present studies was to analyze cerebellar structure in developing mice deficient of the TRα1 isoform of thyroid hormone receptors by using two classical and well known thyroid hormone controlled events, granule cell migration and Purkinje cell differentiation. Lack of thyroid hormone during the neonatal period delays granular cell migration and dramatically arrests Purkinje cell differentiation (18, 27). As a consequence, the external germinal layer persists longer than normal, and the Purkinje cells show severe alterations, including lack of alignment of cell bodies, stunted growth of the dendritic tree, elongated primary dendrites, and delayed disappearance of transient perisomatic processes. The relative contribution of T3 receptor subtypes to these effects of thyroid hormone is unknown.
Previous estimates showed that, in the adult rat, TRα1 accounts for about 75% of T3 binding capacity in the cerebrum and for almost 80% in the cerebellum (28–30). The relative contribution of TRα1 is probably much higher during the neonatal period, given the patterns of mRNA expression. In the cerebellum, the granular cells express TRα1 and TRα2, whereas the Purkinje cells express mainly TRβ (31–33). Furthermore, there is an up-regulation of TRα1 in the granule cells at the time when they undergo the last cell divisions and become prepared to migrate inwardly from the inner part of the external germinal layer (31). In mice, unpublished data from our laboratory show that the patterns of expression of TRα1 and TRβ1 are very similar to rats. Proliferating and migrating granule cells express TRα1 but not TRβ1 not only in rodents, but also in chicken (7).
From its expression data, it would be presumed that TRα1 should have a prominent role in mediating the dramatic effects of thyroid hormone on brain development. This possibility is consistent with recent data from knock-in mice showing that expression of a dominant-negative form of TRα1, but not TRβ1, strongly impairs brain glucose utilization (34). Therefore, it is most intriguing that TRα1 knockout mice do not develop the severe phenotype of profound hypothyroidism. Gross cerebellar structure in intact TRα1−/− mice was not different from that of wild-type mice, and both migration of granule cells and the morphological appearance of Purkinje cells were apparently normal. Our data, therefore, indicate that TRα1 is dispensable for normal cerebellar development. Why then, does the lack of thyroid hormone, as occurs in hypothyroidism, interfere so profoundly with cerebellar development? The possibility of extranuclear actions of T3 cannot be discounted (35), but it would not easily explain the lack of cerebellar alterations in hypothyroid TRα1−/− mice. On the other hand, this latter finding strongly suggests an alternative explanation, that the developmental brain defects observed in severe hypothyroidism are the result of the strong transrepressor activity mediated by unliganded TRα1 (36, 37).
Recent in vivo studies have confirmed the specificity of GC-1 as a TRβ-selective ligand and the validity of its use to link TRα or TRβ with specific thyroid hormone-dependent pathways (20, 21, 26). Our results with this compound indicate that the effect of thyroid hormone on granule cell migration is TRα1 isoform-specific. They also give an additional strong support to the idea that unliganded TRα1 is responsible for the migration defect present in neonatal hypothyroidism.
As for granule cell migration, Purkinje cell maturation was not altered in TRα1−/− mice. In principle, this result is what should be expected, because the predominant TR expressed in these cells is TRβ. However, it also was found that Purkinje cells were not affected in the TRα1−/− mice after induction of hypothyroidism. This finding was, indeed, another unexpected one and argues that unliganded TRβ does not exert dominant-negative effects in these cells. GC-1 treatment was as effective as T3 in increasing PCP-2 gene expression, but had only partial effects on Purkinje cell maturation. This result is not entirely surprising, because maturation of Purkinje cells depends strongly on granule cells. The axons of these cells, the parallel fibers, establish connections with Purkinje cell dendrites as they differentiate and migrate inward, and the ensuing synaptic activity promotes dendritic maturation (38, 39). Therefore, in the absence of any effects on granule cells, it is not entirely surprising that full differentiation of the Purkinje cells could not be achieved with GC-1 treatment.
Therefore, we propose that the effect of thyroid hormone on migration of granule cells of the cerebellum is a TRα1 response, whereas differentiation of Purkinje cells is a mixed TRα1 and TRβ response. Further analysis of specific genetically modified animals should determine the role of other products of the TRα gene, namely TRα2 and the truncated forms. The study of cerebellar development in these animals should provide fundamental insights into the biological role of thyroid hormone and its receptors in physiology and evolution.
Acknowledgments
We thank Professor Gabriella Morreale de Escobar for T4 and T3 determinations. This work has been supported by Grants DGICYT-PM18–0118 and Community of Madrid 08.5/0044./2000 (to J.B.), by the Swedish Cancer Society (to B.V.), and by Grant DK-52798 from the National Institutes of Health (to T.S.).
Abbreviations
- TR
thyroid hormone receptor
- T3
l-triiodothyronine
- T4
l-thyroxine
- GC-1
TRβ-selective T3 analog
- egl
external germinal layer
- ml
molecular layer
- igl
internal granule cell layer
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Izumo S, Mahdavi V. Nature (London) 1988;334:539–542. doi: 10.1038/334539a0. [DOI] [PubMed] [Google Scholar]
- 3.Koening R J, Lazar M A, Holdin R A, Brent G A, Larsen P R, Chin W W, Moore D D. Nature (London) 1989;337:659–661. doi: 10.1038/337659a0. [DOI] [PubMed] [Google Scholar]
- 4.Macchia P E, Takeuchi Y, Kawai T, Cua K, Gauthier K, Chassande O, Seo H, Hayashi Y, Samarut J, Murata Y, et al. Proc Natl Acad Sci USA. 2001;98:349–354. doi: 10.1073/pnas.011306998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chassande O, Fraichard A, Gauthier K, Flamant F, Legrand C, Savatier P, Laudet V, Samarut J. Mol Endocrinol. 1997;11:1278–1290. doi: 10.1210/mend.11.9.9972. [DOI] [PubMed] [Google Scholar]
- 6.Williams G R. Mol Cell Biol. 2000;20:8329–8342. doi: 10.1128/mcb.20.22.8329-8342.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrest D, Hallbook F, Persson H, Vennstrom B. EMBO J. 1991;10:269–275. doi: 10.1002/j.1460-2075.1991.tb07947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gauthier K, Chassande O, Plateroti M, Roux J P, Legrand C, Pain B, Rousset B, Weiss R, Trouillas J, Samarut J. EMBO J. 1999;18:623–631. doi: 10.1093/emboj/18.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu J-H, Brent G A. Trends Endocrinol Metab. 1998;9:103–112. doi: 10.1016/s1043-2760(98)00026-5. [DOI] [PubMed] [Google Scholar]
- 10.Forrest D, Erway L C, Ng L, Altschuler R, Curran T. Nat Genet. 1996;13:354–357. doi: 10.1038/ng0796-354. [DOI] [PubMed] [Google Scholar]
- 11.Forrest D, Hanebuth E, Smeyne R J, Everds N, Stewart C L, Wehner J M, Curran T. EMBO J. 1996;15:3006–3015. [PMC free article] [PubMed] [Google Scholar]
- 12.Gloss B, Trost S, Bluhm W, Swanson E, Clark R, Winkfein R, Janzen K, Giles W, Chassande O, Samarut J, Dillmann W. Endocrinology. 2001;142:544–550. doi: 10.1210/endo.142.2.7935. [DOI] [PubMed] [Google Scholar]
- 13.Wikström L, Johansson C, Salto C, Barlow C, Campos Barros A, Baas F, Forrest D, Thoren P, Vennström B. EMBO J. 1998;17:455–461. doi: 10.1093/emboj/17.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plateroti M, Gauthier K, Domon-Dell C, Freund J N, Samarut J, Chassande O. Mol Cell Biol. 2001;21:4761–4772. doi: 10.1128/MCB.21.14.4761-4772.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauthier K, Plateroti M, Harvey C B, Williams G R, Weiss R E, Refetoff S, Willott J F, Sundin V, Roux J P, Malaval L, et al. Mol Cell Biol. 2001;21:4748–4760. doi: 10.1128/MCB.21.14.4748-4760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gothe S, Wang Z, Ng L, Kindblom J M, Barros A C, Ohlsson C, Vennström B, Forrest D. Genes Dev. 1999;13:1329–1341. doi: 10.1101/gad.13.10.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calza L, Forrest D, Vennström B, Hökfelt T. Neuroscience. 2000;101:1001–1012. doi: 10.1016/s0306-4522(00)00420-6. [DOI] [PubMed] [Google Scholar]
- 18.Legrand J. In: Neurobehavioral Teratology. Yanai J, editor. Amsterdam: Elsevier Science; 1984. pp. 331–363. [Google Scholar]
- 19.Chiellini G, Apriletti J W, al Yoshihara H, Baxter J D, Ribeiro R C, Scanlan T S. Chem Biol. 1998;5:299–306. doi: 10.1016/s1074-5521(98)90168-5. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro M O, Carvalho S D, Schultz J J, Chiellini G, Scanlan T S, Bianco A C, Brent G A. J Clin Invest. 2001;108:97–105. doi: 10.1172/JCI12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trost S U, Swanson E, Gloss B, Wang-Iverson D B, Zhang H, Volodarsky T, Grover G J, Baxter J D, Chiellini G, Scanlan T S, Dillmann W H. Endocrinology. 2000;141:3057–3064. doi: 10.1210/endo.141.9.7681. [DOI] [PubMed] [Google Scholar]
- 22.Escobar-Morreale H F, Escobar del Rey F E, Obregon M J, Morreale de Escobar G. Endocrinology. 1996;137:2490–2502. doi: 10.1210/endo.137.6.8641203. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 24.Iñiguez M A, Delecea L, Guadaño-Ferraz A, Morte B, Gerendasy D, Sutcliffe J G, Bernal J. Endocrinology. 1996;137:1032–1041. doi: 10.1210/endo.137.3.8603571. [DOI] [PubMed] [Google Scholar]
- 25.Altman J, Bayer S A. Development of the Cerebellar System in Relation to its Evolution, Structure and Functions. Boca Raton, FL: CRC; 1996. [Google Scholar]
- 26.Baxter J D, Dillmann W H, West B L, Huber R, Furlow J D, Fletterick R J, Webb P, Apriletti J W, Scanlan T S. J Steroid Biochem Mol Biol. 2001;76:31–42. doi: 10.1016/s0960-0760(01)00052-8. [DOI] [PubMed] [Google Scholar]
- 27.Hajós F, Patel A J, Balázs R. Brain Res. 1973;50:387–401. doi: 10.1016/0006-8993(73)90740-3. [DOI] [PubMed] [Google Scholar]
- 28.Ercan-Fang S, Schwartz H L, Oppenheimer J H. Endocrinology. 1996;137:3228–3233. doi: 10.1210/endo.137.8.8754744. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz H L, Lazar M A, Oppenheimer J H. J Biol Chem. 1994;269:24777–24782. [PubMed] [Google Scholar]
- 30.Strait K A, Schwartz H L, Perezcastillo A, Oppenheimer J H. J Biol Chem. 1990;265:10514–10521. [PubMed] [Google Scholar]
- 31.Bradley D J, Towle H C, Young W S., III J Neurosci. 1992;12:2288–2302. doi: 10.1523/JNEUROSCI.12-06-02288.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellström B, Naranjo J R, Santos A, González A M, Bernal J. Mol Endocrinol. 1991;5:1339–1350. doi: 10.1210/mend-5-9-1339. [DOI] [PubMed] [Google Scholar]
- 33.Strait K A, Schwartz H L, Seybold V S, Ling N C, Oppenheimer J H. J Cell Biochem. 1991;15, Suppl. 1:17. [Google Scholar]
- 34.Itoh Y, Esaki T, Kaneshige M, Suzuki H, Cook M, Sokoloff L, Cheng S Y, Nunez J. Proc Natl Acad Sci USA. 2001;98:9913–9918. doi: 10.1073/pnas.171319498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis P J, Davis F B. Thyroid. 1999;6:497–504. doi: 10.1089/thy.1996.6.497. [DOI] [PubMed] [Google Scholar]
- 36.Hu X, Lazar M A. Trends Endocrinol Metab. 2000;11:6–10. doi: 10.1016/s1043-2760(99)00215-5. [DOI] [PubMed] [Google Scholar]
- 37.Sap J, Muñoz A, Schmitt J, Stunnenberg H, Vennström B. Nature (London) 1989;340:242–244. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- 38.Baptista C A, Hatten M E, Blazeski R, Mason C A. Neuron. 1994;12:243–260. doi: 10.1016/0896-6273(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 39.Hirai H, Launey T. J Neurosci. 2000;20:5217–5224. doi: 10.1523/JNEUROSCI.20-14-05217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]