Abstract
Objective
: We tested the null hypothesis that vesical neck descent is the same during a cough and during a Valsalva maneuver. We also tested the secondary null hypothesis that differences in vesical neck mobility would be independent of parity and continence status.
Methods
Three groups were included: 17 nulliparous continent (31.3 ± 5.6; range 22–42 years), 18 primiparous continent (30.4 ± 4.3; 24–43), and 23 primiparous stress-incontinent (31.9 ± 3.9; 25–38) women. Measures of vesical neck position at rest and during displacement were obtained by ultrasound. Abdominal pressures were recorded simultaneously using an intravaginal microtransducer catheter. To control for differing abdominal pressures, the stiffness of the vesical neck support was calculated by dividing the pressure exerted during a particular effort by the urethral descent during that effort.
Results
The primiparous stress-incontinent women displayed similar vesical neck mobility during a cough effort and during a Valsalva maneuver (13.8 mm compared with 14.8 mm; P = .49). The nulliparous continent women (8.2 mm compared with 12.4 mm; P = .001) and the primiparous continent women (9.9 mm compared with 14.5 mm; P = .002) displayed less mobility during a cough than during a Valsalva maneuver despite greater abdominal pressure during cough. The nulliparas displayed greater pelvic floor stiffness during a cough compared with the continent and incontinent primiparas (22.7, 15.5, 12.2 cm H2O/mm, respectively; P = .001).
Conclusion
There are quantifiable differences in vesical neck mobility during a cough and Valsalva maneuver in continent women. This difference is lost in the primiparous stress-incontinent women.
Stress urinary incontinence is characterized by loss of urine during increased abdominal pressure. Prevalence estimates of urinary incontinence range from 8.5% to 38% of women, depending on the population studied.1,2 Although no single factor completely explains stress-incontinence etiology, greater-than-normal vesical neck mobility during increased abdominal pressure is considered to be an important associated factor, particularly in women with no evidence of urethral sphincter deficiency.3,4
The muscular and connective tissue structures that support the urethra provide resistance to downward displacement during increases in abdominal pressure. The resistance of these supporting tissues determines the degree of urethral descent that occurs when abdominal pressure rises. The resistance of this structural mechanism to displacement depends on the integrity of the connective tissue and the state of pelvic muscle contraction during the pressure increase.5
As part of a study of stress urinary incontinence and vaginal delivery, we looked at urethral support during cough and during Valsalva maneuver, activities that increase abdominal pressure. Our goal was to test the null hypothesis that there is no significant difference between vesical neck descent during cough and descent occurring during Valsalva maneuver, when the subject is instructed to relax her pelvic floor muscles. Three groups of women representing the primiparous and nulliparous as well as the continent and incontinent were tested. In addition, we tested the secondary null hypothesis that any difference in vesical neck mobility between cough and Valsalva maneuver would be independent of parity and continence status.
Methods
Observations were made in a group of young women who gave informed consent to participate in an Institutional Review Board–approved, National Institutes of Health–sponsored study of the stress continence mechanism. Three groups were included: continent nulliparous women (n = 17; mean age 31.3 years), continent primiparous women (n = 18; mean age 30.4 years), and stress-incontinent primiparous women (n = 23; mean age 31.9 years). The primiparous stress-incontinent women represented cases and the other two groups were controls, age-matched to ±5 years of the cases. All of the subjects were white. All were healthy primiparous women who were 18 years of age or older, at 35 or more weeks’ gestation, and had their first vaginal delivery at the University of Michigan Hospital; all were sent letters approximately 6 months postpartum. The letters described our study of stress urinary incontinence and included a one-page questionnaire that elicited symptoms of stress incontinence. The women were asked to return the questionnaire if they were interested in being involved in the study. Those women who had symptoms of stress incontinence or those without any urinary symptoms were contacted for enrollment. The study protocol was completed at 9–12 months postpartum. One of the primiparous continent women and one of the primiparous stress-incontinent women had a cesarean delivery before this first vaginal delivery. The nulliparous women were recruited from advertisements in the university and local newspaper. Exclusion criteria included major medical conditions such as diabetes, severe preeclampsia, hypertension, or connective tissue disorders. Other exclusion criteria included recurrent or persistent urinary tract infection, known urogenital tract disease, and known neurologic disorder.
A standing paper towel test6 and provocative stress testing were used to confirm continence status. A cotton swab test was performed to measure urethral mobility during a Valsalva maneuver. An 8-French dual-tip Gaeltec catheter (Medical Measurements Inc., Hackensack, NJ) was inserted into the bladder, and the Aquarius Urodynamic System (Laborie Medical Technologies Co., Williston, VT) was used to perform a cystometrogram. The bladder was filled at a rate of 100 mL per minute to a maximum of 300 mL or maximum bladder capacity if this was less than 300 mL. While remaining in the supine position, patients were asked to cough and strain with leak-point pressures recorded if leakage was observed. If leakage was not observed in the supine position, these maneuvers were repeated standing.
Measures of vesical neck position at rest and during displacement were obtained using the Sonoline SI-400 ultrasound (Siemens AG, Erlangen, Germany) with a 5-MHz curved array probe from which videotaped recordings of the urethral mobility were recorded. The ultrasound measurements were a modification of the method developed by Schaer et al.7 The ultrasound examinations were performed by the member of the research team who was assigned to data collection that day. These people included either a urogynecology faculty member or a urogynecology fellow. After completion of the cystometrics, the women were asked to stand with their feet apart so the ultrasound probe could be placed against the vulva in the sagittal plane. Care was taken to keep the probe’s location, orientation, and application pressure constant. Keeping the symphysis located to the right of the screen controlled the location of the probe. The orientation was attained the same way with each individual, keeping the vesical neck and symphysis pubis in the same location on the screen. Enough pressure was applied to have good visualization but not so excessive as to cause the image to blur. Figure 1 demonstrates how the measurements were taken. These measurements were taken from the recorded study once the data-collection session was completed. The central axis of the symphysis pubis and its inferior border are used to establish measurement axes. The vesical neck and its relation to the symphysis pubis are seen. The X and Y coordinates are demonstrated. The resting position of the vesical neck is measured using these coordinates. During cough or Valsalva maneuver, the vesical neck moves from its resting position to the new position as demonstrated by the arrow. The distance that the vesical neck moves during a hard cough and maximal Valsalva maneuver is then measured directly. Abdominal pressures were recorded simultaneously using an 8-French dual-tip Gaeltec catheter placed intravaginally. The stiffness of the vesical neck support was calculated by dividing the change in abdominal pressure exerted during a particular effort by the urethral descent recorded during that effort. This calculation controls for differing abdominal pressures.
Figure 1.
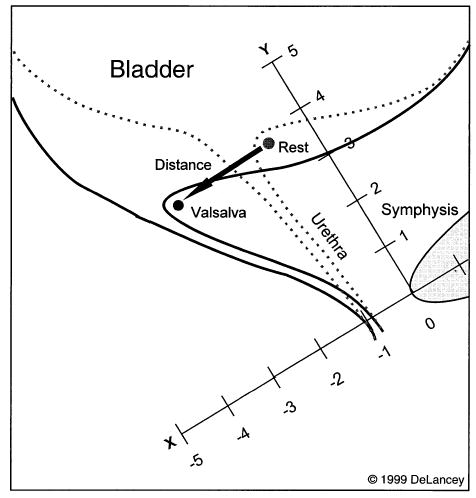
Ultrasound assessment of vesical neck position at rest and during Valsalva.
Within-group comparisons of continuous variables were tested using the two-sided paired t test, and between-group comparisons were tested using one-way analysis of variance. Fisher protected least significant difference was used for post hoc testing. P values of ≤.05 were considered statistically significant.
Methods, definitions, and units conform to the standards proposed by the International Continence Society, except where specifically noted.
Results
All groups of women were of similar age and body mass index (Table 1). Table 1 also contains the gestational age, birth weight, and number of months postpartum for the primiparous women. All of the women defined as being stress incontinent reported a variable number of leakage episodes ranging from a few times per week to a few times per month. Thirteen of these women had both a positive paper towel test and provocative stress test with the leak-point pressures recorded. The mean volume lost during the paper towel testing was 1 mL. The mean leak-point pressure was 108 cm H2O with a standard deviation (SD) of 36. One woman had a negative paper towel test but positive provocative stress test with leak-point pressure recorded, and nine women had a positive paper towel test but leak-point pressures could not be recorded. All of the continent women denied any episodes of leakage and had a negative paper towel test and negative provocative stress testing.
Table 1.
Demographic and Baseline Parameters for Each Group
| Nulliparous continent
|
Primiparous continent
|
Primiparous stress incontinent
|
|||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | (range) | Mean ± SD | (range) | Mean ± SD | (range) | P | |
| Age (y) | 31.3 ± 5.6 | (22–42) | 30.4 ± 4.3 | (24–43) | 31.9 ± 3.9 | (25–38) | .57 |
| Body mass index (kg/m2) | 23.5 ± 2.9 | (19.5–31.2) | 24.9 ± 4.3 | (19.2–35.0) | 25.8 ± 5.1 | (18.7–35.0) | .23 |
| Gestational age (wk) | 39.2 ± 2.0 | (35–42) | 39.6 ± 1.2 | (37–42) | .40 | ||
| Infant weight (g) | 3631 ± 531 | (2540–4580) | 3558 ± 521 | (2500–4690) | .66 | ||
| Months postpartum | 9.9 ± 1.4 | (7.9–12.1) | 9.3 ± 1.1 | (6.9–10.9) | .10 | ||
| Cotton swab test (degrees) | 36.5 ± 17.9 | (10–70) | 47.5 ± 11.7 | (25–70) | 54.6 ± 16.5 | (25–90) | .003 |
| Resting position on X-coordinate (mm)* | 0.4 ± 5.8 | (−13.0–8.4) | −2.2 ± 6.4 | (−18.0–8.0) | −4.8 ± 7.7 | (−21.0–9.6) | .06 |
| Resting position on Y-coordinate (mm)* | 22.9 ± 3.0 | (18–30) | 24.3 ± 4.6 | (18–33) | 22.3 ± 6.8 | (11–39) | .48 |
SD = standard deviation.
Resting X- and Y-coordinates refer to the position of the vesical neck at rest.
The cotton swab test demonstrated greater mobility during the Valsalva maneuver in the primiparous stress-incontinent group compared with the primiparous continent group. The primiparous continent group also had greater vesical neck mobility than the nulliparous continent group (Table 1). Post hoc analysis revealed this difference to be significant when the nulliparous women were compared with both primiparous groups.
The vesical neck location at rest was closer to the symphysis pubis in the nulliparous continent group (X-coordinate) compared with the primiparous continent and primiparous stress-incontinent women, although this did not reach statistical significance. The measurements taken along the Y-coordinate were not significantly different between groups (Table 1).
Figure 2 is a box plot showing the distance moved during cough and Valsalva maneuver for all groups. In these plots the dot represents the mean, the line depicts the median, and the box encompasses the 25th to the 75th percentile. The whiskers represent the 10th and 90th percentile. Each group demonstrated vesical neck descent with Valsalva maneuver. No significant differences were found between groups in the mean distance moved during Valsalva maneuver (12.4 ± 4.7 mm SD, nulliparous continent compared with 14.5 ± 7.0 mm primiparous continent and 14.8 ± 6.4 mm primiparous stress incontinent; P = .42). Significant differences were found in vesical neck mobility measured during cough when comparing the nulliparous continent (8.2 ± 4.1 mm) and primiparous continent (9.9 ± 4.0 mm) with the primiparous stress incontinent (13.8 ± 5.4 mm; P = .001; Figure 2).
Figure 2.
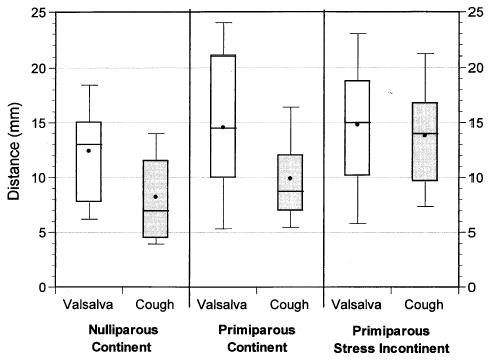
Box plot showing the distance that the vesical neck moves with a Valsalva maneuver and cough effort.
The primary null hypothesis was supported in the primiparous stress-incontinent women: they displayed an equivalent vesical neck mobility during Valsalva maneuver and during cough (14.8 mm compared with 13.8 mm, P = .49; Figure 2). The null hypothesis was rejected in nulliparous and primiparous continent women who displayed significantly less mobility during cough compared with that produced with Valsalva maneuver, as shown in Figure 2 (nulliparous continent: 8.2 during cough compared with 12.4 mm with Valsalva; P = .001, and primiparous continent: 9.9 during cough compared with 14.5 mm during a Valsalva; P = .002). This difference occurred despite the fact that greater pressures were generated during cough than during Valsalva maneuver. When comparing the three groups, there were no significant differences in the cough pressures (160 ± 35 cm H2O in the nulliparous continent compared with 139 ± 29 in the primiparous continent, and 147 ± 48 in the primiparous stress incontinent; P = .36) or Valsalva pressures (111 ± 25 cm H2O in the nulliparous continent compared with 112 ± 41 in the primiparous continent, and 106 ± 45 in the primiparous stress incontinent; P = .87) generated by each group. When comparing the cough stiffness with the Valsalva stiffness, all women were noted to generate greater support during cough than during Valsalva maneuver (nulliparous continent: 22.7 compared with 10.6 cm H2O/mm, P = 0.001; primiparous continent: 15.5 compared with 9.3, P < .001; and primiparous stress incontinent: 12.2 compared with 8.4, P = .001; Figure 3).
Figure 3.
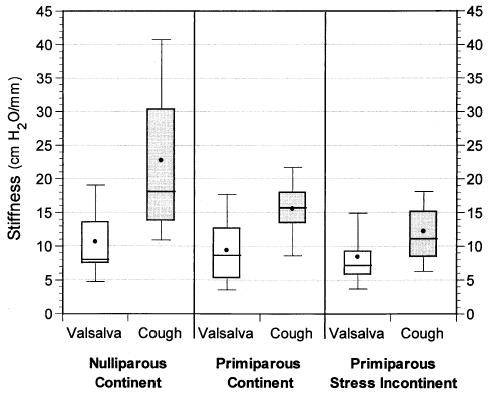
Box plot showing urethral support stiffness during a cough and Valsalva maneuver.
To test the secondary hypothesis that difference in vesical neck mobility would be independent of parity and continence status, we made an intergroup stiffness comparison. The cough stiffness was significantly greater in the nulliparas compared with the primiparous continent and stress incontinent (22.7 ± 11.2 cm H2O/mm, 15.5 ± 4.5, and 12.2 ± 6.7, respectively; P = .001; Figure 3). The stiffness was also greater in the primiparous continent compared with the incontinent women, although this difference did not reach statistical significance. The Valsalva stiffness was not significantly different among groups (10.6 ± 6.2 cm H2O/mm in the nulliparous continent compared with 9.3 ± 4.9 in the primiparous continent and 8.4 ± 4.9 in the primiparous stress incontinent; P = .47).
Finally, we calculated the difference between the mean cough stiffness and the mean Valsalva stiffness. The mean stiffness difference confirmed group variation in support system stiffness (nulliparous continent: 12.1 ± 10.0 cm H2O/mm compared with primiparous continent: 6.2 ± 5.4 and primiparous stress incontinent: 3.8 ± 4.7; P = .002). Post hoc analysis confirmed that this difference was due to a stiffer pelvic floor in nulliparous continent women compared with the two primiparous groups.
Discussion
This report confirms variation in vesical neck mobility during cough and Valsalva maneuver. These differences in mobility are influenced by parity and continence status. The fact that the nulliparous continent and primiparous continent women had less mobility during cough effort than during Valsalva maneuver is interesting. What is even more interesting is the fact that this difference in mobility is lost in the primiparous incontinent women.
Factors affecting vesical neck mobility are poorly understood, but likely involve neuromuscular elements8,9 along with connective tissue integrity and elasticity.10 The occurrence of pelvic muscular activity during cough has been reported.11,12 The significance of this observation may be that this muscular activity results in an increase in stiffness of the urethral support structures, due to contraction of the striated levator ani muscles during cough. The stiffness of a structure is a measure of the resistance that it offers to deformation.13 We know that a 50% increase in striated muscle activity can more than quadruple muscle tensile stiffness.14 By contrast, the Valsalva maneuver is carried out with relaxation of the pelvic muscles. If these observations are true, then the extent of deflection of the urethra under these distinct stressors may differ despite equivalent rises in intra-abdominal pressure.
The standard method for quantifying urethral hyper-mobility has been the cotton swab test, which measures the excursion of the vesical neck during a slow Valsalva maneuver.15 Traditionally, hypermobility is diagnosed when the cotton swab angle exceeds 30°, when measured from rest to maximal strain position. However, hypermobility when slowly straining down is not seen exclusively in patients with genuine stress incontinence; it is also seen in many normal continent women.16,17 This latter observation challenges the prevailing assumption that cotton swab measures made during Valsalva maneuver provide a valid estimate of the hypermobility risk factor purported to correspond with stress incontinence under conditions of cough or sneeze.
The following explanations might address our observations. With the pelvic muscles relaxed, the urethra descends until the elastic limits of its connective tissue supports are reached. In normal nulliparous and parous continent women, activation of the muscles during coughing would stabilize the urethra, preventing its descent. With damage to the muscle, its nerve supply, or the connection between the muscle and connective tissue, the activation of this system is impaired and stabilization of the urethra impossible. Of course, more specific investigations will be needed to test these mechanistic hypotheses, but identification of these cough and Valsalva differences points to interesting aspects of the dynamic operation of the continence mechanism.
This study has several limitations. The women volunteered to participate, and it is likely that women with more severe incontinence will elect to become involved. In addition, the data collector was not masked to a woman’s status. Also, the degree of vesical neck mobility during Valsalva maneuver is dependent upon the degree of pelvic floor relaxation. Relaxation was dependent upon the examiner’s ability to coach the woman and the woman’s comfort during the examination. However, it seems unlikely that these differences would bias the results because any difficulty in coaching would be random.
Results of this preliminary study may shed light on the stabilization of the urethra during increased intra-abdominal pressure. The data also place emphasis on our need to understand the role that pregnancy and childbirth play in the development of pelvic neuromuscular damage. These findings indicate that cough-induced hypermobility may be distinct from Valsalva-induced hypermobility and may have greater selectivity in predicting risk for stress urinary incontinence.
Footnotes
Funded by the National Institutes of Health RO1 DK 51405-01A1.
References
- 1.Thomas TM, Plymat KR, Blannin J, Meade TW. Prevalence of urinary incontinence. BMJ. 1980;281:1243–5. doi: 10.1136/bmj.281.6250.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herzog AR, Diokno AC, Brown MB, Normolle DP, Brock BM. Two-year incidence, remission, and change patterns of urinary incontinence in noninstitutionalized older adults. J Gerontol. 1990;45:M67–74. doi: 10.1093/geronj/45.2.m67. [DOI] [PubMed] [Google Scholar]
- 3.Jeffcoate TNA, Roberts H. Observation on stress incontinence of urine. Am J Obstet Gynecol. 1952;64:721–38. doi: 10.1016/s0002-9378(16)38792-0. [DOI] [PubMed] [Google Scholar]
- 4.Hodgkinson CP. Relationships of the female urethra in urinary incontinence. Am J Obstet Gynecol. 1953;65:560–73. doi: 10.1016/0002-9378(83)90612-9. [DOI] [PubMed] [Google Scholar]
- 5.DeLancey JOL. Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713–23. doi: 10.1016/s0002-9378(94)70346-9. [DOI] [PubMed] [Google Scholar]
- 6.Miller JM, Ashton-Miller JA, DeLancey JOL. Quantification of cough-related urine loss using the paper towel test. Obstet Gynecol. 1998;91:705–9. doi: 10.1016/s0029-7844(98)00045-3. [DOI] [PubMed] [Google Scholar]
- 7.Schaer GN, Koechli OR, Schuessler B, Haller U. Perineal ultrasound for evaluating the bladder neck in urinary stress incontinence. Obstet Gynecol. 1995;85:220–4. doi: 10.1016/0029-7844(94)00369-O. [DOI] [PubMed] [Google Scholar]
- 8.Snooks SJ, Badenoch DF, Tiptaft RC, Swash M. Perineal nerve damage in genuine stress urinary incontinence. An electrophysiological study. Br J Urol. 1985;57:422–6. doi: 10.1111/j.1464-410x.1985.tb06302.x. [DOI] [PubMed] [Google Scholar]
- 9.Smith ARB, Hosker GL, Warrell DW. The role of pudendal nerve damage in the aetiology of genuine stress incontinence in women. Br J Obstet Gynaecol. 1989;96:29–32. doi: 10.1111/j.1471-0528.1989.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 10.Richardson AC, Edmonds PB, Williams NL. Treatment of stress urinary incontinence due to paravaginal fascial defect. Obstet Gynecol. 1981;57:357–62. [PubMed] [Google Scholar]
- 11.Taverner D. An electromyographic study of the normal function of the external anal sphincter and pelvic diaphragm. Dis Colon Rectum. 1959;2:153–60. doi: 10.1007/BF02616708. [DOI] [PubMed] [Google Scholar]
- 12.Constantinou CE, Govan DE. Spatial distribution and timing of transmitted and reflexly generated urethral pressures in healthy women. J Urol. 1982;127:964–9. doi: 10.1016/s0022-5347(17)54148-8. [DOI] [PubMed] [Google Scholar]
- 13.Skalak R, Chien S. Handbook of bioengineering. New York: McGraw-Hill, 1987.
- 14.Sinkjaer T, Toft E, Andreassen S, Hornemann B. Muscle stiffness in human ankle dorsiflexors: Intrinsic and reflex components. J Neurophysiol. 1988;60:1110–21. doi: 10.1152/jn.1988.60.3.1110. [DOI] [PubMed] [Google Scholar]
- 15.Crystle CD, Charme LS, Copeland WE. Q-tip test in stress urinary incontinence. Obstet Gynecol. 1971;38:313–5. [PubMed] [Google Scholar]
- 16.Bergman A, McCarthy TA, Ballard CA, Yanai J. Role of the Q-tip test in evaluating stress urinary incontinence. J Reprod Med. 1987;32:273–5. [PubMed] [Google Scholar]
- 17.Ala-Ketola L. Roentgen diagnosis of female stress urinary incontinence. Roentgenological and clinical study. Acta Obstet Gynecol Scand Suppl. 1973;23:1–59. [PubMed] [Google Scholar]


