Abstract
The nucleus basalis (NB) has been implicated in memory formation indirectly, by lesions, pharmacological manipulations, and neural correlates of learning. Prior findings imply that engagement of the NB during learning promotes memory storage. We directly tested this NB-memory hypothesis by determining whether stimulation of the NB induces behavioral associative memory. Rats were trained either with paired tone (6 kHz) and NB stimulation or with the two stimuli unpaired. We later determined the specificity of cardiac and respiratory behavioral responses to the training tone and several other acoustic frequencies. Paired subjects exhibited frequency generalization gradients with a peak of 6 kHz for both cardiac and respiratory behavior. Unpaired subjects exhibited no generalization gradient. The development of such specific, associative behavioral responses indicates that tone paired with NB stimulation induced behavioral associative memory. The discovery of memory induction by direct activation of the NB supports the NB-memory hypothesis and provides a potentially powerful way to control and investigate neural mechanisms of memory.
The capacity to remember provides the fundamental ability to benefit from experience, by bridging the gap over minutes to years between transient sensory events and subsequent adaptive behavior. Although the storage of experience is undoubtedly a property of many brain systems, the cerebral cortex has drawn special attention for several reasons, including its dominant size and critical role in human cognition and behavior. However, the mechanisms underlying the storage of information in the cerebral cortex are not well understood. Although sensory receptors receive continual environmental stimulation, only a fraction of sensory events enter into memory, suggesting that other, nonsensory brain systems selectively modulate the cortex to enable the storage of experiences that are behaviorally important. Pharmacological evidence indicates that the cholinergic system is one of several neuromodulatory systems that may be directly involved in memory processes (reviewed in ref. 1). Also, many noncholinergic treatments that facilitate memory, such as adrenergic agents and stress hormones, affect memory by means of cholinergic actions (2).
The nucleus basalis (NB) is a candidate for such modulatory functions because it is the major source of cortical acetylcholine (ACh) (3, 4). Stimulation of the NB increases the release of cortical ACh and produces electroencephalographic (EEG) activation (“desynchronization”) (5–7), which is the waking state affiliated with learning (8). Conversely, NB lesions reduce cortical ACh and impair cortical activation (9–11). NB cells respond increasingly to behaviorally significant stimuli during learning (12–15), and ACh is preferentially released in relevant sensory cortical areas at the time of learning (16, 17). Additionally, NB neurons projecting to the primary auditory cortex (ACx) selectively increase transcription of the gene for the synthetic enzyme of ACh, choline acetyltransferase, during behavioral acoustic conditioning (18).
Lesions of the NB using excitatory amino acid agonists disrupt learning in many tasks (reviewed in refs. 19 and 20), and the effects may be specific to certain memory processes, e.g., impairment of acquisition but not retrieval of conditioned taste aversion (21). However, excitotoxic lesions are not selective to cholinergic neurons, also affecting GABAergic (γ-aminobutyric acid, GABA) and other noncholinergic cells. In contrast, selective immunotoxic cholinergic lesions apparently impair attention rather than learning or memory (reviewed in refs. 19 and 20). Yet, recent findings indicate that immunotoxic lesions leave a small amount of ACh that is sufficient to support learning (22). Thus, the role of NB cholinergic neurons in learning and memory is unresolved, although the involvement of the NB region in general seems less controversial.
Complementary to lesion studies, the NB is also implicated in learning and memory by specific neural correlates of learning. The ACx develops receptive field (RF) plasticity that includes shifts of tuning to the acoustic frequency used as a conditioned stimulus (CS). RF plasticity exhibits the major characteristics of associative behavioral memory, including specificity, rapid induction, long-term retention, and consolidation over hours and days (23, 24). Tuning shifts are large enough to increase the area of representation of behaviorally significant frequencies in the tonotopic map of the ACx (25). As with appetitive or aversive reinforcers, NB stimulation can lead to both RF and map plasticity (26–29). Moreover, NB-induced RF plasticity is blocked by direct application of atropine to the cortex, showing that it requires the engagement of cortical muscarinic receptors (30).
These findings support the hypothesis that acquisition processes can be modulated by activation of the NB and the resultant engagement of muscarinic receptors in the cortex (see also ref. 31). However, cortical plasticity itself does not constitute memory, because memory is a property of the organism and can be inferred only from the analysis of behavior (32). (The term “behavioral memory” is sometimes used here to highlight the difference between genuine memory and “physiological memory,” i.e., neural plasticity.) The goal of this experiment is to provide a direct link between activation of the NB and behavioral memory. Specifically, we sought to induce associative memory by pairing a tone with NB stimulation in the absence of normal reinforcers.
The inference of associative memory from behavioral change must meet two criteria, whether memory is induced in normal learning situations or by stimulation of the brain. First, nonassociative factors must be ruled out; in the present situation, we used an unpaired control group. Second, the behavior must be specific to the signal stimulus (e.g., CS). We approached the issue of specificity by using the well established metric of the stimulus generalization gradient, obtained when an animal trained with one stimulus is subsequently tested with many stimuli (33). We reasoned that if NB stimulation paired with a tone induces memory, then the CS should later elicit the largest behavioral responses to all tones tested, i.e., occupy the peak of the frequency generalization function. We selected changes in heart rate and respiration as behavioral indices of newly formed memory because they are highly sensitive, reliable, and robust indicators of behavioral conditioning (34).
Methods
Subjects and Preparation.
Adult male Sprague–Dawley rats (350–690 g), under sodium pentobarbital anesthesia (35–40 mg/kg, i.p.), were fitted with a concentric bipolar stimulating electrode in the right NB, an epidural stainless steel screw recording electrode over the ipsilateral ACx, a thoracic EKG electrode inserted s.c., and a reference screw overlying the frontal sinus. (For a more detailed description see ref. 27). Electrode leads were attached to a multichannel connector that could be connected to a cable from a commutator and embedded in a dental acrylic pedestal that contained threaded spacers for the attachment of a respiration recording assembly. All procedures were performed in accordance with University of California at Irvine Animal Research Committee and National Institutes of Health animal welfare guidelines. NB placements were verified neurohistologically as lying in the caudal NB (medial globus pallidus and dorsal substantia inominata), sites that have cholinergic NB projections to the ACx (35).
Training and Testing.
After recovery from surgery (7–10 days of daily handling), animals were adapted for 2 days to a training box (25 × 22 × 26 cm containing the same bedding as their individual home cages) within an acoustic chamber. After exploring and grooming, animals generally remained quiet. Minimum NB thresholds were determined for eliciting 2–5 s of reliable, visually identifiable EEG activation (i.e., a shift from higher-voltage slower waves to lower-voltage faster waves). Levels were 50–100 μA across animals, constant for each animal throughout training.
Training started the next day; rats were assigned to either the paired (n = 4) or unpaired (n = 4) groups. Paired trials consisted of a 6-kHz tone (2 s, 70 dB, overhead speaker) paired with NB stimulation (0.2 s, 50–100 μA), 1.8 s after tone onset (0.2 s overlap), intertrial intervals averaged 45 s (30–60 s, randomly programmed). Unpaired animals received the same overall density of randomly programmed tone and NB stimulation explicitly unpaired (8-s minimum interstimulus interval). Training consisted of 200 trials per day for 15 days. The EEG was recorded continually to ensure that NB stimulation was effective throughout training. Stimulation effectiveness was verified by quantitative analysis of the EEG (36). NB stimulation levels were not significantly different between groups (t6 = −0.48, P > 0.05). Videotape analyses by two observers blind to the experiment revealed no detectable movement to NB stimulation during training.
Testing was conducted in a single session 24 h after the end of training, in the absence of NB stimulation. Animals were tested in a different lab room and chamber (to reduce potential contextual cues) while the EEG, electrocardiogram, and respiration were recorded. Testing was identical for both groups. After a 10-min period of adaptation, subjects received 105 test trials, consisting of 15 repetitions of the same pseudorandom sequence of 7 tones (15, 1, 12, 2, 10, 3, 6 kHz, each = 2 s, 70 dB). Trials were presented at intervals of >30 s, and only following at least 2 s of visually judged stable EEG and respiration. On the day after testing, the unconditioned effects of NB stimulation were assessed for heart rate and respiration for unpaired animals. The same train of stimulation used in training was presented by using the same protocol used in testing, except that NB stimulation was presented instead of tones. Animals were unrestrained and unanesthetized during training, testing sessions, and posttesting NB-alone sessions. They rested quietly, occasionally grooming or stretching; their EEGs were seldom dominated by delta waves, theta and alpha being most prominent. The general lack of slow-wave sleep may have been caused by the cortical arousal that was induced continually by NB or tone stimulation throughout sessions.
Recording and Data Analysis.
The EEG was recorded conventionally (amplified ×1000, 0.1–100 Hz bandpass), digitized, and analyzed offline by using a fast Fourier transform to extract power in the EEG gamma band (30–58 Hz). The effect of tone-elicited EEG changes during testing was quantified as the ratio of the average gamma-band power during the 2 s immediately preceding tone presentation divided by the average power during tone presentation, on a trial by trial basis.
The EKG signal was amplified, filtered (×1000, 1.0–100 Hz), digitized, and heart rate-calculated offline based on interbeat intervals. As cardiac response to tone was generally biphasic (bradycardia followed by tachycardia) and could last several seconds, overall response was calculated as the range of heart rate change (“peak to peak”) for 10 s after tone onset, for each trial.
Respiration was recorded by means of a lightweight head-mounted assembly of local design, consisting of an adjustable bracket that positioned a sensor (a 3-s time-constant glass encapsulated 250 Ω thermistor) 1–2 mm adjacent to the nares. The sensor, serving as one arm of a bridge circuit, measured the temperature of inspired and expired air. Respiration was analyzed by using a fast Fourier transform performed on a 2-s pretone period and for 10 s beginning with tone onset. The bandwidth of analysis was 1.63–4.88 Hz, which was found to contain greater than 98% of the power in pilot studies of spontaneous respiration. The Fourier transform results were used to calculate a “respiration change index” (RCI) that was sensitive to increases and decreases of both frequency and amplitude. RCI = (|Post − Pre|)/(Pre + Post). A value of 1.0 would indicate complete suppression of respiration and a value of 0 would indicate no change.
Statistical analysis was based on pooling responses to each test frequency across a session because there was no significant trend across serial order of stimulus presentation (ANOVAs, all P > 0.05; runs tests, all P > 0.05). Data for each frequency were then pooled across animals within the paired and unpaired groups. Effects were assessed by ANOVAs. Significant frequency effects were partitioned into components of variance and tested by using F ratios, to determine whether there was a significant quadratic component, i.e., if the generalization gradient had the general form of an inverted “V”; orthogonal contrasts (6 kHz vs. mean of other frequencies) were also performed (37).
Results
EEG Activation.
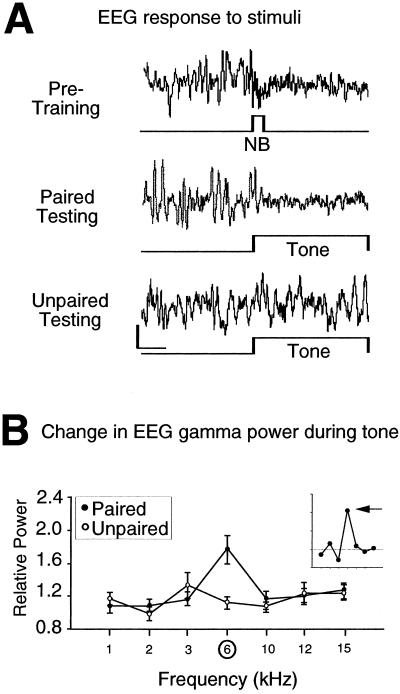
The EEG was recorded during testing to determine whether paired training had produced specific, associative plasticity in the cortex. NB stimulation alone had produced EEG activation at the time current levels were determined and during training. Examples of individual records during testing showed that the 6-kHz tone elicited greater EEG activation in the paired group than in the unpaired control group, suggesting that pairing induced conditioned EEG responses (Fig. 1A). Quantitative analyses of the EEG confirmed this conclusion. An increase of power in the gamma band is a critical component of EEG activation (38). Pairing significantly altered the EEG across acoustic frequency, increasing gamma band activity (F6, 485 = 4.86, P < 0.0001) (Fig. 1B). The increase was greatest to 6 kHz. No effect of training on gamma was exhibited by the unpaired group (F6, 376, = 1.49 P > 0.05) (Fig. 1B). Such an increase in gamma is consistent with reports of EEG activation during spontaneous state changes (38).
Figure 1.
EEG for animals receiving either paired or unpaired 6-kHz tone and NB stimulation during training. (A) Examples of EEG records from individual animals. The first record shows that, during the pretraining determination of threshold level, NB stimulation alone (60 μA) elicited a shift of the EEG from higher amplitude, slower activity to lower amplitude, faster activity, i.e., EEG activation. The second record shows that the paired tone (6 kHz) produced EEG activation during testing. The third example shows a lack of activation to 6 kHz in the unpaired group. Calibrations: 2 s, 400 μV. (B) Group changes (mean ± SE) in the EEG of relative power in the gamma band (30–58 Hz) for all test tones. The paired group exhibited a maximum increase in gamma activity at the frequency of the paired tone, 6 kHz. The Inset presents the group difference function (paired minus unpaired functions) to show the specificity of EEG changes resulting from the difference in training. (Difference function for illustrative, not statistical, purposes.) Horizontal line denotes level of no change. Arrow indicates difference in response to 6 kHz.
The finding of specific, conditioned EEG activation in the cortex confirms that tone-NB pairing was an effective treatment for the induction of neural plasticity. This finding is essentially the same as the well established finding of EEG-conditioned responses in classical conditioning with appetitive or aversive reinforcers, where they have been established to reflect a component of effective learning processes (39). However, this NB-induced EEG plasticity, like other neurophysiological correlates of learning and memory, is insufficient to determine whether stimulation of the NB actually induces memory.
Behavioral Memory
Heart Rate.
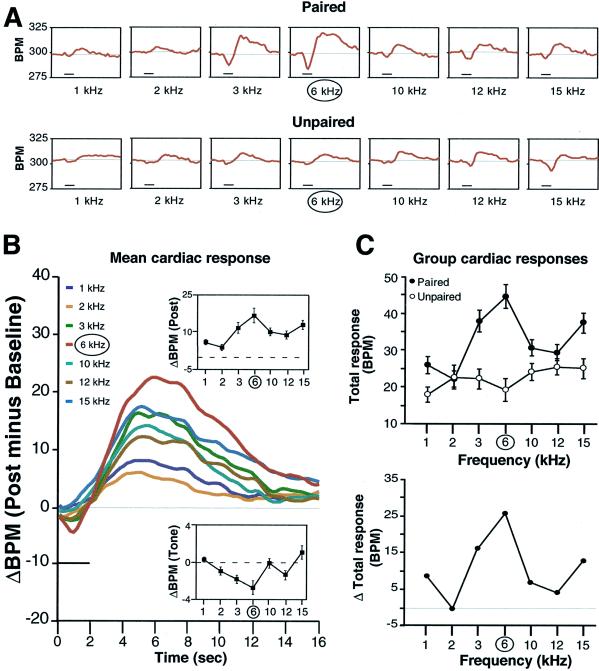
Tones elicited changes in heart rate. The typical cardiac response was biphasic, consisting of a brief bradycardia followed by a larger and more sustained tachycardia. Records of individual animals indicated that the magnitude of both response components was largest for the frequency of the paired tone in the paired group but not in the unpaired group (Fig. 2 A and B). A two-factor ANOVA (group × frequency) revealed that both group (F1, 721 = 8.0, P = < 0.0001) and frequency (F6, 721 = 25.2, P = < 0.0001) were significant, as was the interaction (F6, 721 = 6.4, P = < 0.05).
Figure 2.
Cardiac behavior (heart rate) during the test period. (A) Examples of individual records of heart rate (beats per minute, BPM) for one animal each in the paired and unpaired groups. Deflections below the baseline indicate bradycardia, those above show tachycardia. Horizontal bar indicates test tone duration (2 s). The largest response in the paired animal was to 6 kHz, which was not the case for the unpaired animal. (B) Average changes in heart rate to each test tone for the paired group. The Insets show mean (±SE) amplitude of response during (lower function) and after (upper function) the tones, respectively. Both functions show maximum change at the frequency of the paired tone. (C Upper) Biphasic cardiac response magnitude (mean ± SE peak to peak values) for each test frequency in the two groups. The paired group generalization gradient was significantly quadratic (P < 0.01), and 6 kHz was at the apex of the gradient. (Lower) The difference between group functions (paired minus unpaired) indicates the degree of specificity of cardiac response attributable to pairing per se.
The frequency generalization functions for both groups are shown in Fig. 2C. Inspection of this figure indicates that the responses of the paired group were larger than those in the unpaired group. In addition, the frequency function of the paired group formed a generalization gradient with the CS frequency (6 kHz) at its apex. This gradient is statistically significant (quadratic component of variance, F3, 56 = 8.20, P < 0.01). Furthermore, an orthogonal contrast indicated that response to 6 kHz was significantly greater than to the other frequencies combined (t = 2.77, P < 0.02). However, the unpaired group exhibited no significant gradient (quadratic component F3, 56 = 0.002, P > 0.05). Responses to 6 kHz were significantly smaller than responses to other test frequencies (t = −4.78, P < 0.01).
The NB-memory hypothesis predicts that the CS frequency should be at the apex of the frequency generalization gradient for the paired group only. This outcome was obtained. Therefore, cardiac behavior meets the criteria of associativity and specificity required for the inference of memory induction.
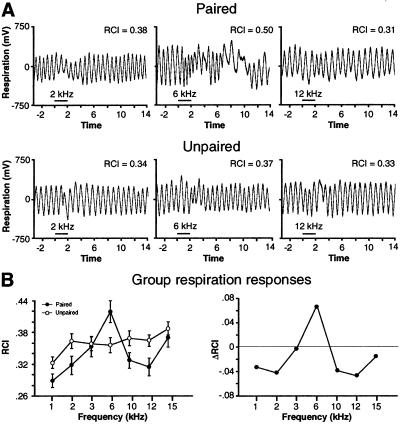
Respiration.
Test tones also interrupted respiration. Examples of individual records are presented in Fig. 3A. The findings were similar to those for heart rate, i.e., the largest responses were at the CS frequency in the paired group. The unpaired group exhibited smaller responses and no specificity to 6 kHz. A two-factor ANOVA (group × frequency) was significant for both group (F1, 721 = 6.5, P = 0.01) and frequency (F6, 721 = 5.4, P < 0.0001) and their interaction (F6, 721 = 2.7, P < 0.05). As with heart rate, the paired group generalization gradient has a significant quadratic component (F3, 56 = 7.44, P < 0.01) with 6 kHz at the apex (Fig. 3B). An orthogonal contrast indicated that responses to 6 kHz were significantly greater than other frequencies combined (t = 2.61, P < 0.05).
Figure 3.
Effects of test tones on respiration. (A) Examples of individual respiration records (with value of RCI) to three frequencies (2, 6, and 12 kHz) for one animal each from the paired and unpaired groups. The largest response was at 6 kHz for the paired animal (RCI = 0.50). Horizontal bar indicates tone duration. (B Left) Group mean (±SE) change in respiration to all tones for both groups. Left shows that the maximal response was at 6 kHz for the paired group but not for the unpaired group. The generalization gradient for only the paired group was significantly quadratic (P < 0.01), with responses to 6 kHz being of greatest magnitude. The group difference function (Right) shows a high degree of specificity of respiratory responses to 6 kHz.
The unpaired group exhibited no significant generalization gradient (quadratic component, F3, 56 = 0.23, P > 0.05), and responses to 6 kHz were not significantly different from responses to other test frequencies (t = −0.2, P > 0.05).
Responses to 15 kHz tended to be larger than responses to other test frequencies in both paired and unpaired groups for respiration and also heart rate. This high degree of stimulus salience may reflect species-specific distress vocalizations within the 15–25 kHz frequency band (40).
Unconditioned Responses.
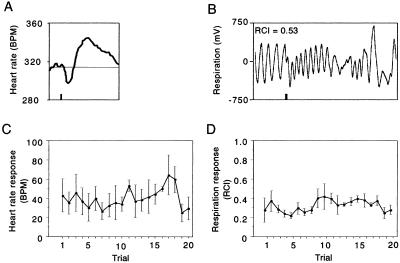
The failure of the unpaired group to exhibit specific CS-peaked generalization gradients for heart rate and respiration might be assumed to reflect the difference in conditioned stimulus–unconditioned stimulus contingency between the paired and unpaired groups. An alternative explanation is that NB stimulation in the unpaired group was inadequate to support cardiac and respiratory responses, although it was adequate to elicit EEG activation during pretraining determination of stimulation thresholds. To resolve this issue, we assessed behavioral responses to NB stimulation alone. On the day after testing, unpaired subjects (n = 3) received 20 trials of NB stimulation. The stimulation elicited clear unconditioned responses, both for heart rate and respiration (Fig. 4 A and B). These resembled the CS-specific responses observed in the paired group (Figs. 2 and 3). The unconditioned responses were consistent across trials, i.e., did not habituate (Fig. 4 C and D), indicating that they should have been adequate to support the induction of memory [one-factor ANOVAs: heart rate, (F19, 40 = 0.59, P > 0.05); respiration, (F19, 40 = 0.78, P > 0.05)].
Figure 4.
Unconditioned effects of NB stimulation on heart rate and respiration. (A) Example of an individual heart rate response to stimulation (beats per minute, BPM). Vertical bar indicates 200-ms stimulation duration. (B) Example of an individual respiration response to stimulation (RCI = 0.53). Vertical bar indicates 200-ms stimulation duration. (C) Group heart rate response (peak to peak ± SE) across trials. (D) Group respiration response (RCI ± SE) across trials. The magnitude of heart rate and respiration response does not significantly change with repeated stimulation.
Discussion
The results show that pairing a tone with NB stimulation is sufficient to induce behavioral associative memory. The animals in the paired group behaved as though they had learned that 6 kHz had acquired increased behavioral significance. Viewing the behavioral data alone, one could not determine whether training had involved a standard reinforcer or NB stimulation. The behavioral effects are associative as they did not develop in the unpaired group. The requisite degree of behavioral specificity is provided by the generalization gradients for heart rate and respiration, both of which were peaked at the CS frequency of 6 kHz.
The use of an unpaired group raises the issue of whether NB stimulation produced learning of a negative contingency, i.e., that the CS predicts the absence of NB stimulation for subjects in the unpaired group (“negative” learning for short). If so, the specificity in the paired group could be a statistical artifact of comparing it with a reduced response to 6 kHz in the unpaired group. In fact, such learning did develop for heart rate in the unpaired group. This finding shows that NB stimulation can act as a reinforcer for negative as well as positive contingencies, as do peripheral sensory reinforcers.
Nevertheless, this finding does not mitigate the results for the paired group. The specificity and increased response of the paired group to 6 kHz vs. other test frequencies was statistically independent of the data for the unpaired group, as evidenced by its significant quadratic (inverted-V) function and significant contrast of 6 kHz vs. other frequencies, both for heart rate and respiration. Of interest, negative learning did not develop for respiration in the unpaired group. Thus, had only respiration been monitored, the learning of the negative contingency between tone and NB stimulation (evident in the cardiac data) would have been missed. This difference in outcome exemplifies the advantages of simultaneously recording the behavior of more than one response system. Overall, the results meet the dual criteria of associativity and specificity that are the long-accepted standard for the inference of associative memory from behavioral change.
The mechanisms of NB-induced memory are beyond the scope of this initial study, but some issues can be addressed in a preliminary manner. The first concerns the nature of NB stimulation as a reinforcer. Commonly used appetitive or aversive reinforcers, such as food or shock, have two basic characteristics: they have positive or negative motivational effects and they elicit unconditioned responses. In contrast, there is at least one circumstance in which classical associative effects can be established for which the reinforcer has neither motivational effects nor elicits a standard unconditioned response: sensory preconditioning (33, 41).
NB stimulation does elicit consistent, nonhabituating, unconditioned responses, both for the EEG and, as reported here, in the cardiac and respiratory systems. However, elicitation of unconditioned responses does not force the conclusion that NB stimulation is in the same category as standard reinforcers because it is not part of any known motivational (or sensory or motor) system (42). This contrasts starkly with the ventral tegmental area (VTA), which is part of a well established, powerful positive reward system (reviewed in ref. 43). For example, pairing a tone with either stimulation of the NB (26–29) or the VTA (44) produces CS-specific tuning plasticity in the ACx, similar to that induced during behavioral conditioning (23), via cortical cholinergic muscarinic receptors (30) and dopaminergic mechanisms (44), respectively. Thus, although activation of both the cholinergic and dopaminergic modulatory systems can produce auditory cortical plasticity, they do not have comparable motivational effects.
Stimulation of certain brain systems, including the NB, may constitute a third class of reinforcers that elicit unconditioned responses but are neither appetitive nor aversive. For example, Olds and Peretz (45) reported that there were separable positive, negative, and motivationally neutral regions of the mesencephalon; the positive area was later found to involve the dopaminergic reward system (43). Motivationally neutral sites produced nonhabituating EEG arousal in areas now identified as involving the ascending projections of brainstem cholinergic nuclei (46). Wester (47, 48) extended this line of inquiry, finding sites that were neither motivationally positive nor negative, but whose stimulation elicited not only nonhabituating EEG activation but also nonhabituating behavioral arousal. These neutral sites were in the same area of the midbrain found by Olds to be neutral, and also in the midline and intralaminar nuclei of the thalamus, the target of brainstem cholinergic projections (45, 46). The involvement or close relationship to the cholinergic system is common to the sites in the midbrain, thalamus, and the NB. Whether the cholinergic substrate is an essential feature for nonhabituating apparently motivational neutral sites remains to be determined.
Stimulation of the NB has another unconditioned effect not yet considered; it increases cerebral blood flow (CBF) (e.g., ref. 49). Conceivably, a sudden modification of CBF might produce a motivationally negative internal state. However, NB effects on CBF have been demonstrated by using stimulation durations that range from 10 s (reviewed in ref. 50) to 1–2 min (e.g., ref. 51). We used a single 200-ms train, which is 0.02% as long as the minimal duration of stimulus reported to increase CBF. It is not yet known whether such a brief stimulus altered CBF.
Nonetheless, it would be premature to conclude that stimulation of the NB, as used in this study, has neither positive nor negative motivational consequences. That subjects did not exhibit movement to NB stimulation is consistent with neutrality but not decisive. The issue should be regarded as unsettled at this time. However, it is reasonable to consider that motivational systems, having evaluated the valence of a peripheral sensory reinforcer, are afferent to other brain structures, which themselves are not part of the hedonic substrates. The NB might be such a “downstream” structure, which exerts broad modulatory effects, once engaged by motivational systems.
The present findings raise many other questions. For example, the minimum amount of training sufficient to induce memory should be determined. This initial study used prolonged training (3,000 trials over 15 days) to match the protocol used by other workers for NB-induced map plasticity in the ACx (28). However, pilot studies indicate that one session of 200 trials or less may be adequate (A. Miasnikov, personal communication).
The mechanisms of NB-induced behavioral memory should now be addressed. Although the present findings are compatible with mediation by cholinergic projections to the cortex, NB stimulation also engages γ-aminobutyric acid neurons that project to the cortex; these apparently work in concert with the NB-mediated release of ACh to promote cortical plasticity (52, 53). The NB also projects to subcortical structures, particularly the amygdala (4, 54). Therefore, cortical plasticity induced by paired NB stimulation (e.g., ref. 26) may develop in parallel with plasticity in subcortical systems, and either or both levels of the neuraxis may be involved in the induction of behavioral indices of memory. Selective pharmacological and other interventions of cortical and subcortical targets of NB stimulation will be necessary to resolve this issue. Also, the NB may be only one of several neuromodulatory systems that can also induce behavioral memory. Similar experiments with other systems should be pursued.
A highly speculative, but intriguing, question concerns what aspects of memory might have been induced by tone paired with NB stimulation. Memories normally involve both (i) the sensory content of an experience (e.g., the occurrence and relationship of two stimuli,) and (ii) the level of behavioral importance of the experience (e.g., much greater for a hungry than a satiated animal). If NB stimulation proves to induce memory in the absence of any motivational effect, and because it is not part of any sensory system, then the NB may have had a singular effect. It would have induced only the increased importance of 6 kHz, without storage of the normal conditioned stimulus–unconditioned stimulus sensory–sensory or sensory–motivational relationship, as these were absent. The present approach may provide a way to investigate memory for stimulus importance, relatively isolated from memory of normal sensory-motivational events, thereby allowing the “dissection” of memory components for reductionistic analyses.
Acknowledgments
We thank Tom Carew for insightful critique of the manuscript, Jemmy Chen for help with data analysis, and Jacquie Weinberger for assistance with preparation of the manuscript. This work was supported by National Institute of Mental Health Grant MH-57235, and National Institute of Deafness and Other Communication Disorders Grants DC-02346 and DC-02398 (to N.M.W.).
Abbreviations
- NB
nucleus basalis
- ACh
acetylcholine
- EEG
electroencephalogram
- ACx
auditory cortex
- CS
conditioned stimulus
- RCI
respiration change index
References
- 1.Iversen S D. Compt Rend Acad Sci Sci Vie. 1998;321:209–215. doi: 10.1016/s0764-4469(97)89824-1. [DOI] [PubMed] [Google Scholar]
- 2.Introini-Collison I B, McGaugh J L. Psychopharmacology. 1988;94:379–385. doi: 10.1007/BF00174693. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann J, Nagy J I, Atmadia S, Fibiger H C. Neuroscience. 1980;5:1161–1174. doi: 10.1016/0306-4522(80)90195-5. [DOI] [PubMed] [Google Scholar]
- 4.Mesulam M M, Mufson E J, Wainer B H, Levey A I. Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- 5.Casamenti F, Deffenu G, Abbamondi A L, Pepeu G. Brain Res Bull. 1986;16:689–695. doi: 10.1016/0361-9230(86)90140-1. [DOI] [PubMed] [Google Scholar]
- 6.Kurosawa M, Sato A, Sato Y. Neurosci Lett. 1989;98:45–50. doi: 10.1016/0304-3940(89)90371-6. [DOI] [PubMed] [Google Scholar]
- 7.Rasmusson D D, Clow K, Szerb J C. Neuroscience. 1994;60:665–677. doi: 10.1016/0306-4522(94)90495-2. [DOI] [PubMed] [Google Scholar]
- 8.John E R. Annu Rev Physiol. 1961;23:451–484. doi: 10.1146/annurev.ph.23.030161.002315. [DOI] [PubMed] [Google Scholar]
- 9.Celesia G G, Jasper H H. Neurology. 1966;16:1053–1063. doi: 10.1212/wnl.16.11.1053. [DOI] [PubMed] [Google Scholar]
- 10.Lo Conte G, Bartolini L, Casamenti F, Marconcini-Pepeu I, Pepeu G. Pharmacol Biochem Behav. 1982;17:933–937. doi: 10.1016/0091-3057(82)90475-0. [DOI] [PubMed] [Google Scholar]
- 11.Riekkinen P, Jr, Riekkinen M, Sirviö J, Miettinen R, Riekkinen P. Neuroscience. 1992;47:823–831. doi: 10.1016/0306-4522(92)90032-w. [DOI] [PubMed] [Google Scholar]
- 12.Richardson R T, DeLong M R. Adv Exp Med Biol. 1991;295:233–252. doi: 10.1007/978-1-4757-0145-6_12. [DOI] [PubMed] [Google Scholar]
- 13.Pirch J H. Brain Res Bull. 1993;31:73–83. doi: 10.1016/0361-9230(93)90013-2. [DOI] [PubMed] [Google Scholar]
- 14.Whalen P J, Kapp B S, Pascoe J P. J Neurosci. 1994;14:1623–1633. doi: 10.1523/JNEUROSCI.14-03-01623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maho C, Hars B, Edeline J M, Hennevin E. Psychobiology. 1995;23:10–25. [Google Scholar]
- 16.Orsetti M, Casamenti F, Pepeu G. Brain Res. 1996;724:89–96. doi: 10.1016/0006-8993(96)00292-2. [DOI] [PubMed] [Google Scholar]
- 17.Butt A E, Testylier G, Dykes R W. Psychobiology. 1997;25:18–33. [Google Scholar]
- 18.Oh J D, Edwards R H, Woolf N J. Exp Neurol. 1996;140:95–99. doi: 10.1006/exnr.1996.0119. [DOI] [PubMed] [Google Scholar]
- 19.Wenk G L. Neurobiol Learn Mem. 1997;67:85–95. doi: 10.1006/nlme.1996.3757. [DOI] [PubMed] [Google Scholar]
- 20.Everitt B J, Robbins T W. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 21.Miranda M I, Bermúdez-Rattoni F. Proc Natl Acad Sci USA. 1999;96:6478–6482. doi: 10.1073/pnas.96.11.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutiérrez H, Gutiérrez R, Silva-Gandarias R, Estrada J, Miranda M I, Bermúdez-Rattoni F. Brain Res. 1999;834:136–141. doi: 10.1016/s0006-8993(99)01519-x. [DOI] [PubMed] [Google Scholar]
- 23.Weinberger N M. Neurobiol Learn Mem. 1998;70:226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- 24.Galvan V V, Weinberger N M. J Assoc Res Otolaryngol. 2002;77:78–102. doi: 10.1007/s101620010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Recanzone G H, Schreiner C E, Merzenich M M. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakin J S, Weinberger N M. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjordahl T S, Dimyan M A, Weinberger N M. Behav Neurosci. 1998;112:467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- 28.Kilgard M P, Merzenich M M. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 29.Dimyan M A, Weinberger N M. Behav Neurosci. 1999;113:691–702. doi: 10.1037//0735-7044.113.4.691. [DOI] [PubMed] [Google Scholar]
- 30.Miasnikov A A, McLin D E, III, Weinberger N M. NeuroReport. 2001;12:1537–1542. doi: 10.1097/00001756-200105250-00047. [DOI] [PubMed] [Google Scholar]
- 31.Rasmusson D D. Behav Brain Res. 2000;115:205–218. doi: 10.1016/s0166-4328(00)00259-x. [DOI] [PubMed] [Google Scholar]
- 32. Cahill, L., McGaugh, J. L. & Weinberger, N. M. (2002) Trends Neurosci., in press. [DOI] [PubMed]
- 33.Mackintosh N J. The Psychology of Animal Learning. New York: Academic; 1974. [Google Scholar]
- 34.Lennartz R C, Weinberger N M. Psychobiology. 1992;20:93–119. [Google Scholar]
- 35.Moriizumi T, Hattori T. Neuroscience. 1992;46:701–710. doi: 10.1016/0306-4522(92)90156-v. [DOI] [PubMed] [Google Scholar]
- 36.McLin D E., III . Dissertation. Irvine: Univ. of California; 2001. [Google Scholar]
- 37.Kirk R E. Experimental Design: Procedures for the Behavioral Sciences. Pacific Grove, CA: Brooks/Cole; 1995. [Google Scholar]
- 38.Maloney K J, Cape E G, Gotman J, Jones B E. Neuroscience. 1997;76:541–555. doi: 10.1016/s0306-4522(96)00298-9. [DOI] [PubMed] [Google Scholar]
- 39.Morrell F. Physiol Rev. 1961;41:443–494. doi: 10.1152/physrev.1961.41.3.443. [DOI] [PubMed] [Google Scholar]
- 40.Busnel R G. Acoustic Behaviour of Animals. New York: Elsevier; 1963. [Google Scholar]
- 41.Gewirtz J C, Davis M. Neuropharmacology. 1998;37:453–459. doi: 10.1016/s0028-3908(98)00036-7. [DOI] [PubMed] [Google Scholar]
- 42.Pennartz C M A. Brain Res Rev. 1995;21:219–245. doi: 10.1016/0165-0173(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 43.McBride W J, Murphy J M, Ikemoto S. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 44.Bao S, Chan V T, Merzenich M M. Nature (London) 2000;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- 45.Olds J, Peretz B. Electroencephalogr Clin Neurophysiol. 1960;12:445–454. doi: 10.1016/0013-4694(60)90020-1. [DOI] [PubMed] [Google Scholar]
- 46.Steriade M, Buzsaki G. In: Brain Cholinergic Systems. Steriade M, Biesold D, editors. Oxford: Univ. of Oxford Press; 1990. pp. 3–64. [Google Scholar]
- 47.Wester K. Brain Res. 1972;43:139–145. doi: 10.1016/0006-8993(72)90279-x. [DOI] [PubMed] [Google Scholar]
- 48.Wester K. Electroencephalogr Clin Neurophysiol. 1971;30:52–61. doi: 10.1016/0013-4694(71)90204-5. [DOI] [PubMed] [Google Scholar]
- 49.Biesold D, Inanami O, Sato A, Sato Y. Neurosci Lett. 1989;98:39–44. doi: 10.1016/0304-3940(89)90370-4. [DOI] [PubMed] [Google Scholar]
- 50.Sato A, Sato Y, Uchida S. Int J Dev Neurosci. 2001;19:327–337. doi: 10.1016/s0736-5748(01)00017-x. [DOI] [PubMed] [Google Scholar]
- 51.Lacombe P, Sercombe R, Verrecchis C, Philipson V, MacKenzie E T, Seylaz J. Brain Res. 1989;491:1–14. doi: 10.1016/0006-8993(89)90083-8. [DOI] [PubMed] [Google Scholar]
- 52.Jimenez-Capdeville M E, Dykes R W, Myasnikov A A. J Comp Neurol. 1997;381:53–67. [PubMed] [Google Scholar]
- 53.Gritti I, Mainville L, Mancia M, Jones B E. J Comp Neurol. 1997;383:163–177. [PubMed] [Google Scholar]
- 54.Woolf N J, Butcher L L. Brain Res Bull. 1982;8:751–763. doi: 10.1016/0361-9230(82)90102-2. [DOI] [PubMed] [Google Scholar]